Abstract
Background and Objectives:
India is among the largest countries to implement the revised National Tuberculosis Control Program (RNTCP). This program provides intermittent regimens to the patients, where the doses of isoniazid and ethambutol are more as compared to the daily regimen, which is a cause of concern, particularly with regard to the ocular toxicity of ethambutol. The present study was undertaken to explore the ocular toxicity in the patients registered under the program.
Materials and Methods:
This was a prospective single center cohort study of 64 patients of categories I and II, coming to the RNTCP-Directly Observed Treatment Strategy (DOTS) center at a tertiary care referral hospital. The detailed history, best corrected visual acuity, fundus examination, and color vision test were carried out in all patients at the start of treatment and then at the first and second month of treatment.
Results:
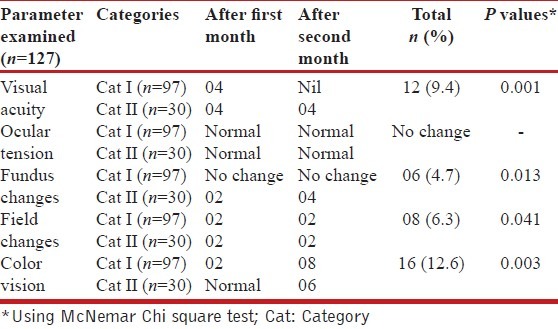
Loss in visual acuity from the baseline was noted at the second month follow up in 12 (9.4%) eyes (P = 0.001), visual field defects were seen in eight (6.3%) eyes (P = 0.0412), and optic disc abnormalities were observed in six (4.7%) (P = 0.013) eyes. Color vision abnormalities were noted in 16 (12.6%) eyes (P = 0.003), four eyes showed impairment in red–green color perception, and the others showed impairment in blue–yellow color perception as well. Patients with ocular symptoms were advised to stop ethambutol and they showed improvement in visual acuity after follow up of one to two months. The overall outcome of treatment was not affected by discontinuation of ethambutol in these patients.
Conclusion:
Ethambutol when taken according to program could cause ocular toxicity. The early recognition of ocular symptoms is important to prevent unnecessary delay in diagnosis and probable irreversible visual loss.
Keywords: Ethambutol toxicity, ocular symptoms, revised national tuberculosis control program, visual defects
INTRODUCTION
India is among the countries that carry the highest burden for tuberculosis, and accounts for one-fifth of the global burden. To combat this gigantic problem a revised National Tuberculosis Control Program (RNTCP) has been implemented throughout the country. Under this program patients receive intermittent treatment under supervision, thrice weekly. The program caters to different regimens and durations, for different categories of patients with tuberculosis. The combination of drugs used in these categories is the same as in the daily regimens, but the dosage of ethambutol and isoniazid is almost double as compared to the daily regimen. The safety and efficacy of the intermittent regimens have been well-documented, but there are still concerns regarding the ocular toxicity of ethambutol, because of its increased dose.
Ethambutol is a bacteriostatic drug, developed in 1962. Since then, mild-to-severe toxic amblyopia due to ethambutol has been reported by several authors.[1,2,3] Toxic optic neuritis may be of early or late onset, may be reversible or irreversible, and axial or peri-axial.[4,5] Ethambutol can cause visual impairment as a result of retrobulbar neuritis, which is related to the dose and duration of treatment. There are several controversial reports in medical literature regarding the safety of ethambutol and fear of its use as a routine anti-tubercular drug, especially in children. Due to the paucity of medical literature regarding the incidence and clinical manifestations of ethambutol-induced optic neuritis given as an intermittent therapy under RNTCP, this study was planned.
MATERIALS AND METHODS
Study design
Prospective single center cohort study of category I and category II patients registered at the RNTCP-DOTS center between May 2011 and April 2012.
Setting
The study was done at the Department of Ophthalmology, at Era's Lucknow Medical College − a tertiary care referral hospital and a teaching medical institution, after approval from the Institutional Ethics Committee.
Patient selection
All consecutive patients of categories I and II, coming to the institution's RNTCP-DOTS center were enrolled in the study after their informed consent. The category I regimen comprised of two months of rifampicin (450 mg), isoniazid (600 mg), ethambutol (1200 mg), and pyrazinamide (1500 mg), thrice weekly, followed by four months of rifampicin and isoniazid in the same dose. In the category II regimen, patients received two months of streptomycin (750 mg), isoniazid (600 mg), rifampicin (450 mg), ethambutol (1200 mg), and pyrazinamide (1500 mg), with one month of the same drugs, except streptomycin, in the intensive phase, and five months of rifampicin, isoniazid, and ethambutol − all these drugs were given thrice weekly.
Exclusion criteria for participants
Participants with other systemic diseases such as renal failure, diabetes, hypertension, addiction to tobacco, alcohol, and patients having other ocular diseases that might affect visual acuity or may possibly contribute to color vision defects like diabetic retinopathy, sickle cell retinopathy, retinitis pigmentosa, previous retinal detachment, optic neuropathies, gluacoma, optic neuritis or optic atrophy, or cataract with more than +2 nuclear sclerosis were excluded from the study. Also patients on medications implicated in causing color vision deficiency like oral contraceptives, digoxin, and indomethacin, and those who had color vision deficiency at the baseline were excluded from the study.
Clinical evaluation of participants
The participants underwent an eye evaluation consisting of detailed history, best corrected visual acuity by Snellen chart, direct fundoscopy examination, anterior segment slit-lamp biomicroscopy, color vision by the Ishihara Chart and Farnsworth Panel D-15 hue test, and field charting using the Humphrey field analyzer with the C-30-2 threshold program.
All the enrolled patients were examined before the start of the anti-tubercular treatment and after the first and second months of treatment. Visual acuity loss was counted, to see if it had exceeded two Snellen chart lines between the last ophthalmological examination before ethambutol was started and the first examination after the medication was started, in the absence of other causal factors.
A single investigator assessed the color vision using the full series of Ishihara Color Vision Plates (Ishihara) and Farnsworth Panel D-15. The participants were tested binocularly while wearing the best correction. If a participant read nine or fewer plates correctly, his/her color vision was regarded as being deficient. The FD-15 test was done using the Color Vision Recorder software, version 4. The screen color was calibrated based on the standards set by the International Color Consortium (ICC). In these color-arrangement tests, the participant was offered a series of colors that needed to be sorted either in a sequence or in groups. The final diagnosis consisted of two parts−the pass and fail diagnosis, which was automatically set by the software.
Differences between baseline and follow-up findings were noted. The student's t-test and Chi-square tests were used, where a P value equal to or less than 0.05 was considered statistically significant.
RESULTS
There were 69 participants of categories I and II, who were enrolled in the study. Two participants developed severe intolerance to the drug after two weeks of DOTS therapy and refused to continue the treatment and three participants did not turn up after the first week of registration. Thus, 64 participants completed the prescribed number of follow ups and constituted the study group.
There were 39 males and 25 females, of age 13 to 70 years, with a mean of 34.23 ± 15.54 years. At the baseline, the visual acuity ranged from 6/6 (log MAR 0.0) (log minimum angle of resolution) to 6/60 (log MAR 1), with a mean of 0.26 ± 0.39 [Table 1]. Anterior segment examination by slit lamp, direct ophthalmoscopy, visual fields, and color vision were all normal. Visual acuity loss was seen in six eyes, two each in categories I and II after one month, and two eyes in category II after two months of starting the therapy. On using McNemar Chi-Square Test, there was a statistically significant difference in visual acuity in terms of MAR values at the second month after the start of therapy (mean 0.0460 ± 0.14687, P < 0.001).
Table 1.
Characteristics of the study subjects

Visual field defects were seen in eight (6.3%) eyes of four participants. One participant of category I showed centrocecal scotoma on the Humphrey perimeter, while the remaining eyes showed peripheral constriction. The defects were bilateral in all cases. Visual field defects noted after two months showed the exact significance of 0.0412 by McNemar Chi square test. Optic disc abnormalities were observed in six (4.7%) eyes, all from category II. Two eyes had disc edema, while the other four had temporal pallor only. These changes were statistically significant after two months of therapy (P = 0.013). Color vision abnormalities were noted in 16 eyes of eight patients, four eyes showed impairment in red-green color perception and the others showed impairment in blue-yellow color perception. All abnormalities were noted by Farnsworth Panel D-15 test, while the Ishihara pseudoisochromatic test showed abnormality in only one participant. This difference of color vision was statistically significant (P = 0.003). There was no change observed in ocular tension after the second month vide [Table 2]. Six patients had ocular symptoms and they were advised to stop ethambutol and all of them showed improvement in visual acuity, fundus findings, and color vision after follow up of one to two months. The overall outcome of treatment was not affected by discontinuation of ethambutol in these patients.
Table 2.
Ophthalmological examination during follow up distributed category-wise
DISCUSSION
Ethambutol is being used to treat tuberculosis since the 1960s. The potential for visual impairment was recognized soon after its introduction. Ocular toxicity due to ethambutol usually develops after two months of therapy[6] and is related to the dose,[7] as was also evident in the present study. The dose-related toxicity of this drug as reported by Liebold[7] and Bobrowitz[6] was 18% at 35 mg/kg body weight/day, 5% among patients on 25 mg/kg/day, and 3% among those on 20 mg/kg/day, while negligible toxicity was seen in patients taking ethambutol 15 mg/kg/day. In the present study patients received ethambutol >20 mg/kg body weight. Krishnaswamy[8] and Mittal et al.[9] did not find any case of retrobulbar neuritis in their series, while Roy et al.[10] and Sharma et al.[11] found 3% toxicity in cases using 25 mg/kg/day of ethambutol. On the other hand Narang and Verma[12] in their study of 640 cases treated by ethambutol of 25 mg/kg/day of the drug, along with a companion drug came across only four cases (0.62%), among which retrobulbar neuritis developed during six to eight months of therapy. Retrobulbar neuritis was reversible in all the four cases after two to four months of withdrawal of the drug, as was seen in our case, and also where improvement, both in terms of visual acuity and fundus changes, was seen as early as one month of withdrawal of the drug.
A study by Menon et al.,[13] done on patients treated with ethambutol under DOTS, showed no effect on visual acuity, color vision or fundus of any patient, although visual field defects were seen in 7.69% of the eyes, with increased latency of the P wave in Pattern VER after one and two months of therapy. In recent times, another similar study by Kandel et al.[14] revealed a statistically significant change in the visual acuity, as was found in our study.
The limitation of this study could be the ocular toxicity contributed by isoniazid, as revealed by a recent study by Sahin et al., which was not taken into consideration.[15]
However, this recent study has been performed on an animal model and needs further evaluation in humans before its results can be recommended for the same.
CONCLUSION
This study on the evaluation of visual functions in patients receiving ethambutol as a part of DOTS therapy does hint at the fact that ethambutol when taken according to the dose and duration, as prescribed under DOTS Regimen, can cause ocular toxicity. It is also seen that a reversal of these toxic effects occurs if the drug is immediately stopped. Thus, early diagnosis using a sensitive indicator is necessary and may be helpful in preventing irreversible visual loss that may occur if the drug is used continuously.
The study findings have shown that there is a need to formulate guidelines for mandatory routine ophthalmic checkups in patients receiving ethambutol and compulsory education to the patient about blurring of vision, problems in appreciating different colors, and any non-seeing areas in the central field. This can be carried out by the DOTS provider and will not require an ophthalmologist consultation at the base level. It is important to preserve the sight while treating tuberculosis.
ACKNOWLEDGEMENTS
The authors would like to thank Sri. Zeeshan Zaidi for providing help with regard to the statistical analysis and evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Place VA, Black H. New antituberculous agents: Laboratory and clinical studies. Ann N Y Acad Sci. 1966;135:681–709. doi: 10.1111/j.1749-6632.1966.tb45515.x. [DOI] [PubMed] [Google Scholar]
- 2.Inocencio FP, Castillo TR. Toxic optic neuropathy secondary to ethambutol. Philipp J Ophthalmol. 1999;24:65–8. [Google Scholar]
- 3.World Health Organization. Ethambutol efficacy and toxicity: Literature review and recommendations for daily and intermittent dosage in children. Geneva: World Health Organization; [Google Scholar]
- 4.Adel A. Ophthalmological side-effects of ethambutol. Scand J Respir Dis Suppl. 1969;69:55–8. [PubMed] [Google Scholar]
- 5.Schild HS, Fox BC. Rapid-onset reversible ocular toxicity from ethambutol therapy. Am J Med. 1991;90:404–6. [PubMed] [Google Scholar]
- 6.Bobrowitz ID. Ethambutol in the retreatment of pulmonary tuberculosis. Ann N Y Acad Sci. 1966;135:796–822. doi: 10.1111/j.1749-6632.1966.tb45523.x. [DOI] [PubMed] [Google Scholar]
- 7.Leibold JE. The ocular toxicity of ethambutol and its relation to dose. Ann N Y Acad Sci. 1966;135:904–9. doi: 10.1111/j.1749-6632.1966.tb45532.x. [DOI] [PubMed] [Google Scholar]
- 8.Krishnaswamy KV. Proceedings 24 th National Cong. Tubercle and Chest Dis.; Trivandrum. 1969. p. 254. [Google Scholar]
- 9.Mittal OP, Narang RK, Sachan AS. Ethambutol in retreatment of pulmonary tuberculosis. Indian J Tuberc. 1975;22:142–146. [Google Scholar]
- 10.Roy DC, Sen PC, Bajpai BK. Ethambutol in the re-treatment of mult-resistant advanced pulmonary tuberculosis (salvage cases) Indian J Chest Dis. 1974;16:153–62. [PubMed] [Google Scholar]
- 11.Sharma GS, Purohit SD, Lodha SC. Comparative study of isoniazid and ethambutol with isoniazid ethambutol and pyrazinamide in the retreatment of pulmonary tuberculosis. Ind Med Gaz. 1975;15:140. [Google Scholar]
- 12.Narang RK, Varma BM. Occular toxicity of ethambutol (a clinical study) Indian J Ophthalmol. 1979;27:37–40. [PubMed] [Google Scholar]
- 13.Menon V, Jain D, Saxena R, Sood R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93:1251–4. doi: 10.1136/bjo.2008.148502. [DOI] [PubMed] [Google Scholar]
- 14.Kandel H, Adhikari P, Shrestha GS, Ruokonen EL, Shah DN. Visual function in patients on ethambutol therapy for tuberculosis. J Ocul Pharmacol Ther. 2012;28:174–8. doi: 10.1089/jop.2011.0095. [DOI] [PubMed] [Google Scholar]
- 15.Şahin A, Kürşat Cingü A, Kaya S, Türkcü G, Arı Ş, Evliyaoğlu O, et al. The protective effects of caffeic acid phenethyl ester in isoniazid and ethambutol-induced ocular toxicity of rats. Cutan Ocul Toxicol. 2013;32:228–33. doi: 10.3109/15569527.2012.759958. [DOI] [PubMed] [Google Scholar]


