Abstract
In our previous study, we reported that prenatal restraint stress could induce cognitive deficits, which correlated with a change in expression of growth-associated protein 43 in the hippocampus. In this study, we investigated the effects of enriched environment on cognitive abilities in prenatally stressed rat offspring, as well as the underlying mechanisms. Reverse transcription-PCR and western blot assay results revealed that growth-associated protein 43 mRNA and protein levels were upregulated on postnatal day 15 in the prenatal restraint stress group. Growth-associated protein 43 expression was significantly lower in the prenatal restraint stress group compared with the negative control and prenatal restraint stress plus enriched environment groups on postnatal days 30 and 50. Morris water maze test demonstrated that cognitive abilities were noticeably increased in rats from the prenatal restraint stress plus enriched environment group on postnatal day 50. These results indicate that enriched environment can improve the spatial learning and memory ability of prenatally stressed offspring by upregulating growth-associated protein 43 expression.
Keywords: prenatal restraint stress, growth-associated protein 43, hippocampus, Morris water maze, enriched environment, cognition, neural regeneration
Research Highlights
-
(1)
Prenatal restraint stress impairs spatial learning and memory abilities in rat offspring.
-
(2)
Enriched environment enhances cognitive abilities in prenatally stressed rat offspring by upregulating growth-associated protein 43 expression in the hippocampus.
Abbreviation
P, postnatal day
INTRODUCTION
Adversity during pregnancy can compromise the development of the fetus and cause physiological and behavioral alterations in the offspring. Prenatal restraint stress induces profound behavioral changes and learning and memory deficits and causes mental and systemic disorders, including decreased social interaction, deficits in sensory gating, heightened responses to psychomotor stimuli, memory impairment, increased anxiogenic and depressive behavior, and altered sleep-wake cycles[1,2]. All these changes have been shown to be associated with elevated levels of corticosterone[3]. Considerable morphological evidence has shown that exposure to 21 days of chronic restraint stress can change the structure of neurons in the hippocampus, and these changes have been associated with the impairment of hippocampus-dependent memory[4].
Growth-associated protein 43 has been termed a “growth” or “plasticity” protein, because it is expressed at a high level in neuronal growth cones during development and axonal regeneration. Growth-associated protein 43 is considered a crucial component of the axon and presynaptic terminal, and a null mutation in the gene encoding it leads to death within days of birth due to axon pathfinding defects[5,6]. Prenatal restraint stress can alter the expression of several genes, including growth-associated protein 43[7], but its effects on the cognitive ability of prenatally stressed pups has not been studied.
Enriched environment has proven to be an effective method for the remodeling of the nervous system[8]. Research in nonhuman primates has shown that a more stimulating environment can aid the treatment of and recovery from a diverse variety of brain dysfunctions[9]. Enriched environment has been shown to change the expression of growth-associated protein 43[10]. To gain further insight into prenatal restraint stress-induced alteration of growth-associated protein 43 expression and the effects of enriched environment on brain functional recovery, we investigated growth-associated protein 43 mRNA and protein levels in the hippocampus and assessed the cognitive ability of prenatally stressed rat pups.
RESULTS
Quantitative analysis of experimental animals
A total of 18 pregnant rats were equally and randomly divided into negative control, prenatal restraint stress and prenatal restraint stress plus enriched environment groups. Rats from the prenatal restraint stress and prenatal restraint stress plus enriched environment groups were exposed to restraint stress from embryonic days 13 to 19. Furthermore, pups from the prenatal restraint stress plus enriched environment group were exposed to enriched environment from postnatal days 10 (P10) to 30. A total of 28 male pups from each group were studied at P15, P30 and P50. A total of 84 pups were included in the final analysis.
Growth-associated protein 43 mRNA expression in the pup hippocampus
To study the effects of prenatal restraint stress and/or enriched environment on the expression of the growth-associated protein 43 gene in pup hippocampus, we examined the levels of growth-associated protein 43 mRNA in the hippocampus with reverse transcription (RT)-PCR at P15, P30 and P50 (Figure 1). At the early stage of rat brain development (P15), growth-associated protein 43 mRNA expression in the hippocampus of prenatal restraint stress and prenatal restraint stress plus enriched environment pups was significantly higher than in the hippocampus of negative control pups (P < 0.05; Figure 1A). Moreover, there was no significant difference between prenatal restraint stress and prenatal restraint stress plus enriched environment pups (P > 0.05). Growth-associated protein 43 mRNA expression decreased in the prenatal restraint stress pups, and was significantly lower than in prenatal restraint stress plus enriched environment and negative control pups on P30 (Figure 1B; P < 0.05 or P < 0.01) and P50 (Figure 1C; P < 0.01). Growth-associated protein 43 mRNA expression in prenatal restraint stress plus enriched environment pups was lower than in negative control pups on P30 and P50 (Figure 1B, P < 0.05; Figure 1C, P < 0.05).
Figure 1.
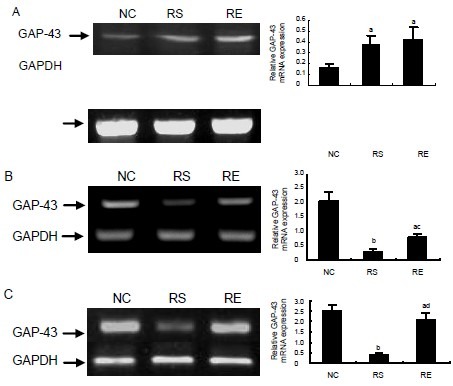
Growth-associated protein 43 (GAP-43) mRNA expression in pup hippocampus.
(A–C) GAP-43 mRNA expression on postnatal day 15 (P15), P30 and P50.
Left panel shows PCR products for GAP-43 (705 bp) and GAPDH (382 bp).
Right panel exhibits the statistical results of the data in the left panel (relative expression of GAP-43 mRNA was normalized to GAPDH).
The data are expressed as mean ± SEM from 3–4 rats per group. aP < 0.05, bP < 0.01, vs. negative control (NC) group; cP < 0.05, dP < 0.01, vs. prenatal restraint stress (RS) group by one-way analysis of variance.
RE: Prenatal restraint stress plus enriched environment.
Growth-associated protein 43 protein levels in the pup hippocampus
To investigate the effects of prenatal restraint stress and/or enriched environment on growth-associated protein 43 protein levels in the hippocampus, we determined the levels of growth-associated protein 43 protein in the hippocampus with western blot analysis on P15, P30 and P50. Restraint stress-induced changes in protein expression levels were equivalent to the changes in mRNA levels observed by RT-PCR analysis (Figure 2). Growth-associated protein 43 was abundant in the prenatal restraint stress and prenatal restraint stress plus enriched environment pups on P15, and significantly higher than in negative control pups (Figure 2A; P < 0.05). However, the level of growth-associated protein 43 protein in the prenatal restraint stress pups decreased markedly on P30 (Figure 2B) and P50 (Figure 2C). Higher levels of growth-associated protein 43 were detected in the negative control and prenatal restraint stress plus enriched environment groups compared with the prenatal restraint stress group (P < 0.01 or P < 0.05).
Figure 2.
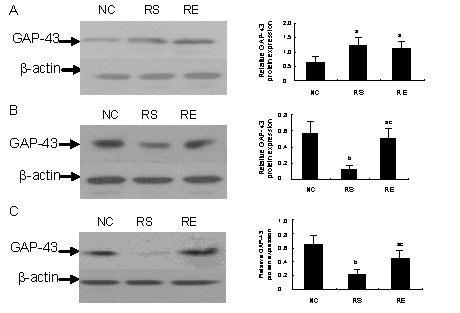
Growth-associated protein 43 (GAP-43) protein levels in pup hippocampus.
(A–C) GAP-43 protein expression on postnatal day 15 (P15), P30 and P50.
Left panel shows western blots for GAP-43 (46 kDa) and β-actin (45 kDa).
Right panel exhibits the statistical results of the data in the left panel (relative expression of GAP-43 protein was normalized to β-actin).
The data are expressed as mean ± SEM from 3–4 rats from each group. aP < 0.05, bP < 0.01, vs. negative control (NC) group; cP < 0.05, vs. prenatal restraint stress (RS) group by one-way analysis of variance.
RE: Prenatal restraint stress plus enriched environment.
Spatial learning and memory ability of pups
Average latency in finding the platform
To investigate the spatial learning and memory ability of pups, we recorded the swimming latency of all groups on P50. The latency of the prenatal restraint stress pups was significantly longer than that of negative control pups (P < 0.01) and prenatal restraint stress plus enriched environment pups (P < 0.05; Figure 3). The latency of negative control pups was significantly shorter than that of prenatal restraint stress plus enriched environment pups (Figure 3; P < 0.05).
Figure 3.
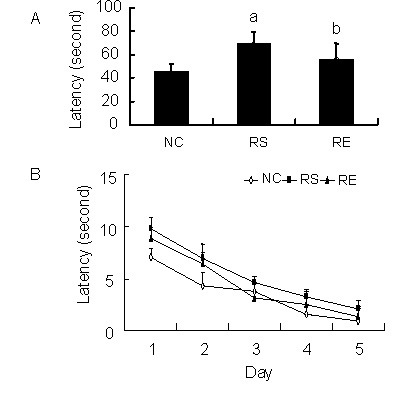
Average latency in finding the platform.
Panel (A) and (B) show the average latency of pups in all groups. (A) is the average of (B). The data are expressed as mean ± SEM from 12 rats in each group.
aP < 0.01, bP < 0.05, vs. negative control (NC) group; cP < 0.05, vs. prenatal restraint stress (RS) group using repeated-measures analysis of variance with Tukey's post-hoc test. RE: Prenatal restraint stress plus enriched environment.
Swimming speed in finding the platform
The swimming speed was also compared among the different groups, and the data are shown in Figure 4A. There were no significant differences in swimming speed to reach the platform among negative control, prenatal restraint stress and prenatal restraint stress plus enriched environment groups (P > 0.05).
Figure 4.
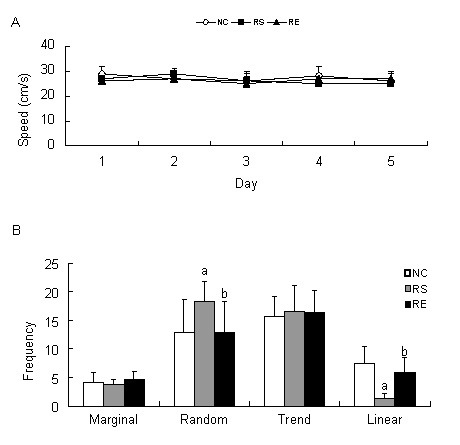
Swimming speed and probe strategies in finding the platform.
(A) The swimming speed of pups in finding the platform. There is no significant difference in swimming speed among the groups (P > 0.05) using repeated-measures analysis of variance.
(B) The search strategies used by the pups in finding the platform on postnatal day 50 (P50).
The data are expressed as mean ± SEM from 12 rats in each group. aP < 0.05, vs. negative control (NC) group; bP < 0.05, vs. prenatal restraint stress (RS) group using repeated-measures analysis of variance with Tukey's post-test. RE: Prenatal restraint stress plus enriched environment.
Search strategies in finding the platform
To study the effects of prenatal restraint stress on the spatial learning and memory ability of pups and the effects of enriched environment, we compared the search strategies of pups used in the Morris water maze on P50. The prenatal restraint stress group was more inclined to use a random mode compared with the negative control and prenatal restraint stress plus enriched environment groups (P < 0.05). In contrast, the negative control and prenatal restraint stress plus enriched environment pups tended to use a linear mode (P < 0.05). No significant difference was found in the choice of random or linear mode between negative control and prenatal restraint stress plus enriched environment pups (P > 0.05). There was no significant difference among the three groups with regard to the selection of either the marginal mode or the trend mode (P > 0.05; Figure 4B).
DISCUSSION
Three important novel findings were obtained in the present study: (1) growth-associated protein 43 mRNA and protein levels in the pup hippocampus are upregulated by prenatal restraint stress during the early stages of development and downregulated in later life. (2) prenatal restraint stress impairs the spatial learning and memory ability of pups. (3) Enriched environment upregulates growth-associated protein 43 expression and enhances spatial learning and memory ability of stressed rat pups. Some studies have shown that prenatal stress can lead to alterations in the expression of several genes in the rat hippocampus, which induces cognitive and behavioral disorders in the offspring[11,12].
Prenatal restraint stress regulates the hypothalamic-pituitary-adrenal axis, and the effect is similar to corticosterone injection (40 mg/kg)[3,7]. It is widely known that days 13 to 19 of embryonic development is an important period for the development of the nervous system. This is the peak period of synaptogenesis in the brain, and abnormal axon sprouting and reorganization may lead to defects in synaptic pruning at later stages of life[13]. Growth-associated protein 43 is an important protein in neurite formation, regeneration and plasticity, and has a key role in stress-induced damage to the hippocampus[14,15]. In vitro, growth-associated protein 43 can regulate dendritic branching and filopodia formation[16]. Protective high-level expression of growth-associated protein 43 is also found in chronic and acute injury[17,18]. Therefore, we examined Growth-associated protein 43 expression in the hippocampus of male pups, as there is a sex-dependent effect on the expression of the gene[19]. The results showed that in pups of the prenatal restraint stress group, Growth-associated protein 43 level increased significantly on P15 and decreased to a low level from P30 to P50. These results indicate that during the early stages of brain development, growth-associated protein 43 expression is evoked by prior stress to repair damaged structures, which might be a protective mechanism against the toxicity of maternal stress hormone. However, this change was transient, and growth-associated protein 43 expression dropped below normal levels in later life. The increase in growth-associated protein 43 levels in the early stage of development did not reverse the impairment of brain structure and function. Results of our Morris water maze test showed that the spatial learning and memory ability of negative control pups was better than that of prenatal restraint stress pups. The stressed pups had a longer average latency and a preference for the random strategy, whereas the negative control group preferred the linear strategy.
Enriched environment has been shown to improve learning and memory ability[20]. It is possible that enriched environment can affect the expression of genes that determine neuronal structure in the cerebral cortex and hippocampus[21]. Therefore, we examined the expression of the growth-associated protein 43 gene and protein in stressed pups that had received enriched environment on P15, P30 and P50. No significant difference was observed compared with stressed pups on P15, but growth-associated protein 43 gene and protein expression increased significantly on P30 and P50. We also found pups in the prenatal restraint stress plus enriched environment group performed better in the Morris water maze test compared with prenatal restraint stress pups. The average latency of pups in the prenatal restraint stress plus enriched environment group was shorter than that of prenatal restraint stress pupes, but still longer than negative control pups. In terms of selection of search strategies, there was no significant difference between the prenatal restraint stress plus enriched environment and negative control groups. These results suggest that enriched environment enhances the spatial learning and memory ability of pups by upregulating growth-associated protein 43 expression (a potential mechanism by which enriched environment improves brain function). However, the cognitive ability of pups did not recover to the normal level, as shown by the longer average latency of pups from the prenatal restraint stress plus enriched environment group.
These findings significantly advance the current understanding of the effects of enriched environment on prenatal stressed pups and the underlying mechanism. A remarkable improvement was observed in the pups’ learning and memory ability (cognitive ability), and this improvement correlated with the expression of growth-associated protein 43. Our results indicate that growth-associated protein 43 may have a key role in the recovery of brain function. Moreover, our findings provide further evidence to support a critical role for growth-associated protein 43 in brain development. However, further studies are needed to identify upstream molecules that affect growth-associated protein 43 expression in the brains of offspring.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
Experiments were performed at the Department of Histology and Embryology, Guangzhou Medical University, China in October 2011.
Materials
A total of 18 female rats weighing 220–250 g and 6 male rats weighing 250–270 g were obtained from the Experimental Animal Center of Sun Yat-Sen University, China (license No. SCXK (Yue) 2009-0011). The present study was conducted in accordance with the NIH Guidelines on the Care and Use of Animals.
Methods
Intervention and group management
Rats were put into a cage to mate at night. Vaginal smears were taken on the second morning. The rats with sperm present in the smears were housed individually in static cages and were designated as G0. Pregnant rats were divided into three groups: a negative control group, a prenatal restraint stress group and a prenatal restraint stress plus enriched environment group. Rats in the prenatal restraint stress and prenatal restraint stress plus enriched environment groups were exposed to restraint stress. After parturition, dams and their pups were left in large static cages until weaning on P21, and male pups were selected for further study. All rats were allowed free access to food and water and were maintained under a 12-hour light/dark cycle at 25 ± 1°C.
Prenatal stress procedure
All pregnant dams were exposed to restraint stress from days 13 (G13) to 19 (G19)[3]. Specifically, dams from the prenatal restraint stress and prenatal restraint stress plus enriched environment groups were restrained in a well-ventilated cylindrical Plexiglas restrainer (7 cm in diameter, 19 cm in length; home-made) for 45 minutes. Stress was repeated three times daily. Negative control dams were left in the cages. After delivery, each dam and her pups were left in the same cage until weaning. Pups were selected randomly from litters in the experiment.
Enriched environment
The pups were subjected to an enriched environment from P10 to P30. Pups in the prenatal restraint stress plus enriched environment group were put into a large cage (120 cm × 60 cm × 50 cm) containing wood shavings, colorful toys, ladders, tunnels, running wheels and bells for 2 hours every day[9]. The toys were changed once a week at the time of cage cleaning. Pups in the negative control and prenatal restraint stress groups were raised in an ordinary cage (40 cm × 30 cm × 30 cm) containing only wood shavings.
Tissue specimen preparation
A total of 8, 8 and 12 male pups from each group were decapitated on P15, P30 and P50, respectively. Hippocampal tissues were obtained for RT-PCR and western blot assay.
RT-PCR analysis of growth-associated protein 43 mRNA expression
The procedures used for RT-PCR were similar to those described by Kage et al[22]. Briefly, hippocampal tissues were collected for total RNA preparation using Trizol (Gibco-BRL, Gaithersburg, MD, USA) according to the manufacturer's instructions. The expression of growth-associated protein 43 mRNA in rat hippocampus was assessed using RT-PCR. From each sample, 1 μg of total RNA was converted to cDNA, and PCR was performed with oligonucleotide primers complementary to the cDNA. Primers and the length of the PCR product were as follows: growth-associated protein 43 (705 bp), 5’-TGT CAA ACC GGA GGA TAA GGC TCA-3’ (forward), 5’-AGC AGG ACA GGA GAG GAA ACT TCA-3’ (reverse). The reverse transcription reaction involved incubation at room temperature for 10 minutes, then at 42°C for 15 minutes, followed by heating for 5 minutes at 95°C. 30 cycles were chosen to compare different levels of expression of various mRNA. The housekeeping gene GAPDH was chosen as an endogenous standard. PCR primers for the amplification of growth-associated protein 43 and GAPDH cDNA were synthesized by Life Technologies (Shanghai, China).
Western blot analysis of growth-associated protein 43 protein expression
Hippocampal tissues were dissected out, put into liquid nitrogen and ground to a powder. Tissues were collected and mixed with buffer A (20 mM N-2-hydroxyethylplperazine-N’-2-ethanesulfonic acid at pH 7.4, 2 mM ethylenediamine tetraacetic acid, 50 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM Na3VO4, 1% Triton X-100 and 10% glycerol). The suspension was incubated on ice for 20 minutes with vortexing every 5 minutes. Cellular debris was then pelleted by centrifugation for 30 minutes at 13 000 r/min (Eppendorf centrifuge) at 4°C, and the supernatant was collected. Protein concentrations were determined using Bicinchoninic Acid Protein Assay (Bio-Rad, Tokyo, Japan)28. Lysates were isolated by 12% SDS-PAGE (50 μg/lane) and blotted with mouse monoclonal antibodies against growth-associated protein 43 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), at 4°C, overnight. The same blots were subsequently stripped and reblotted with rabbit anti-mouse IgG (1:1 000; Cell Signaling Technology, Danvers, MA, USA) for 2 hours at room temperature. β-actin served as the loading control.
Morris water maze test of cognitive abilities
The spatial learning and memory abilities of the rats were investigated using the Morris water maze[9]. The Morris water maze consisted of a round tank (pool) (1.2 m in diameter, 0.5 m in depth; Bio-well, Shanghai, China) and a video system (Bio-well). The tank was filled with water maintained at 25 ± 1°C and made opaque with non-toxic tempera paint. The tank was arbitrarily divided into southeast, northeast, northwest and southwest quadrants. A circular Perspex escape platform (9 cm in diameter) was submerged 35 cm away from the wall in the southeast quadrant of the pool (the target quadrant). The position of the platform was held constant during the 5-day period. A camera situated above the pool was used to capture video footage of the animals’ swim trace. The video was then transferred to computer for further analysis.
Animals underwent one training session which consisted of four trials every day for 5 successive days (from P45 to P49; each experimental group consisted of 12 rats). The trial was started by placing a rat into the pool facing the wall of the tank. Each of the four starting positions (north, east, south and west) was used once in a series of four trials, and the order of the starting position was randomized. Each trial was terminated as soon as the rat climbed onto the platform or when 120 seconds had elapsed. The rat was allowed to stay on the platform for 15 seconds after which it was taken away from the platform, and the next trial started after 20 seconds. Rats that did not find the platform within 120 seconds were put on the platform by the experimenter and were allowed to stay there for 15 seconds. The average latency and swimming speed to reach the platform were compared among the groups. All training sessions were conducted between 8:00 and 18:00.
The test session took place 24 hours after the last training session. The platform was not present and the animal only swam one trial of 120 seconds. Four different search strategies were defined as follows: random mode, linear mode, trend mode and marginal mode. Consider the line between the starting position and the platform as the axis. If more than 70% of the swim trace was no more than 15 cm from the axis, the strategy was called linear mode; within 50 cm was considered the trend mode. Consider a circle of radius 75 cm, with its center at the center of the round tank. If more than 70% of the swim trace was outside the circle, it was defined as the marginal mode. Swimming strategies other than the ones described above were designated as random mode. The search strategies were compared among the groups.
Statistical analysis
Data were analyzed with SPSS 11.5 (SPSS, Chicago, IL, USA) and expressed as mean ± SEM. Behavior test data were analyzed by repeated-measures analysis of variance with Tukey's post-hoc test. Western blot and RT-PCR data were analyzed by one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: The work was supported by a grant from Guangzhou Medical University in China, No. 2010-2012.
Conflicts of interest: None declared.
Ethical approval: The animal study protocol was approved by the Animal Care Committee of Guangzhou Medical University in China.
(Edited by Lou Y, Yu YQ/Qiu Y/Song LP)
REFERENCES
- [1].Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65(5):427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- [2].Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175(2):323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [3].Afadlal S, Polaboon N, Surakul P, et al. Prenatal stress alters presynaptic marker proteins in the hippocampus of rat pups. Neurosci Lett. 2010;470(1):24–27. doi: 10.1016/j.neulet.2009.12.046. [DOI] [PubMed] [Google Scholar]
- [4].Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat. 1997;13(3):149–167. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- [5].Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- [6].Kaneda M, Nagashima M, Nunome T, et al. Changes of phospho-growth-associated protein 43 (phospho-GAP43) in the zebrafish retina after optic nerve injury: a long-term observation. Neurosci Res. 2008;61(3):281–288. doi: 10.1016/j.neures.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [7].Jutapakdeegul N, Afadlal S, Polaboon N, et al. Repeated restraint stress and corticosterone injections during late pregnancy alter GAP-43 expression in the hippocampus and prefrontal cortex of rat pups. Int J Dev Neurosci. 2010;28(1):83–90. doi: 10.1016/j.ijdevneu.2009.09.003. [DOI] [PubMed] [Google Scholar]
- [8].Sun H, Zhang J, Zhang L, et al. Environmental enrichment influences BDNF and NR1 levels in the hippocampus and restores cognitive impairment in chronic cerebral hypoperfused rats. Curr Neurovasc Res. 2010;7(4):268–280. doi: 10.2174/156720210793180819. [DOI] [PubMed] [Google Scholar]
- [9].Kozorovitskiy Y, Gross CG, Kopil C, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102(48):17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomez-Pinilla F, Ying Z, Agoncillo T, et al. The influence of naturalistic experience on plasticity markers in somatosensory cortex and hippocampus: effects of whisker use. Brain Res. 2011;1388:39–47. doi: 10.1016/j.brainres.2011.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neeley EW, Berger R, Koenig JI, et al. Strain dependent effects of prenatal stress on gene expression in the rat hippocampus. Physiol Behav. 2011;104(2):334–339. doi: 10.1016/j.physbeh.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Markham JA, Taylor AR, Taylor SB, et al. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2010;4:173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dupouy JP, Coffigny H, Magre S. Maternal and foetal corticosterone levels during late pregnancy in rats. J Endocrinol. 1975;65(3):347–352. doi: 10.1677/joe.0.0650347. [DOI] [PubMed] [Google Scholar]
- [14].Casoli T, Di Stefano G, Gracciotti N, et al. Cellular distribution of GAP-43 mRNA in hippocampus and cerebellum of adult rat brain by in situ RT-PCR. J Histochem Cytochem. 2001;49(9):1195–1196. doi: 10.1177/002215540104900917. [DOI] [PubMed] [Google Scholar]
- [15].Chao HM, McEwen BS. Glucocorticoids and the expression of mRNAs for neurotrophins, their receptors and GAP-43 in the rat hippocampus. Brain Res Mol Brain Res. 1994;26(1-2):271–276. doi: 10.1016/0169-328x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- [16].Gauthier-Campbell C, Bredt DS, Murphy TH, et al. Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs. Mol Biol Cell. 2004;15(5):2205–2217. doi: 10.1091/mbc.E03-07-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu HX, Zhang JJ, Zheng P, et al. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005;139(1):169–177. doi: 10.1016/j.molbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- [18].Tagaya M, Matsuyama T, Nakamura H, et al. Increased F1/GAP-43 mRNA accumulation in gerbil hippocampus after brain ischemia. J Cereb Blood Flow Metab. 1995;15(6):1132–1136. doi: 10.1038/jcbfm.1995.140. [DOI] [PubMed] [Google Scholar]
- [19].Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23(4):320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]
- [20].Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- [21].Rampon C, Jiang CH, Dong H, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A. 2000;97(23):12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kage M, Ikemoto A, Akiguchi I, et al. Primary structure of GAP-43 mRNA expressed in the spinal cord of ALS patients. Neuroreport. 1998;9(7):1403–1406. doi: 10.1097/00001756-199805110-00028. [DOI] [PubMed] [Google Scholar]


