Abstract
Astrocytes perform many functions in the brain and spinal cord. Glucose metabolism is important for astroglial cells and astrocytes are the only cells with insulin receptors in the brain. The common antibiotic penicillin is also a chemical agent that causes degenerative effect on neuronal cell. The aim of this study is to show the effect of insulin and glucose at different concentrations on the astrocyte death induced by penicillin on primer astroglial cell line. It is well known that intracranial penicillin treatment causes neuronal cell death and it is used for experimental epilepsy model commonly. Previous studies showed that insulin and glucose might protect neuronal cell in case of proper concentrations. But, the present study is about the effect of insulin and glucose against astrocyte death induced by penicillin. For this purpose, newborn rat brain was extracted and then mechanically dissociated to astroglial cell suspension and finally grown in culture medium. Clutters were maintained for 2 weeks prior to being used in these experiments. Different concentrations of insulin (0, 1, 3 nM) and glucose (0, 3, 30 mM) were used in media without penicillin and with 2 500 μM penicillin. Penicillin decreased the viability of astroglial cell seriously. The highest cell viability appeared in medium with 3 nM insulin and 3 mM glucose but without penicillin. However, in medium with penicillin, the best cell survival was in medium with 1 nM insulin but without glucose. We concluded that insulin and glucose show protective effects on the damage induced by penicillin to primer astroglial cell line. Interestingly, cell survival depends on concentrations of insulin and glucose strongly. The results of this study will help to explain cerebrovascular pathologies parallel to insulin and glucose conditions of patient after intracranial injuries.
Keywords: astrocyte, penicillin, insulin, glucose, rat, newborn, brain, cell culture, cell death, cell survival
Research Highlights
-
(1)
Astrocytes are the only cells with insulin receptors in the brain. Penicillin may cause neuronal degeneration.
-
(2)
Penicillin decreased the viability of astroglial cell seriously. Insulin and glucose seemed to be protective against the damage induced by penicillin on the primer astrocyte cell line.
Abbreviation
GABA, gamma aminobutyric acid
INTRODUCTION
Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical support of endothelial cells that form the blood-brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, and repair and scarring process of the brain and spinal cord following traumatic injuries. Furthermore, astrocytes express plasma membrane transporters such as glutamate transporters for several neurotransmitters, including glutamate, ATP, and gamma aminobutyric acid (GABA). More recently, astrocytes were shown to release glutamate or ATP in a vesicular Ca2+-dependent manner[1,2,3]. On the other hand, the common antibiotic penicillin is a chemical convulsant[4,5]. It causes neuronal degeneration and neuronal loss[6,7] by decreasing the GABAergic action[8], increasing excitatory glutamatergic neurotransmission[6], or activating the Ca2+ and Na+ conduction in ion channels[9]. It is commonly beleived that glutamate excitotoxicity relates to neuronal stress[10,11,12]. Also it has been known that extracellular glutamate level rises in a glucose-free condition[13]. Glucose metabolism is selective because of phosphofructokinase activity difference in different brain areas[14]. Insulin has a double-edged effect on the neuronal cell death dependent on glucose concentration, and that the CA1 and the dentate gyrus have a different sensitivity to insulin in terms of cell survival[15]. The relationship between the penicillin-induced astrocyte death and the protective effect of different insulin-glucose concentrations has to be studied. The information is crucial to explain complex mechanism of neuronal pathologies and treatment. Thus, this study was designed to evaluate the relationship between penicillin-induced astrocyte death and suitable concentration of glucose and insulin for increasing the astrocyte viability on astroglial cell culture. This may also allow us to determine the suitable glucose and insulin concentrations for the best astrocyte viability.
RESULTS
Astrocyte cell line without penicillin
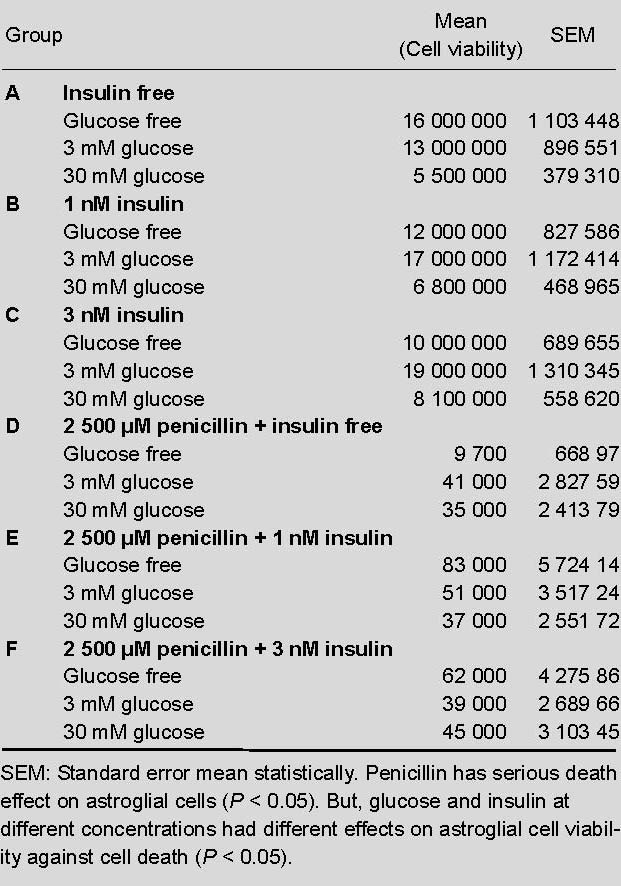
In insulin free composition (A), cell viability decreased significantly as the glucose concentrations increased (P < 0.05). Whereas in 1 nM insulin composition (B), cell viability was the best in 3 mM glucose (P < 0.05). Among all groups, the best result for cell viability was 3 nM insulin (C) and 3 mM glucose (Table 1).
Table 1.
Insulin and glucose concentrations used in the experiment
Astrocyte cell line with 2 500 μM (500 IU) penicillin
In insulin free composition (D), decreased cell viability due to penicillin (P < 0.05) seemed to be compensated by glucose. Glucose levels correlated with increased viability (P < 0.05). In the 1 nM insulin group (E), viability was the best in the absence of glucose. Controversial to group D, increased glucose levels depressed the viability in groups E and F.
DISCUSSION
In this study, the effects of different insulin and glucose concentrations on the astrocyte death induced by penicillin were examined. Previously penicillin was frequently used for experimental epilepsy[4,5]. The amount of penicillin is important in an epilepsy model. It means that higher penicillin dose causes higher number of hippocampal pyramidal neuronal loss in the intracortical penicillin rat model[16]. It seems to be the same in neuronal cell culture. At least 100–5 000 μM penicillin is required to block GABA[17]. So, we used 2 500 μM (500 IU) of penicillin concentration to cause a damage in astrocyte cell culture.
GABAergic action is important in astrocyte cell death[8]. Increased excitatory glutamatergic neurotransmission, activated Ca2+ and Na+ conduction in ion channels may also be the mechanisms of astrocyte cell death[7,8,9,10,11].
Astrocytes propagate intercellular Ca2+ waves over long distances in response to stimulation, and, similar to neurons, release transmitters (called gliotransmitters) in a Ca2+-dependent manner. Data suggest that astrocytes also signal to neurons through Ca2+-dependent release of glutamate[18]. Calcium elevations are the primary known axis of activation in astrocytes, and are necessary and sufficient for some types of astrocytic glutamate release[19,20,21].
Recent researches indicate that astrocyte is the tight junctions and basal lamina of the cerebral endothelial cells that play the most substantial role in maintaining the barrier[22,23]. Astrocytes express potassium channels at a high density. When neurons are active, they release potassium, increasing the local extracellular concentration. Because astrocytes are highly permeable to potassium, they rapidly clear the excess accumulation in the extracellular space. If this function is interfered, the extracellular concentration of potassium will rise, leading to neuronal depolarization. Abnormal accumulation of extracellular potassium is well known to result in epileptic neuronal activity[24].
In the hippocampus, astrocytes suppress synaptic transmission by releasing ATP, which is hydrolyzed by ectonucleotidases to yield adenosine[25,26]. Astrocytes may serve as intermediaries in neuronal regulation of blood flow[27]. Electrical activity in neurons causes them to release ATP, which serves as an important stimulus for myelin to form. This suggests that astrocytes have an executive-coordinating role in the brain[28].
Furthermore, studies are underway to determine whether astroglia plays an instrumental role in depression, based on the link between diabetes and depression. Altered central nervous system glucose metabolism is seen in both conditions, and the astroglial cells are the only cells with insulin receptors in the brain. They may have a role in regulating the response of the hypothalamus to glucose[29,30,31,32,33].
The results of this study will help to explain cerebrovascular pathologies parallel to insulin and glucose conditions of patient after intracranial injuries. Head injury is one of the major causes of trauma-related morbidity and mortality in all age groups all over the world. Despite a better understanding of the pathophysiological processes following traumatic brain injury and a wealth of research, there is currently no specific treatment. Within critical care, the importance of controlling blood glucose is becoming clearer, along with the potential beneficial effects of hyperoxia[34,35]. Peripheral nerve axotomy in adult mice elicits a complex response that includes increased glucose uptake in regenerating nerve cells. This work analyses the expression of the neuronal glucose transporters GLUT3, GLUT4 and GLUT8 in the facial nucleus of adult mice during the first days after facial nerve axotomy. The current results are also very important related to glucose level in penumbra[36]. Glycemic control in the treatment of patients with stroke, international guidelines recommend treating this subset of critically ill patients for hyperglycemia in the hospital setting. This treatment regime is, however, particularly challenging in patients with stroke, and is associated with an increased risk of the patient developing hypoglycemia[37]. There is an ongoing discussion as to which treatment algorithm, if any, provides the most effective prospective intervention[38]. Acute brain ischemia is a dynamic process susceptible to multiple modulating factors, such as blood glucose level. During acute ischemic brain injury, hyperglycemia exacerbates multiple deleterious derangements. Timely and sufficient correction of hyperglycemia during acute brain ischemia may limit the brain injury and improve clinical outcomes[39]. As discussion above, the effects of glucose and insulin level are important in neuronal case, but discussion is ongoing about them[40].
Epilepsies are common, with a major genetic contribution to etiology. But a complete understanding of precipitation is lacking. Reid at al[41] investigated that if lowering blood glucose increases spike-wave activity in mouse models with varying seizure susceptibility. They concluded that low blood glucose can precipitate seizures in genetically predisposed animal models and should be considered as a potential environmental risk factor in patients with absence epilepsy.
Astrocytes detect neuronal activity and can release chemical transmitters, which in turn control synaptic activity. The astroglial cell-specific glutamate transporter subtype 2 (excitatory amino acid transporter 2, GLT1) plays an important role in excitotoxicity that develops after damage to the central nervous system is incurred[42]. Our results suggest that insulin may modulate the expression of astrocytic excitatory amino acid transporter 2, which might play a role in reactive astrocytes after penicillin-induced injuries. Insulin enters the brain across the blood-brain barrier by a receptor-mediated transport system[43].
Conclusion
It was clear that penicillin had serious death effect on astroglial cells. Glucose and insulin at different concentrations had different effects on astroglial cell viability against cell death induced by penicillin. This situation might be up to the different mechanism of the astroglial cells related to the brain. Then, it might help to explain central nervous system disorders parallel to insulin and glucose conditions.
MATERIALS AND METHODS
Design
A randomized and controlled isolated cell culture study to determine the effect of insulin and glucose at different concentrations on cell death induced by penicillin.
Time and setting
This study was performed at Pamukkale University, School of Medicine between February and August 2011.
Materials
Primary astrocytes were derived from 1 to 5 days postnatal Sprague-Dawley rats. Briefly, cerebral cortices were dissected out. After removal of the meninges and blood vessels, the cerebral cortices were collected and minced with scalpel in a solution containing 20 μg/mL DNase and 0.3% bovine serum albumin in Hanks balanced PBS.
Methods
The tissues were centrifuged and incubated in 0.25% trypsin/EDTA solution for 30 minutes at 37°C. The suspension was filtered through a 70 μm nylon filter, pelleted by centrifugation to remove trypsin, and then suspended in 10% (v/v) fetal bovine serum in Dulbecco's modified Eagle's medium/F12 containing penicillin and streptomycin antibiotic mixture. And transferred to culture flasks and maintained at 37°C, 5% CO2 and 90% relative humidity. When cells reached confluence, flasks were gently shaken to remove microglia and oligodendrocytes. After shaking, cells were rinsed three times with Hanks balanced PBS and then trypsinized, then pat flasks firmly to loosen cells. Take the medium and put it a new flasks and add the astrosite culture media (Dulbecco's modified Eagle's medium with F12, 15% fetal bovine serum, L-glutamine and 500 ng/mL insulin). Astrosit cells were cultured in the astrosit medium until they were confluent (Figure 1). The cells were tyripsinized and counted for experiments.
Figure 1.
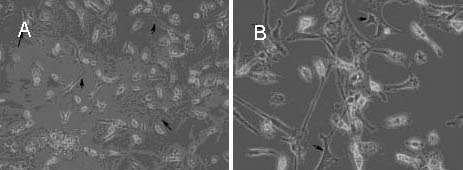
Astrocyte cell line cultured in the astrosit medium (light microscope).
(A) Astrocyte cell line without penicillin, and black arrows indicate the astrocytes (× 400).
(B) Astrocyte cell line with 2 500 μM penicillin, and black arrows indicate the astrocytes (× 1 000).
Statistical analysis
Values were expressed as mean ± SEM from 18 independent experiments. The statistical significance was established by analysis of variance followed by a post-hoc test, and then the non-paired t test was employed using StatView software (Abacus concepts, Berkeley, CA, USA). P < 0.05 was considered to be statistically significant.
Footnotes
Conflicts of interest: None declared.
(Edited by Poulsen DJ/Akgün H/Song LP)
REFERENCES
- [1].Agulhon C, Fiacco T, McCarthy K. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327(5970):1250–1257. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- [2].Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93(2):182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [3].Santello M, Volterra A. Synaptic modulation by astrocytes via Ca(2+)-dependent glutamate release. Neuroscience. 2008;22(1):253–259. doi: 10.1016/j.neuroscience.2008.03.039. [DOI] [PubMed] [Google Scholar]
- [4].Biziere K, Chambon JP. Animal models of epilepsy and experimental seizures. Rev Neurol. 1987;143(5):329–340. [PubMed] [Google Scholar]
- [5].Fisher RS. Animal models of the epilepsies. Brain Res Rev. 1989;14(3):245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- [6].Babb TL, Brown WJ, Pretorius J, et al. Temporallobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25(6):729–740. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- [7].Dam AM. Hippocampal neuron loss in epilepsy and after experimental seizures. Acta Neurol Scand. 1982;66(6):601–642. doi: 10.1111/j.1600-0404.1982.tb04528.x. [DOI] [PubMed] [Google Scholar]
- [8].Arzimanoglou A, Hirsch E, Nehlig A, et al. Epilepsy and neuroprotection: an illustrated review. Epileptic Disord. 2002;4(3):173–182. [PubMed] [Google Scholar]
- [9].Bagirici F, Gokce FM, Marangoz C. Depressive effect of nicardipine on penicillin-induced epileptiform activity in rats. Neurosci Res Commun. 1999;24(3):149–154. [Google Scholar]
- [10].Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26(4):438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- [11].Nakamura N, Negishi K, Hirano A, et al. Real-time monitoring of L-glutamate release from mouse brain slices under ischemia with a glass capillary-based enzyme electrode. Anal Bioanal Chem. 2005;383(4):660–667. doi: 10.1007/s00216-005-0033-6. [DOI] [PubMed] [Google Scholar]
- [12].Tomiyama M, Kimura T, Maeda T, et al. Expression of metabotropic glutamate receptor mRNAs in the human spinal cord: implications for selective vulnerability of spinal motor neurons in amyotrophic lateral sclerosis. J Neurol Sci. 2001;189(1-2):65–69. doi: 10.1016/s0022-510x(01)00561-5. [DOI] [PubMed] [Google Scholar]
- [13].Takata T, Hirai H, Shigemoto T, et al. The release of glutamate and accumulation of intracellular calcium in the guinea pig hippocampal slices during glucose deprivation. Neurosci Lett. 1995;189(1):21–24. doi: 10.1016/0304-3940(95)11439-4. [DOI] [PubMed] [Google Scholar]
- [14].Li X, Yokono K, Okada Y. Phosphofructokinase, a glycolytic regulatory enzyme has a crucial role for maintenance of synaptic activity in guinea pig hippocampal slices. Neurosci Lett. 2000;294(2):81–84. doi: 10.1016/s0304-3940(00)01535-4. [DOI] [PubMed] [Google Scholar]
- [15].Tanaka Y, Takata T, Satomi T, et al. The double-edged effect of insulin on the neuronal cell death associated with hypoglycemia on the hippocampal slice culture. Kobe J Med Sci. 2008;54:E97–107. [PubMed] [Google Scholar]
- [16].Akdogan I, Adiguzel E, Yilmaz I, et al. Penicillin-induced epilepsy model in rats: dose-dependant effect on hippocampal volume and neuron number. Brain Res Bull. 2008;77(4):172–177. doi: 10.1016/j.brainresbull.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [17].Twyman RE, Green RM, MacDonald RL. Kinetics of open channel block by penicillin of single GABAA receptor channels from mouse spinal cord neurones in culture. J Physiol. 1992;445:97–127. doi: 10.1113/jphysiol.1992.sp018914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fiacco TA, Agulhon C, McCarthy KD. Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol. 2009;49:151–174. doi: 10.1146/annurev.pharmtox.011008.145602. [DOI] [PubMed] [Google Scholar]
- [19].Bennett M, Contreras J, Bukauskas F, et al. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26(11):610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Parpura V, Haydon P. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97(15):8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kimelberg HK, Jalonen T, Walz W. Regulation of the brain microenvironment: transmitters and ions. In: Murphy S, editor. Astrocytes: pharmacology and function. San Diego, CA: Academic Press; 1993. [Google Scholar]
- [23].Swaminathan N. Brain-scan mystery solved. Sci Am Mind. 2008:7. doi:10.1038/scientificamericanmind1008-7b. [Google Scholar]
- [24].Durand DM, Park EH, Jensen AL. Potassium diffusive coupling in neural networks. Philos Trans R Soc Lond B Biol Sci. 2010;365(1551):2347–2362. doi: 10.1098/rstb.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- [26].Piet R, Vargová L, Syková E, et al. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101(7):2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parri R, Crunelli V. An astrocyte bridge from synapse to blood flow. Nat Neurosci. 2003;6(1):5–6. doi: 10.1038/nn0103-5. [DOI] [PubMed] [Google Scholar]
- [28].Ishibashi T, Dakin K, Stevens B, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brawer JR. Composition of Gomori-positive inclusions in astrocytes of the hypothalamic arcuate nucleus. Anatomical Record. 1994;240:407–415. doi: 10.1002/ar.1092400313. [DOI] [PubMed] [Google Scholar]
- [30].Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10(2):201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- [31].Marty N. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115(12):3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Young JK, McKenzie JC. GLUT2 immunoreactivity in Gömöri-positive astrocytes of the hypothalamus. J. Histochemistry & Cytochemistry. J Histochem Cytochem. 2004;52(11):1519–1524. doi: 10.1369/jhc.4A6375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15(11):7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Protheroe RT, Gwinnutt CL. Early hospital care of severe traumatic brain injury. Anaesthesia. 2011;66(11):1035–1047. doi: 10.1111/j.1365-2044.2011.06874.x. [DOI] [PubMed] [Google Scholar]
- [35].MacDougall NJ, Muir KW. J Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and meta-analysis. Cereb Blood Flow Metab. 2011;31(3):807–818. doi: 10.1038/jcbfm.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gómez O, Ballester-Lurbe B, Mesonero JE, et al. Glucose transporters GLUT4 and GLUT8 are upregulated after facial nerve axotomy in adult mice. J Anat. 2011;219(4):525–530. doi: 10.1111/j.1469-7580.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bruno A, Liebeskind D, Hao Q, et al. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol. 2010;12(6):492–503. doi: 10.1007/s11940-010-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kreisel SH, Berschin UM, Hammes HP, et al. Pragmatic management of hyperglycaemia in acute ischaemic stroke: safety and feasibility of intensive intravenous insulin treatment. Cerebrovasc Dis. 2009;27(2):167–175. doi: 10.1159/000185608. [DOI] [PubMed] [Google Scholar]
- [39].Kruyt ND, Biessels GJ, Devries JH, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- [40].Quinn TJ, Lees KR. Hyperglycaemia in acute stroke-to treat or not to treat. Cerebrovasc Dis. 2009;27(Suppl 1):148–155. doi: 10.1159/000200453. [DOI] [PubMed] [Google Scholar]
- [41].Reid CA, Kim TH, Berkovic SF, et al. Low blood glucose precipitates spike-and-wave activity in genetically predisposed animals. Epilepsi. 2011;52(1):115–120. doi: 10.1111/j.1528-1167.2010.02911.x. [DOI] [PubMed] [Google Scholar]
- [42].Ji YF, Xu SM, Zhu J, et al. Insulin increases glutamate transporter GLT1 in cultured astrocytes. Biochem Biophys Res Commun. 2011;405(4):691–696. doi: 10.1016/j.bbrc.2011.01.105. [DOI] [PubMed] [Google Scholar]
- [43].Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species specific radioimmunoassays. Peptides. 1997;18(8):1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]


