Abstract
Connexin 43, a gap junction protein, is expressed mainly in glia in the central nervous system. Neuroinflammation plays an important role in central nervous system injury. Changes to glial connexin 43 levels and neuroinflammation may trigger brain injury and neurodegenerative diseases. To illustrate the relationship between connexin 43 and neuroinflammation, this study investigated how connexin 43 expression levels change in lipopolysaccharide-stimulated rat C6 glioma cells. C6 cells were treated with 0.05, 0.25, 0.5, 1, 2.5 and 5 μg/mL lipopolysaccharide for 24 hours. The nitrite estimation-detected nitric oxide release level was elevated substantially after lipopolysaccharide stimulation. To test the transcriptional level changes of inducible nitric oxide synthase, tumor necrosis factor-α and connexin 43 mRNA, C6 cells were treated with 5 μg/mL lipopolysaccharide for 3–48 hours. Reverse transcription-PCR showed that the expression of inducible nitric oxide synthase and tumor necrosis factor-α mRNA increased over time, but connexin 43 mRNA levels increased in lipopolysaccharide-stimulated C6 cells at 3 and 6 hours, and then decreased from 12 to 48 hours. Connexin 43 protein expression was detected by immunofluorescence staining, and the protein levels matched the mRNA expression levels. These results suggest that connexin 43 expression is biphasic in lipopolysaccharide-induced neuroinflammation in C6 cells, which may be correlated with the connexin 43 compensatory mechanism.
Keywords: connexin, lipopolysaccharide, C6 cells, neuroinflammation, central nervous system, inducible nitric oxide synthase, tumor necrosis factor-α, neural regeneration
Research Highlights
-
(1)
After stimulating inflammation using lipopolysaccharide, this study investigated the dynamic changes of connexin 43 mRNA expression in the central nervous system over time.
-
(2)
Released levels of nitric oxide, inducible nitric oxide synthase and tumor necrosis factor-α mRNA expression increased in C6 cells under lipopolysaccharide stimulation.
-
(3)
Connexin 43 mRNA and protein expression increased significantly at 3 and 6 hours, and then decreased at 12, 24 and 48 hours after lipopolysaccharide treatment.
Abbreviations
iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-α; CNS, central nervous system
INTRODUCTION
Connexins are a family of membrane proteins. At least 21 human genes and 20 mouse genes for connexins have been identified[1], and they are commonly named according to their molecular weight (e.g. connexin 43 is 43 kDa). All connexin subtypes share a common structure, having four trans-membrane domains with two extracellular loops, an intracellular loop and the cytoplasmic C- and N-termini. Six connexins cluster into a connexon, and two connexons dock together to form a gap junction channel[2]. Between neighboring cells, gap junction channels allow for the diffusion of small molecules (< 1 kDa), which include molecules such as adenosine triphosphate, charged ions, cyclic adenosine monophosphate and peptides. Astrocytic gap junctions play a vital role in maintaining ionic and metabolic stability in the central nervous system (CNS)[3,4,5,6]. Connexin 43 is the most ubiquitously expressed protein and is predominantly found in astrocytes[7]. The functional syncytium formed by astrocytes via connexin 43 gap junction intercellular communication has been implicated in maintaining the homeostasis of the extracellular milieu of neurons[8].
Lipopolysaccharide, also known as endotoxin, constitutes the lipid portion of the outer leaflet of gram-negative bacteria[9]. Lipopolysaccharide functions as a potent inducer of innate immunity and triggers an inflammatory reaction[10]. Tumor necrosis factor-α (TNF-α), as a single nuclear factor, can be generated in lipopolysaccharide stimulation, and lead to the inflammatory response. Inducible nitric oxide synthase (iNOS) does not express in resting cells, while the cells were stimulated by cytokines or immunologic microbes, such as lipopolysaccharide-induced; iNOS synthesizes high concentration of nitric oxide, and it generates a series of pathological effects. A bacterial lipopolysaccharide-induced brain injury mouse model was created after intracerebral injection of lipopolysaccharide, but the pathogenesis of inflammation in the CNS is poorly understood. Inflammation in the CNS contributes to numerous neurodegenerative diseases and results in encephalopathy[11].
Sayyah et al[12] showed that connexin 43 containing gap junctions in the hippocampus was down-regulated in response to chronic injection of lipopolysaccharide. Haupt et al[13] reported that reactive proliferating astrocytes formed gliotic tissue and exhibited up-regulated connexin 43 expression at the mRNA and protein levels after photothrombosis. An increase in connexin 43 and connexin 43 mRNA-positive cells in the gray matter adjacent to the lesion was observed as early as 4 hours post-injury and remained high for 4 weeks[14]. To analyze the effect of the neuroinflammation on connexin 43 expression in glial cells, and to address the relationship between connexin 43 and neuroinflammation, the present study investigated the dynamic expression of connexin 43 in neuroinflammatory events over time. Reverse transcription-PCR and immunofluorescence staining were used to detect the mRNA and protein expression levels of connexin 43 in lipopolysaccharide-stimulated C6 cells.
RESULTS
Lipopolysaccharide-induced neuroinflammation in C6 cells
The nitrite estimation showed that 50, 250, 1 000, 2 500, and 5 000 ng/mL lipopolysaccharide increased the release of nitric oxide (Figure 1).
Figure 1.
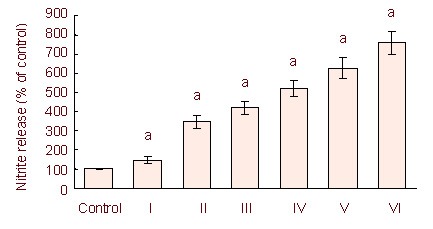
Lipopolysaccharide stimulation for 24 hours induced a dose-dependent release of nitrite in C6 cells.
Independent sample t- test and one-way analysis of variance were used to analyze the data. aP < 0.05, vs. control group. Histograms represent the mean ± SD of three independent experiments (n = 3).
I–VI: 50, 250, 1 000, 2 500, and 5 000 ng/mL lipopolysaccharide treatment.
Lipopolysaccharide-induced nitric oxide release was dose dependent. We used 5 μg/mL of lipopolysaccharide for 3–48 hours to stimulate C6 cells and induce neuroinflammation.
iNOS and TNF-α mRNA expression in C6 cells treated with lipopolysaccharide
To further confirm lipopolysaccharide-induced neuroinflammation in C6 cells, and to observe the changes of inflammatory factors, we observed iNOS and TNF-α mRNA expression. Using reverse transcription-PCR, C6 cells treated with 5 μg/mL lipopolysaccharide, showed increased expression of iNOS and TNF-α mRNA compared with the control group which was treated with the same volume of PBS. The expression of iNOS and TNF-α mRNA increased in a time-dependent manner (Figure 2).
Figure 2.
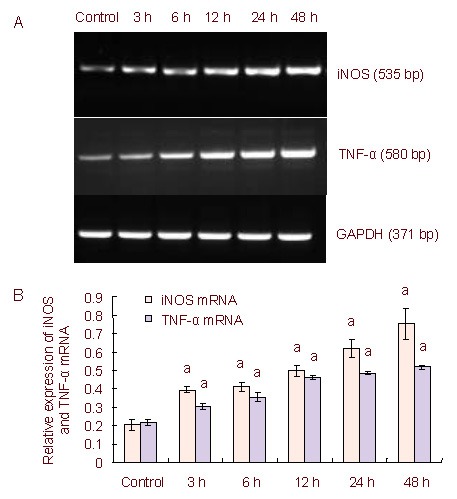
Inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α (TNF-α) mRNA expression in lipopolysaccharide-stimulated C6 cells over time.
(A) Reverse transcription-PCR iNOS and TNF-α mRNA expression levels in lipopolysaccharide-induced C6 cells. Lane 1: Control; lane 2: 3 hours; lane 3: 6 hours; lane 4: 12 hours; lane 5: 24 hours; lane 6: 48 hours.
(B) Quantification of iNOS and TNF-α mRNA expression. The data are expressed as mean ± SD of the band absorbance ratio of the target gene to GAPDH, and analyzed by one-way analysis of variance and the least significant difference method.
The experiments were repeated at least three times (n = 3). Independent sample t-tests and one-way analysis of variance were used to analyze the data. aP < 0.05, vs. control group.
3, 6, 12, 24, 48 h: After 3, 6, 12, 24, 48 hours of lipopolysaccharide treatment.
Connexin 43 mRNA expression in C6 cells
The expression of connexin 43 mRNA was detected by reverse transcription-PCR. The results showed that the expression of connexin 43 mRNA increased significantly at 3 and 6 hours compared with the control group, but decreased significantly at 12, 24 and 48 hours compared with the control group (Figure 3).
Figure 3.
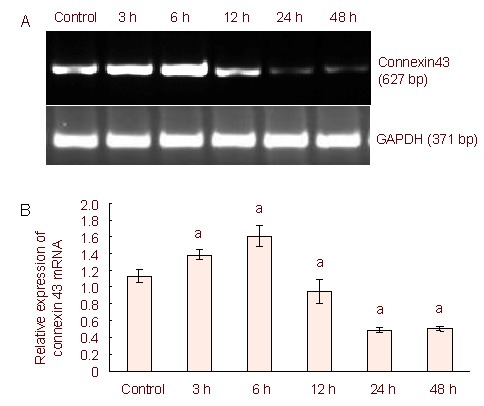
Connexin 43 mRNA expression in lipopolysaccharide-stimulated C6 cells using reverse transcription-PCR.
(A) Reverse transcription-PCR connexin 43 mRNA expression levels in lipopolysaccharide-induced C6 cells.
Connexin 43 mRNA expression increased at 3 and 6 hours compared with the control group, but then decreased at the 12, 24, and 48 hour time points compared with the control group.
(B) Quantification of connexin 43 mRNA expression. The data were expressed as mean ± SD of the band absorbance ratio of the target gene to GAPDH, and analyzed by one-way analysis of variance and the least significant difference method.
The experiments were repeated at least three times (n = 3). Independent sample t- tests and one-way analysis of variance were used to analyze the data. aP < 0.05, vs. control group.
3, 6, 12, 24, 48 h: After 3, 6, 12, 24, 48 hours of lipopolysaccharide treatment.
Connexin 43 protein expression in C6 cells
We used immunofluorescence staining to observe connexin 43 protein expression levels. Connexin 43 protein expression was detectable in the cytoplasm and in protrusions in the control group. At 3 and 6 hours, connexin 43 protein expression increased significantly in the cytoplasm and the protrusions compared with the control group (P < 0.05). Connexin 43 protein expression decreased in a time-dependent manner (Figure 4) at 12, 24 and 48 hours compared with the control group (P < 0.05), especially in the protrusions. Combined with the iNOS and TNF-α mRNA expression, proinflammatory cytokines severely reduced connexin 43 expression, and aggravated the neuroinflammation after 12 hours.
Figure 4.
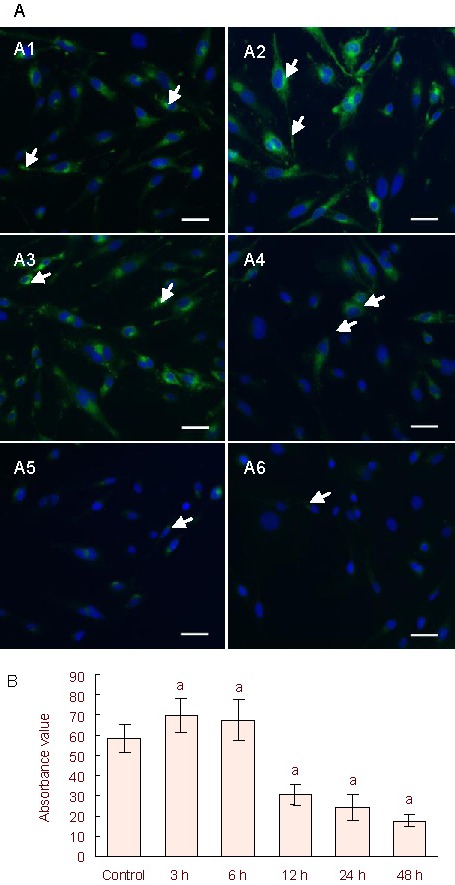
Connexin 43 protein expression in C6 cells.
(A) Immunofluorescence staining detected connexin 43 protein expression in lipopolysaccharide-treated C6 cells. The nuclei were stained blue using 4’, 6-diamidino- 2-phenylindole.
(A1) Control group: connexin 43 expression (fluorescein isothiocyanate, green) was detected in the cytoplasm and protrusions (arrows). (A2–A6) 5 μg/mL lipopolysaccharide-treated groups (for 3, 6, 12, 24, and 48 hours), with arrows showing connexin 43 protein expression. Scale bars: 25 μm.
(B) Semi-quantification of immunofluorescence staining. Six non-overlapping fields were randomly selected and measured for absorbance values using ImageJ for each group.
The experiments were repeated three independent times. Independent sample t-tests and one-way analysis of variance were used to analyze the data. aP < 0.05, vs. control group.
3, 6, 12, 24, 48 h: After 3, 6, 12, 24, 48 hours of lipopolysaccharide treatment.
DISCUSSION
Neuroinflammation plays a vital role in the pathophysiology of neurodegenerative diseases and CNS injury[15]. Neuroinflammation is a process that results primarily from the presence of chronically activated glial cells (astrocytes and microglia) in the brain, and is a common feature of several neurodegenerative conditions. Activated glia release a variety of nerve excitability substances that potentiate neurotransmission, especially proinflammatory cytokines[16].
Connexin 43 expression changes during neuroinflammation. Kozoriz et al[17] showed that connexin 43 protein exhibited decreased cerebral infarct size and inflammatory cell invasion in the peri-infarct region with reduced astrogliosis in an mouse model of middle cerebral artery occlusion.
Haupt et al[13] reported that reactive proliferating astrocytes exhibited up-regulated connexin 43 expression at the mRNA and protein levels with CNS injury. To explain the role of connexin 43 expression in neuroinflammatory events, we stimulated C6 cells with lipopolysaccharide to establish a neuroinflammatory cell model. In this study, our data showed that nitric oxide release gradually increased with different concentrations of lipopolysaccharide-stimulated C6 cells for 24 hours.
Gap junctions between astrocytes have been implicated in traumatic brain injury and brain infarcts[18]. Astrocytes are abundant in the brain, and compose a glial network which supports neurons both physically and chemically. Astrocytes play an important role in the maintenance of neuronal activity[19]. Activated astrocytes can secrete cytokines, such as interleukin-1, interleukin-6, and TNF-α[20] and interact with inflammatory cells. Along with iNOS and TNF-α, interleukin-1β has been reported to influence astrocyte gap junction/hemichannel activity in vitro[21]. However, the role of astrocytic gap junctions is still controversial in inflammatory, traumatic and ischemic conditions. Astrocytic gap junctions may enhance an expansion of lesion volume[22]. Alternatively, another previous study demonstrated that blocking astrocytic gap junctions increased the vulnerability of neurons to ischemic insults[23]. Astrocytic gap junctions may reduce apoptosis and inflammation in the penumbra following ischemic insults[24]. Because connexin 43 is the major protein forming astrocytic gap junctions, we evaluated connexin 43 expression levels. In the present study, we selected rat C6 glioma cells, because C6 cells demonstrate astrocytic characteristics.
Our data demonstrated that connexin 43 mRNA expression increased at 3 and 6 hours, and then declined from 12 to 48 hours in lipopolysaccharide-stimulated C6 cells. Connexin 43 protein was expressed in the cytoplasm and protrusions in normal C6 cells. In lipopolysaccharide-stimulated C6 cells, connexin 43 protein expression increased at 3 and 6 hours, but decreased from 12 to 48 hours, especially in the protrusions. We hypothesize that the mechanism regulating connexin 43 expression in the early stage of neuroinflammation may be different than in the late stage.
The significantly decreased protein levels of connexin 43 in the protrusions may be associated with decreased gap junction function. Similar to the iNOS and TNF-α mRNA expression levels changes, connexin 43 expression may be affected by pro-inflammatory cytokines in neuroinflammation after 12 hours. The change of connexin 43 expression may be associated with a connexin 43 compensatory mechanism.
Early in neuroinflammation injury, a connexin 43 compensatory mechanism begins, which enhances connexin 43 mRNA and protein expression. This increased expression may allow the formation of functional gap junction channels to promote the exchange of relevant injury factors among the C6 cells. In the late stage, the neuroinflammation injury is more serious. Then the compensatory mechanisms of connexin 43 could play a lesser role, and the expression levels of connexin 43 mRNA and protein are decreased. Although we observed a dynamic change in the connexin 43 expression levels over time, whether or not connexin 43 formed the functional gap junctions in this model of neuroinflammation is unknown.
In conclusion, connexin 43 expression increased at 3 and 6 hours, and declined from 12 to 48 hours in lipopolysaccharide-stimulated rat C6 glioma cells. This dynamic change of the major component of gap junctions requires further investigation to elucidate the role of the changing levels of connexin 43 in neuroinflammation and CNS injury models.
MATERIALS AND METHODS
Design
This is an in vitro parallel experiment.
Time and setting
The experiments were performed at the Department of Human Anatomy, Norman Bethune College of Medicine, Jilin University, China from October 2011 to April 2012.
Materials
C6 cells (Rattus norvegicus glioma cells) were stored in the Department of Human Anatomy, Norman Bethune College of Medicine, Jilin University, China. Lipopolysaccharide was purchased from Sigma, St. Louis, MO, USA (Product No. L2630).
Methods
C6 cell culture
C6 cells were cultured in Roswell Park Memorial Institute-1640 medium (10% fetal calf serum, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM glutamine; Gibco, Grand Island, NY, USA) in a 5% CO2 humidified incubator at 37°C. The culture medium was replaced every 2 days and C6 cells were observed using an inverted microscope (Olympus, Tokyo, Japan).
Drug dispensation
Lipopolysaccharide was dissolved at 5 mg/mL in distilled water, and stored at –20°C. The final concentrations of lipopolysaccharide used on the C6 cells were 50, 250, 500, 1 000, and 5 000 ng/mL. The cells untreated with lipopolysaccharide were the control groups.
Nitrite estimation
Nitrite estimations in culture supernatants were performed using the Griess reagent. C6 cells were seeded at a cell density of 1 × 104 cells/well in 96 well plates. Each culture supernatant (100 μL) was mixed with an equal amount of Griess reagent (1% p-amino-benzene sulfonamide, 0.01% napthyl ethylenediamine in 2.5% phosphoric acid; Nanjing Jiancheng Bioengineering Institute, China) and incubated at room temperature for 15 minutes in the dark. Absorbance was read at 570 nm using a microplate reader (Sunrise™, Tecan Group Ltd., Männedorf, Switzerland). The release of nitric oxide was expressed as the percent increase from basal levels.
Reverse transcription-PCR for detection of iNOS, TNF-α and connexin 43 mRNA expression
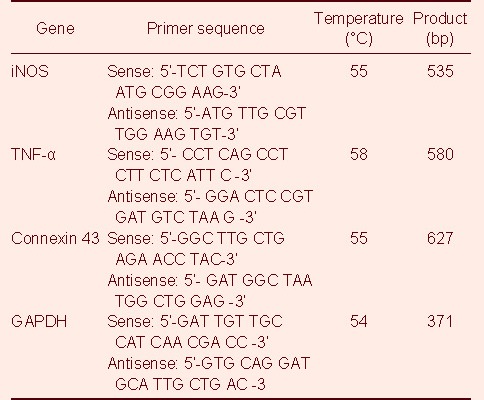
C6 cells were cultured in 6-well cell culture plates (Corning Incorporated, Corning, NY, USA) and then incubated for 3–48 hours after adding 5 μg/mL lipopolysaccharide. Total cell RNA was extracted in Trizol (Invitrogen, Carlsbad, CA, USA) and 1 μg RNA samples were then reverse-transcribed with the specific antisense primer using a one-step reverse transcription-PCR kit according to the manufacturer's protocol (Takara Biotechnology, Dalian, China). Primers were synthesized by the Shanghai Biological Technology Company, China. The primer sequences are shown in Table 1.
Table 1.
The primer sequences for inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), connexin 43 and GAPDH
PCR was performed for 30 cycles, with each cycle consisting of 94°C for 30 seconds, 55°C/58°C/53°C for 30 seconds and 72°C for 40 seconds, followed by a final extension step at 72°C for 10 minutes. PCR products were subjected to 1.5% agarose gel electrophoresis, and stained with ethidium bromide for detection. The specific PCR bands were analyzed using an ImageMaster Video Documentation System (Pharmacia Biotech Company, Uppsala, Sweden).
Immunofluorescence staining for connexin 43 protein expression
Sterilized slides were placed in 6-well culture plates (Corning Incorporated). After an equal amount of cell suspension was dropped onto the slides, the cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C for 3 hours. When the cells firmly adhered to the slides, the slides were carefully fixed with 4% paraformaldehyde and washed three times with PBS. Following non-immune goat serum to block nonspecific antigens, cells were incubated with polyclonal rabbit anti-connexin 43 (1:500; Sigma) at 4°C overnight, washed three times with PBS and then processed with goat anti-rabbit IgG-fluorescein isothiocyanate (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 15 minutes. After the sections were washed three times with PBS, 4’, 6-diamidino-2-phenylindole was used to stain the nuclei. Photographs were taken with a DP72 camera (Olympus, Tokyo, Japan). At least three slides were selected from each group, and six non-overlapping fields of vision were randomly selected from each slide. ImageJ software (ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA) was used to quantitate the expression of target proteins.
Statistical analysis
The data were analyzed using SPSS 11.5 (SPSS, Chicago, IL, USA) software, represented as mean ± SD. The results were analyzed by independent sample t-tests and one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Liankun Sun, Hongyan Li and Jiamei Liu for their comments on the manuscript and helpful discussions.
Footnotes
Funding: This study was sponsored by the National Natural Science Foundation of China, No. 30901323.
Conflicts of interest: None declared.
(Edited by Tong XJ, Li YJ/Qiu Y/Song LP)
REFERENCES
- [1].Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10(4-6):173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- [2].Yeager M, Harris AL. Gap junction channel structure in the early 21st century: Facts and fantasies. Curr Opin Cell Biol. 2007;19(5):521–528. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20(4):299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- [4].Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94(1-2):120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- [6].Gandhi GK, Cruz NF, Ball KK, et al. Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. J Neurochem. 2009;110(3):857–869. doi: 10.1111/j.1471-4159.2009.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Contreras JE, Sánchez HA, Véliz LP, et al. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47(1-3):290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chew SS, Johnson CS, Green CR, et al. Role of connexin43 in central nervous system injury. Exp Neurol. 2010;225(2):250–261. doi: 10.1016/j.expneurol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- [9].Cipolla L, Polissi A, Airoldi C, et al. New targets for antibacterial design: Kdo biosynthesis and LPS machinery transport to the cell surface. Curr Med Chem. 2011;18(6):830–852. doi: 10.2174/092986711794927676. [DOI] [PubMed] [Google Scholar]
- [10].Ianaro A, Tersigni M, D’Acquisto F. New insight in LPS antagonist. Mini Rev Med Chem. 2009;9(3):306–317. doi: 10.2174/1389557510909030306. [DOI] [PubMed] [Google Scholar]
- [11].Grin’kina NM, Karnabi EE, Damania D, et al. Sphingosine kinase 1 deficiency exacerbates LPS-induced neuroinflammation. PLoS One. 2012;7(5):e36475. doi: 10.1371/journal.pone.0036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sayyah M, Kaviani B, Khoshkholgh-Sima B, et al. Effect of chronic intracerebroventricluar administration of lipopolysaccharide on connexin43 protein expression in rat hippocampus. Iran Biomed J. 2012;16(1):25–32. doi: 10.6091/IBJ.1030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Haupt C, Witte OW, Frahm C. Up-regulation of Connexin43 in the glial scar following photothrombotic ischemic injury. Mol Cell Neurosci. 2007;35(1):89–99. doi: 10.1016/j.mcn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [14].Lee IH, Lindqvist E, Kiehn O, et al. Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol. 2005;489(1):1–10. doi: 10.1002/cne.20567. [DOI] [PubMed] [Google Scholar]
- [15].Mosley RL, Benner EJ, Kadiu I, et al. Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson's disease. Clin Neurosci Res. 2006;6(5):261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw. 2012;12(2):41–47. doi: 10.4110/in.2012.12.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kozoriz MG, Bechberger JF, Bechberger GR, et al. The connexin43 C-terminal region mediates neuroprotection during stroke. J Neuropathol Exp Neurol. 2010;69(2):196–206. doi: 10.1097/NEN.0b013e3181cd44df. [DOI] [PubMed] [Google Scholar]
- [18].Giaume C, Kirchhoff F, Matute C, et al. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14(7):1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- [19].Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16(3):877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18(3):351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- [21].John GR, Scemes E, Suadicani SO, et al. IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci U S A. 1999;96(20):11613–11618. doi: 10.1073/pnas.96.20.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22(4):453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- [23].Ozog MA, Siushansian R, Naus CC. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. J Neuropathol Exp Neurol. 2002;61(2):132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- [24].Nakase T, Fushiki S, Naus CC. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34(8):1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]


