Abstract
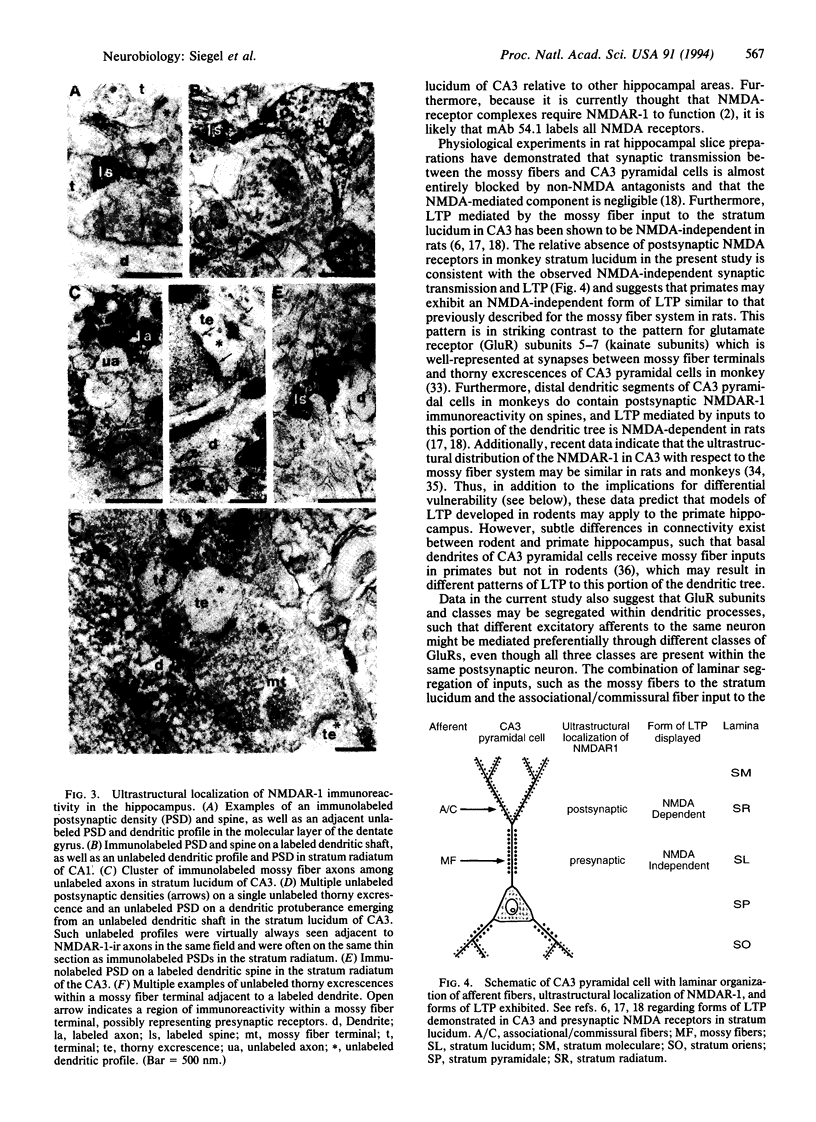
The regional, cellular, and subcellular distributions of N-methyl-D-aspartate (NMDA) receptor subunit 1, NMDAR-1, were investigated in monkey hippocampus by using a monoclonal antibody directed against a fusion protein corresponding to aa 660-811 of NMDAR-1. The data indicate that many neurons in each subfield of the hippocampus contain NMDAR-1 protein, although the intensity and distribution of immunoreactivity varied across regions, strata, and cellular compartments. In stratum lucidum of CA3, mossy fiber axons were immunoreactive for NMDAR-1, which may correspond to previously hypothesized presynaptic receptors. NMDAR-1-labeled postsynaptic profiles were present in stratum radiatum of CA3 but were largely absent from stratum lucidum. Such intraneuronal segregation of glutamate receptor subunits or classes may be spatially correlated with afferent systems that exhibit laminar segregation and terminate in different portions of the postsynaptic dendritic tree. For example, in CA3 pyramidal cells, NMDA receptors are postsynaptic in distal apical dendrites (stratum radiatum) where NMDA-dependent long-term potentiation in rats is mediated by associational/commissural afferents, and are absent from proximal apical dendrites (stratum lucidum), where NMDA-independent long-term potentiation is mediated by the mossy fiber input.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S., Stanton P. K., Sejnowski T. J. Commissural synapses, but not mossy fiber synapses, in hippocampal field CA3 exhibit associative long-term potentiation and depression. Brain Res. 1989 Aug 21;495(1):145–150. doi: 10.1016/0006-8993(89)91228-6. [DOI] [PubMed] [Google Scholar]
- Chazot P. L., Cik M., Stephenson F. A. Immunological detection of the NMDAR1 glutamate receptor subunit expressed in embryonic kidney 293 cells and in rat brain. J Neurochem. 1992 Sep;59(3):1176–1178. doi: 10.1111/j.1471-4159.1992.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Flatman J. A., Ganong A. H., Perkins M. N. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J Physiol. 1986 Sep;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington M. L., Lynch M. A., Bliss T. V. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987 Jan;20(1):279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Target cell specificity of synaptic connections in the hippocampus. Hippocampus. 1991 Apr;1(2):123–130. doi: 10.1002/hipo.450010202. [DOI] [PubMed] [Google Scholar]
- Good P. F., Huntley G. W., Rogers S. W., Heinemann S. F., Morrison J. H. Organization and quantitative analysis of kainate receptor subunit GluR5-7 immunoreactivity in monkey hippocampus. Brain Res. 1993 Oct 8;624(1-2):347–353. doi: 10.1016/0006-8993(93)90102-s. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huntley G. W., Rogers S. W., Moran T., Janssen W., Archin N., Vickers J. C., Cauley K., Heinemann S. F., Morrison J. H. Selective distribution of kainate receptor subunit immunoreactivity in monkey neocortex revealed by a monoclonal antibody that recognizes glutamate receptor subunits GluR5/6/7. J Neurosci. 1993 Jul;13(7):2965–2981. doi: 10.1523/JNEUROSCI.13-07-02965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H., Kaneko S., Tajima A., Satoh M. Separate mechanisms of long-term potentiation in two input systems to CA3 pyramidal neurons of rat hippocampal slices as revealed by the whole-cell patch-clamp technique. Neurosci Res. 1991 Nov;12(3):393–402. doi: 10.1016/0168-0102(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Levey A. I., Huganir R. L., Price D. L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993 Mar;53(2):327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Hughes T. E., Hollmann M., Gasic G. P., Deneris E. S., Heinemann S. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J Neurosci. 1991 Sep;11(9):2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993 Sep;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Seress L., Gulyás A. I., Freund T. F. Parvalbumin- and calbindin D28k-immunoreactive neurons in the hippocampal formation of the macaque monkey. J Comp Neurol. 1991 Nov 1;313(1):162–177. doi: 10.1002/cne.903130112. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological variability and developmental aspects of monkey and human granule cells: differences between the rodent and primate dentate gyrus. Epilepsy Res Suppl. 1992;7:3–28. [PubMed] [Google Scholar]
- Siegel S. J., Ginsberg S. D., Hof P. R., Foote S. L., Young W. G., Kraemer G. W., McKinney W. T., Morrison J. H. Effects of social deprivation in prepubescent rhesus monkeys: immunohistochemical analysis of the neurofilament protein triplet in the hippocampal formation. Brain Res. 1993 Aug 13;619(1-2):299–305. doi: 10.1016/0006-8993(93)91624-2. [DOI] [PubMed] [Google Scholar]
- Sonders M. S., Barmettler P., Lee J. A., Kitahara Y., Keana J. F., Weber E. A novel photoaffinity ligand for the phencyclidine site of the N-methyl-D-aspartate receptor labels a Mr 120,000 polypeptide. J Biol Chem. 1990 Apr 25;265(12):6776–6781. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers J. C., Huntley G. W., Edwards A. M., Moran T., Rogers S. W., Heinemann S. F., Morrison J. H. Quantitative localization of AMPA/kainate and kainate glutamate receptor subunit immunoreactivity in neurochemically identified subpopulations of neurons in the prefrontal cortex of the macaque monkey. J Neurosci. 1993 Jul;13(7):2982–2992. doi: 10.1523/JNEUROSCI.13-07-02982.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky R. A., Nicoll R. A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990 Jun 29;248(4963):1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]