Abstract
A model of focal cerebral ischemic infarction was established in dogs through middle cerebral artery occlusion of the right side. Thirty minutes after occlusion, models were injected with nerve growth factor adjacent to the infarct locus. The therapeutic effect of nerve growth factor against cerebral infarction was assessed using the hemisphere anomalous volume ratio, a quantitative index of diffusion-weighted MRI. At 6 hours, 24 hours, 7 days and 3 months after modeling, the hemisphere anomalous volume ratio was significantly reduced after treatment with nerve growth factor. Hematoxylin-eosin staining, immunohistochemistry, electron microscopy and neurological function scores showed that infarct defects were slightly reduced and neurological function significantly improved after nerve growth factor treatment. This result was consistent with diffusion-weighted MRI measurements. Experimental findings indicate that nerve growth factor can protect against cerebral infarction, and that the hemisphere anomalous volume ratio of diffusion-weighted MRI can be used to evaluate the therapeutic effect.
Keywords: diffusion-weighted MRI, nerve growth factor, hemisphere anomalous volume ratio, cerebral infarction, treatment, neuroprotection, brain, regeneration, neural regeneration
Research Highlights
-
(1)
Imaging, histology, and behavioral performance revealed that nerve growth factor was neuroprotective against cerebral ischemic damage.
-
(2)
The hemisphere anomalous volume ratio of diffusion-weighted MRI was used to quantitatively assess the therapeutic effect of drugs for the treatment of cerebral ischemic injury.
-
(3)
A quantitative method to assess the therapeutic effect of drugs using diffusion-weighted MRI was established.
Abbreviation
MCAO, middle cerebral artery occlusion
INTRODUCTION
Early intervention with neuroprotective agents can significantly improve the prognosis of cerebral infarction[1]. In this study, we aimed to develop a method to quantitatively evaluate the therapeutic effect of drugs following cerebral ischemic injury. The hemisphere anomalous volume ratio, the percentage of abnormal lesion volume to the total volume of the contralateral hemisphere, was first used in the measurement of intracranial tumor volume and to assess surrounding normal brain tissue, thus providing evidence for quantitative analysis of tumor volume, boundary and adjacent tissue conditions[2]. Hemisphere anomalous volume ratio is frequently used in studies investigating intracranial tumor progression[3,4]; however, little attention has been paid to the application of hemisphere anomalous volume ratio in cerebral vascular disease research. Scholars have measured cerebral malacia volume using hemisphere anomalous volume ratio[5]. However, the role of hemisphere anomalous volume ratio in diffusion-weighted imaging measurements for super-acute cerebral infarct volume has not been investigated fully (supplementary Figure 1 online).
Figure 1.
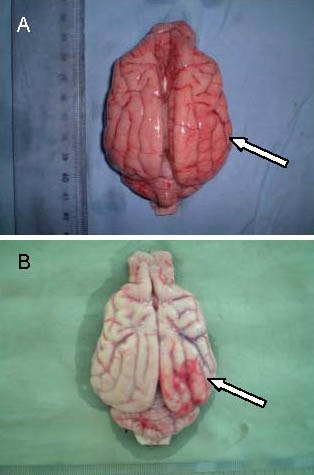
Gross specimens in the treatment group and the control group at 24 hours after cerebral infarction in Beagle dogs.
In the control group (B), the number of thickened blood vessels increased at the peripheral infarct site. In the treatment group (A), hyperplasia was reduced. Arrows indicate blood vessels at the infarct site.
Nerve growth factor can affect the survival and differentiation of certain neurons in the central nervous system[6,7,8,9], and significantly reduce neuronal death after injury, promote the regeneration of nerve fibers, and facilitate neural rehabilitation[10]. This study aimed to evaluate the change in cerebral infarct volume after never growth factor intervention using hemisphere anomalous volume ratio, an important parameter of diffusion-weighted imaging. In addition, the neuroprotective effect of never growth factor was monitored using histology and behavioral testing.
RESULTS
Quantitative analysis of experimental animals
Forty Beagle dogs were randomly divided into two groups, a treatment group (n = 24) and a control group (n = 16). All dogs underwent cerebral infarction by right middle cerebral artery occlusion (MCAO)[11]. Thirty minutes after occlusion, dogs in the treatment group were treated with never growth factor, while control animals were treated with physiological saline. At 6 hours, 24 hours, 7 days and 3 months after MCAO, brain tissue was harvested for observations. All 40 Beagle dogs were involved in the final analysis.
Assessment of neurological function in dogs following MCAO
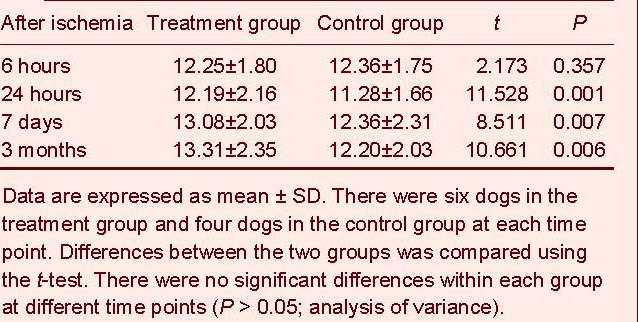
MCAO induced focal neurological impairment in dogs, which showed varying degrees of contralateral hemiplegia, especially in the hind limbs. Unilateral limb weakness, muscle tension reduction, unsteady gait, inclined body and low appetite were evident. In the treatment group at each time point, neurological defects were reduced and the recovery time was shorter. Compared with the control group, the treatment group showed a significant decrease in neurological function scores at 24 hours, 7 days and 3 months after MCAO (P < 0.05 or P < 0.01; Table 1).
Table 1.
Neurological function scores in two groups at different time points
Morphological changes in the brain tissue following MCAO
Gross observations
Brain tissue swelling was visible on the affected side of dogs in the control group at 6 hours after MCAO; at 24 hours, blood vessels were thickened in peripheral infarct tissue (Figure 1), and hemorrhaging was visible within the brain parenchyma; at 3 months, brain atrophy occurred on the surface of the infarct site.
Brain tissue swelling and hyperplasia were slightly reduced in the treatment group when compared with the control group. The observational indices at 24 hours and 3 months after MCAO are shown in Table 2.
Table 2.
Gross observation of infarct brain tissue in two groups
Histological observations
Hematoxylin-eosin staining revealed that ischemic neurons appeared shrunken, triangular in shape, had nuclear pyknosis and structural disappearance was evident in the control group at 6 hours after MCAO. At 24 hours, there was widespread degeneration and necrosis of neurons within the loci (Figure 2).
Figure 2.
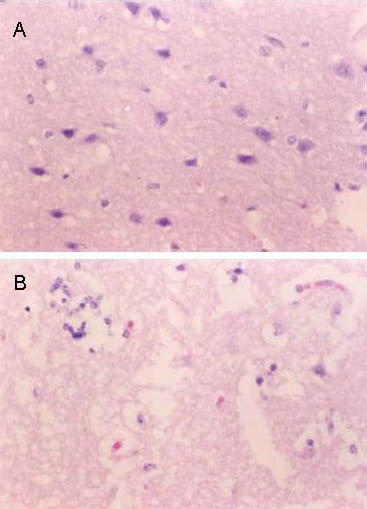
Pathological changes in infarct site at 24 hours after cerebral infarction in the two groups (hematoxylin-eosin staining, light microscope, × 400).
In the control group (B), the majority of neurons within the infarct foci degenerated and were necrotic, neuronal nuclei were pyknotic, neuropil gap widened, and cell vacuolation was observed. In the treatment group (A), these effects were reduced.
Figure 3.
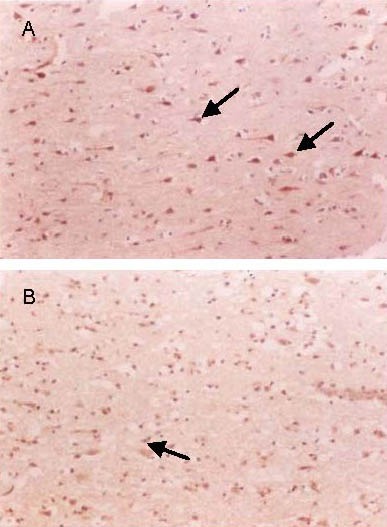
Nerve growth factor expression in infarct brain tissues at 24 hours following cerebral infarction (immunohistochemical staining, light microscope, × 200).
The number of immunoreactive neurons (arrows) increased and cells were deeply stained in the treatment group (A) when compared with the control group (B).
In the treatment group, ischemic neurons showed a lower degree of nuclear pyknosis, neuropil clearance, glial cells, and micro-vascular gap width than neurons in the control group. At 3 months, the malacia residues in the treatment group were smaller than that in the control group, and there were many normal neurons.
Immunohistochemical staining
Results showed a small number of never growth factor-positive cells in the control group and a large number of never growth factor-positive cells in the treatment group following cerebral infarction. Statistical analysis demonstrated that the number of never growth factor-positive cells in the treatment group was significantly higher than that of cells in the control group at 24 hours and 7 days (P < 0.01). In addition, there was no significant difference in the number of positive cells at 6 hours and 3 months between the two groups, although the number of cells in the treatment group was still higher than that in the control group (P > 0.05; Table 3).
Table 3.
Number of nerve growth factor immunopositive cells (n/400-fold visual field) in the infarct tissue at different time points in the two groups
Ultrastructural observations
Transmission electron microscopy revealed mild swelling of neurons and apparent intracellular mitochondrial swelling in the control group at 6 hours after MCAO. At 24 hours, neuronal swelling, cell shrinkage, the cell nucleolus disappeared, cells exhibited swollen mitochondria, spherical dilatation of the chamber, crista fragmentation, and an electron density decrease was visible. Moreover, the rough endoplasmic reticulum degranulated, the Golgi complex expanded and vacuolized, and the cytoplasm was abundant with characteristic lipofuscin granules, which were metabolite cells containing large particles and lipid droplets (Figure 4). At 7 days after MCAO, the swelling of neurons surrounding the infarct site reduced, and the mitochondria, Golgi complex and the rough endoplasmic reticulum greatly improved in morphology. At 3 months, oligodendrocyte proliferation markedly increased, less residual neurons were present, and there was a marked reduction in the number of synapses (P < 0.01). Neuronal ultrastructure improved in the treatment group compared with the control group at each time point, and cells appeared close to normal morphology at 3 months.
Figure 4.
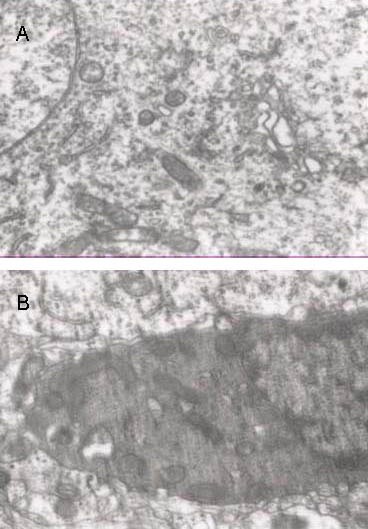
Ultrastructure of infarct brain tissue in the two groups at 24 hours following cerebral infarction (transmission electron microscope, × 15 000).
In the control group (B), neuronal swelling, nuclear shrinkage, rough endoplasmic reticulum degranulation, Golgi complex expansion and vacuolization were visible. In the treatment group (A), these effects improved.
Compared with the control group, the number of surviving neurons in the treatment group significantly increased at 24 hours, 7 days and 3 months following MCAO, and neuronal axons and synapses also increased (P < 0.01), while synaptic number showed no significant difference in the treatment group at each time point (P > 0.05; Table 4).
Table 4.
The number of synapses (n/8 000-fold visual field) in the infarct site at different time points following cerebral infarction
Imaging of brain tissue after MCAO
At 6 hours after MCAO, diffusion-weighted imaging images of both the treatment group and the control group displayed diffusion anomaly areas, located in the right middle cerebral artery (Figure 5).
Figure 5.
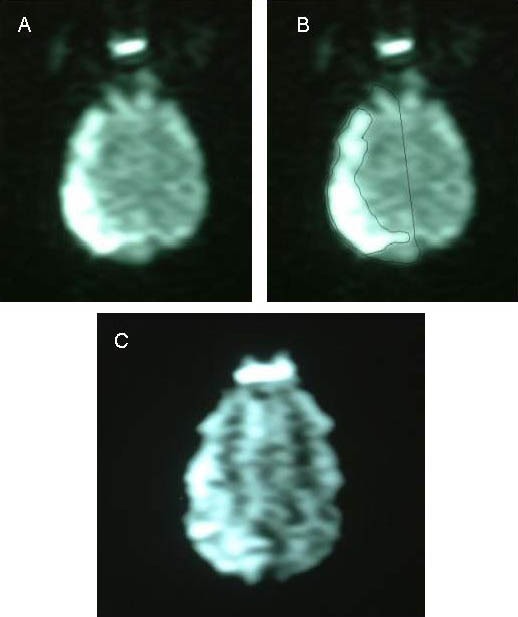
Diffusion-weighted imaging at 24 hours following cerebral infarction in the two groups.
(A) Image of the treatment group; (B) Schematic map of single layer hemisphere edge and infarct edge outlined for the same level; (C) Image of the control group.
The ischemic area in the control group was larger than that of the treatment group, and the occipital lesions invaded the midline.
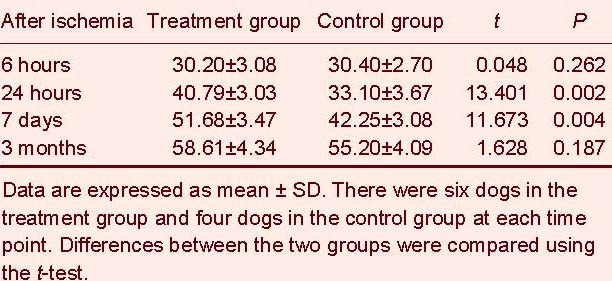
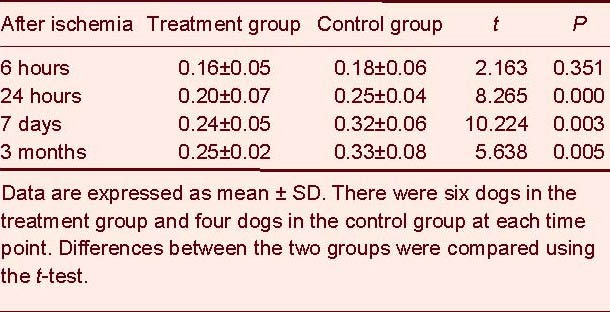
As infarction time progressed, the hemisphere anomalous volume ratio gradually increased, particularly during 6–24 hours and 24 hours–7 days. Compared with the control group, the treatment group had a decreased hemisphere anomalous volume ratio at 24 hours, 7 days and 3 months (P < 0.01; Table 5).
Table 5.
Comparison of hemisphere anomalous volume ratio (%) between the two groups at different time points
DISCUSSION
Ischemic brain injury may lead to an increase in the expression of endogenous never growth factor and its receptor under a natural state[12,13,14,15,16]. However, endogenous never growth factor production is too minimal to result in restoration of damaged neurons. Therefore, administration of exogenous never growth factor is applied to reduce ischemic damage caused by cerebral infarction, and to promote neuronal recovery and regeneration[1]. At present, never growth factor can be separated and purified from many biomaterials, such as snake venom, the mouse submandibular gland, the human full-term placenta and the guinea pig prostate gland[8,9]. Never growth factor, in combination with its presynaptic membrane receptor, binds to form complexes, which then enter and cross the axoplasm, transfer to the cell body, and bind with nuclear receptors, thus resulting in biological effects[17,18,19].
Never growth factor cannot only promote the growth and regeneration of nerve fibers, but can also facilitate synaptic formation[20,21,22]. In this study, treatment of never growth factor induced apparent degeneration of synapses surrounding the cortex lesions, and increased synaptic number, as determined under the electron microscope. These findings indicate that never growth factor can effectively protect damaged neurons, and promote recovery and regeneration.
MRI examination is characterized by high sensitivity to cerebral ischemic injury in the treatment of acute cerebral infarction[23]. This is due to each water molecule containing two protons that generate MR signals. Once ischemia occurs, brain edema forms and causes the MR signal to change, thus generating abnormal signals on the MR image. Prior to the emergence of pathological changes in lesions, MRI signals were apparently visible under the light microscope[24,25]. However, the ultra-high field magnetic resonance diffusion-weighted imaging technique can detect brain ischemic lesions several hours earlier than conventional MRI T2 imaging, thus providing evidence for the potential benefit of early intervention in the treatment of ischemia.
This study compared MRI, cell biology and molecular biology results following ischemic cerebral infarction in a canine model. Results revealed that neuroprotection and repair following never growth factor treatment occurred within 24 hours following MCAO. At this time, the infarct volume was reduced and hemisphere anomalous volume ratio growth was reduced when compared with the control group. This is evidence that the adjacent penumbra was narrowed and that mildly impaired nerve cells recovered. The degree of the hemisphere anomalous volume ratio increase can be used as an indicator to assess the neuroprotective effect of never growth factor and its ability to restore impaired neurons in the penumbra after cerebral infarction.
Although diffusion-weighted imaging axial tomographic images can reflect early ischemic lesions, single layer images cannot indicate the overall situation of lesions as scanning positions can differ depending on the technician. In addition, the location of the lesion depends on the volume of the brain. Therefore, this scanning technique is not adequate to reveal lesion size. Hemisphere anomalous volume ratio refers to the relative area of the hypoxic or ischemic lesion in the brain, which is not affected by scanning technicians and patients. Hemisphere anomalous volume ratio can clearly display the infarct size and volume, and can also be used as an objective index to evaluate disease progression. In this experiment, hemisphere anomalous volume ratio measurements were based on diffusion-weighted imaging images, which detect early infarction lesions and lay the foundation for early hemisphere anomalous volume ratio evaluation of cerebral infarction. In animal experiments, diffusion-weighted imaging could detect abnormal signals as early as 24 hours following MCAO[26,27]. Because of its high sensitivity to cerebral ischemia, diffusion-weighted imaging is a potential means for the early imaging evaluation of cerebral infarction. Here, we adopted hemisphere anomalous volume ratio for diffusion-weighted imaging to clearly determine infarct volume, and compare volumes with other treatments, thereby indirectly determining the therapeutic effect of never growth factor against cerebral ischemia. In addition, we analyzed brain tissue at a relatively low perfusion status within the ischemic zone, an area where never growth factor induces greatest neuroprotection. Results showed significant improvement after never growth factor treatment.
In summary, our study provides preliminarily evidence for the use of diffusion-weighted imaging hemisphere anomalous volume ratio technology for the evaluation of never growth factor in protecting and restoring neurons following acute cerebral infarction. In addition, we showed that hemisphere anomalous volume ratio can be used as a reliable quantitative index to evaluate the therapeutic effect of other neuroprotective agents.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment pertaining to neuroimaging.
Time and setting
Experiments were performed from June 2010 to October 2011 in the Department of Radiology, the Second Hospital of Hebei Medical University, China.
Materials
Forty healthy Beagle dogs, aged 6 months, male and female, weighing 10–15 kg, were provided by the Experimental Animal Center of Hebei Medical University, China (license No. SCXK (Ji) 2011-02-05). Prior to experimentation, each dog underwent MR-diffusion-weighted imaging examination, to eliminate animals presenting abnormal cerebral signals. Experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[28].
Methods
Establishment of MCAO model and intervention
Medical silicone rubber, matrix and catalyst were mixed and the mixture was injected into a 1.1-mm diameter glass tube, which was prepared for 8-mm-long emboli. Under anesthesia, one side of the canine common carotid artery was exposed and inserted with two emboli. Thus, the catheter entered the internal carotid artery and reached the skull, and finally the emboli were pressurized to invade the skull. The MCAO models were successfully prepared and the time at which the emboli was inserted was taken as the starting point of the cerebral infarction[11]. Thirty minutes after model establishment, magnetic resonance examination was performed, according to diffusion-weighted imaging detected lesions, and the reference location map and surface landmarks we determined were used as the administration site for never growth factor injection[29]. Using a skull driller and micro-syringe, the cerebral infarct site was injected with 0.5 mL of solution containing 2 000 biological activity units (BU) of never growth factor (each injection contained 1 000 BU; Geriatric Disease Prevention And Control Research Center of China Medical University, Dalian, Liaoning Province, China). The control group was given the same amount of physiological saline.
MRI examination
MRI was performed using a GE Signa Excite 3.0 T magnetic resonance scanner (Bethesda, MD, USA) and knee coil. At 6 hours, 24 hours, 7 days and 3 months, patients were examined by diffusion-weighted imaging. The scanning sequence included MR scanning conventional sequence and diffusion-weighted imaging. Diffusion-weighted imaging used a single-shot SE-EPI sequence, and a diffused sensitive gradient was added to the x, y and z axes, thus resulting in a diffusion-weighted image. The diffusion sensitive coefficient = 1 000 s/mm2. Repetition time: 2 800 ms, echo time: 60 ms, slice thickness: 5 mm, interval: 0, layers: 12, matrix: 128 × 128, field of view: 24 cm × 24 cm, phase encoding: the front and rear direction, motivation number: 6. After scanning was completed, diffusion-weighted imaging data of all subjects was transferred to an Advantage Workstation 4.2P (GE).
The hemisphere anomalous volume ratio was calculated according to diffusion-weighted imaging images. The infarct volume in each layer of diffusion-weighted imaging images was preliminarily observed, and then the hemisphere and infarct loci (diffusion anomaly area) were outlined on the workstation. The outline area on each layer multiplied by the slice thickness was the volume of the hemisphere and infarct loci on each layer. Finally, all results were accumulated to obtain the hemisphere volume and infarct volume (Figure 5).
hemisphere anomalous volume ratio (%) = (infarct volume / hemisphere volume with diffusion anomaly area) × 100%.
Specimen collection
Under anesthesia, dogs were fixed by cardiac perfusion fixation, and cerebral infarct specimens were harvested according to the diffusion-weighted imaging image (Figure 5)[30]. Specimens were prepared for paraffin embedding, which underwent hematoxylin-eosin staining, immunohistochemical staining and electron microscopic observation.
Immunohistochemical staining
The staining procedures were performed according to the manufacturer's instructions (Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China). Specimens were incubated with rabbit anti-human never growth factor monoclonal antibody (1:500; Beijing Zhongshan Biotechnology) in a wet box for 1.5 hours; with goat anti-rabbit IgG (1:200; Beijing Zhongshan Biotechnology) and SABC complexes (1:100; Beijing Zhongshan Biotechnology) for 30 minutes; and colored with DAB-H2O2 liquid (Beijing Zhongshan Biotechnology). The number of immunoreactive cells was counted under a light microscope (× 400; Olympus, Tokyo, Japan). Three brain slices were collected from each dog and ten fields of vision from each brain slice were used to calculate the average value.
Electron microscopy
1 mm × 1 mm × 3 mm specimens were fixed with 3% (v/v) glutaraldehyde solution, which was precooled to 0–4°C, fixed with osmium, dehydrated in acetone, embedded, and prepared into ultrathin sections at a thickness of approximately 70 nm. Specimens were dyed with uranyl acetate and lead citrate, and observed under a Hitachi H-7500 transmission electron microscope (Tokyo, Japan). Under the transmission electron microscope (× 8 000), the synapses in each field of view was counted.
Neurological function score
At 24 hours, 7 days and 3 months after MCAO, neurological function scores were assessed before the specimens were harvested. Scoring criteria are shown in Table 6[11]. Rats at postoperative 6 hours were excluded because of the interference of anesthesia.
Table 6.
Neurological function scale for cerebral infarction in dogs

Statistical analysis
Data were statistically analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Differences between groups were compared using the t-test, and comparison at different time points was performed with analysis of variance. A P value less than 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Hebei Provincial Medical Science Research Key Youth Project, No. 20100078.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Experimental Animal Ethics Committee of Hebei Medical University in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Huang LA, Sun XJ/Yang Y/Wang L)
REFERENCES
- [1].Ding J, Cheng Y, Gao S, et al. Effects of nerve growth factor and Noggin-modified bone marrow stromal cells on stroke in rats. J Neurosci Res. 2011;89(2):222–230. doi: 10.1002/jnr.22535. [DOI] [PubMed] [Google Scholar]
- [2].Jung DC, Ju W, Choi HJ, et al. The validity of tumour diameter assessed by magnetic resonance imaging and gross specimen with regard to tumour volume in cervical cancer patients. Eur J Cancer. 2008;44(11):1524–1528. doi: 10.1016/j.ejca.2008.04.023. [DOI] [PubMed] [Google Scholar]
- [3].Mitsuda M, Yamaguchi M, Furuta T, et al. Multiple-animal MR imaging using a 3T clinical scanner and multi-channel coil for volumetric analysis in a mouse tumor model. Magn Reson Med Sci. 2011;10(4):229–237. doi: 10.2463/mrms.10.229. [DOI] [PubMed] [Google Scholar]
- [4].Andronesi OC, Gagoski BA, Sorensen AG. Neurologic 3D MR spectroscopic imaging with low-power adiabatic pulses and fast spiral acquisition. Radiology. 2012;262(2):647–661. doi: 10.1148/radiol.11110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tzarouchi LC, Astrakas LG, Zikou A, et al. Periventricular leukomalacia in preterm children: assessment of grey and white matter and cerebrospinal fluid changes by MRI. Pediatr Radiol. 2009;39(12):1327–1332. doi: 10.1007/s00247-009-1389-0. [DOI] [PubMed] [Google Scholar]
- [6].Chen C, Hu Q, Yan J, et al. Early inhibition of HIF-1 alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis. 2009;33(3):509–517. doi: 10.1016/j.nbd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- [7].Chan CB, Liu X, Pradoldej S, et al. Phosphoinositide 3-kinase enhancer regulates neuronal dendritogenesis and survival in neocortex. J Neurosci. 2011;31(22):8083–8092. doi: 10.1523/JNEUROSCI.1129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scarcello E, Morrone F, Piro P, et al. Protein S-100B as biochemical marker of brain ischemic damage after treatment of carotid stenosis. Ann Vasc Surg. 2011;25(7):975–978. doi: 10.1016/j.avsg.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [9].Leconte C, Tixier E, Freret T, et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40(10):3349–3355. doi: 10.1161/STROKEAHA.109.557314. [DOI] [PubMed] [Google Scholar]
- [10].Tonchev AB. The nerve growth factor in health and unhealth neurons. Arch Ital Biol. 2011;149(2):225–231. doi: 10.4449/aib.v149i2.1368. [DOI] [PubMed] [Google Scholar]
- [11].Wu BL, Liu HJ, Wang GS, et al. A new embolism model of cerebral infarction in canine. Zhongguo Yixue Yingxiangxue Zazhi. 2003;11(1):51–55. [Google Scholar]
- [12].Lecht S, Arien-Zakay H, Marcinkiewicz C, et al. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. J Mol Neurosci. 2010;41(1):183–192. doi: 10.1007/s12031-009-9318-0. [DOI] [PubMed] [Google Scholar]
- [13].Himeda T, Tounai H, Hayakawa N, et al. Postischemic alterations of BDNF, NGF, HSP 70 and ubiquitin immunoreactivity in the gerbil hippocampus: pharmacological approach. Cell Mol Neurobiol. 2007;27(2):229–250. doi: 10.1007/s10571-006-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang DY, Pan HC, Yen YJ, et al. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28(6):1220–1229. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [15].Dmitrieva VG, Povarova OV, Skvortsova VI, et al. Semax and Pro-Gly-Pro activate the transcription of neurotrophins and their receptor genes after cerebral ischemia. Cell Mol Neurobiol. 2010;30(1):71–79. doi: 10.1007/s10571-009-9432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamamoto H, Kokame K, Okuda T, et al. NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem. 2011;286(29):26158–26165. doi: 10.1074/jbc.M111.256446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aloe L, Iannitelli A. Neurotrophic factors and brain damage in hypoxic-ischemic encephalopathy: a role of nerve growth factor. Ann Ist Super Sanita. 2001;37(4):573–580. [PubMed] [Google Scholar]
- [18].Chiaretti A, Antonelli A, Mastrangelo A, et al. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25(3):225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- [19].Chiaretti A, Falsini B, Aloe L, et al. Neuroprotective role of nerve growth factor in hypoxicischemic injury-From brain to skin. Arch Ital Biol. 2011;149(2):275–282. doi: 10.4449/aib.v149i2.1364. [DOI] [PubMed] [Google Scholar]
- [20].Chiu AW, Richert N, Ehrmantraut M, et al. Heterogeneity in response to interferon beta in patients with multiple sclerosis: a 3-year monthly imaging study. Arch Neurol. 2009;66(1):39–43. doi: 10.1001/archneur.66.1.noc80047. [DOI] [PubMed] [Google Scholar]
- [21].Fink G, Li M, Lau Y, et al. Chronic activation of endothelin B receptors: new model of experimental hypertension. Hypertension. 2007;50(3):512–518. doi: 10.1161/HYPERTENSIONAHA.107.094821. [DOI] [PubMed] [Google Scholar]
- [22].Lamba S, Ravichandran V, Major E. Glial cell type-specific subcellular localization of 14-3-3 zeta: an implication for JCV tropism. Glia. 2009;57(9):971–977. doi: 10.1002/glia.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu JY. Imaging diagnosis of hyperacute cerebral infarction. Zhongguo Yiyao Daobao. 2007;4(3):14–15. [Google Scholar]
- [24].Arien-Zakay H, Lecht S, Nagler A. Neuroprotection by human umbilical cord blood derived progenitors in ischemic brain injuries. Arch Ital Biol. 2011;149(2):233–245. doi: 10.4449/aib.v149i2.1370. [DOI] [PubMed] [Google Scholar]
- [25].Spitzmiller RE, Phillips T, Meinzen-Derr J, et al. Amplitude-integrated EEG is useful in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol. 2007;22:1069–1078. doi: 10.1177/0883073807306258. [DOI] [PubMed] [Google Scholar]
- [26].Pease A, Miller R. The use of diffusion tensor imaging to evaluate the spinal cord in normal and abnormal dogs. Vet Radiol Ultrasound. 2011;52(5):492–497. doi: 10.1111/j.1740-8261.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- [27].Sutherland-Smith J, King R, Faissler D, et al. Magnetic resonance imaging apparent diffusion coefficients for histologically confirmed intracranial lesions in dogs. Vet Radiol Ultrasound. 2011;52(2):142–148. doi: 10.1111/j.1740-8261.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- [28].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [29].Uriarte A, Moissonnier P, Thibaud JL, et al. Surgical treatment and radiation therapy of frontal lobe meningiomas in 7 dogs. Can Vet J. 2011;52(7):748–752. [PMC free article] [PubMed] [Google Scholar]
- [30].Yang TZ. Technical experience in perfusion through the heart for fixation of animal tissues. Hebei Yixueyuan Xuebao. 1992;13(2):83–84. [Google Scholar]


