Abstract
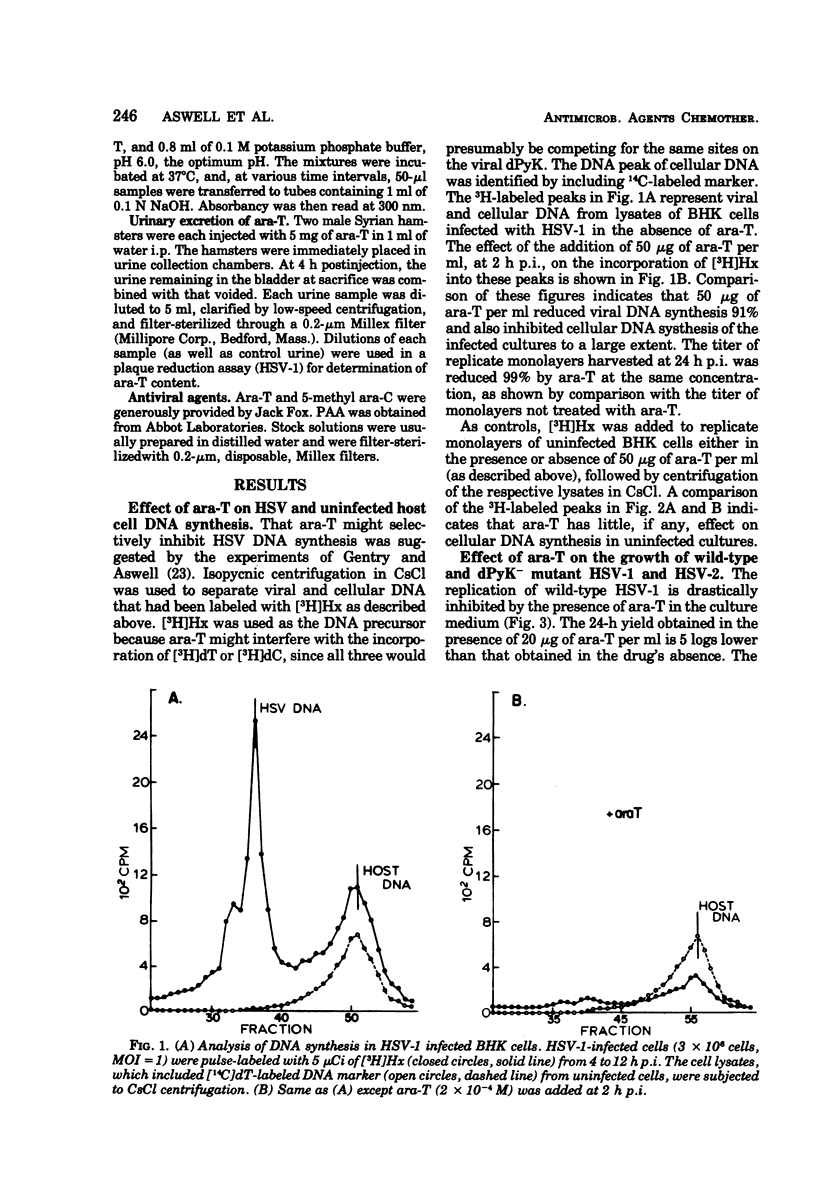
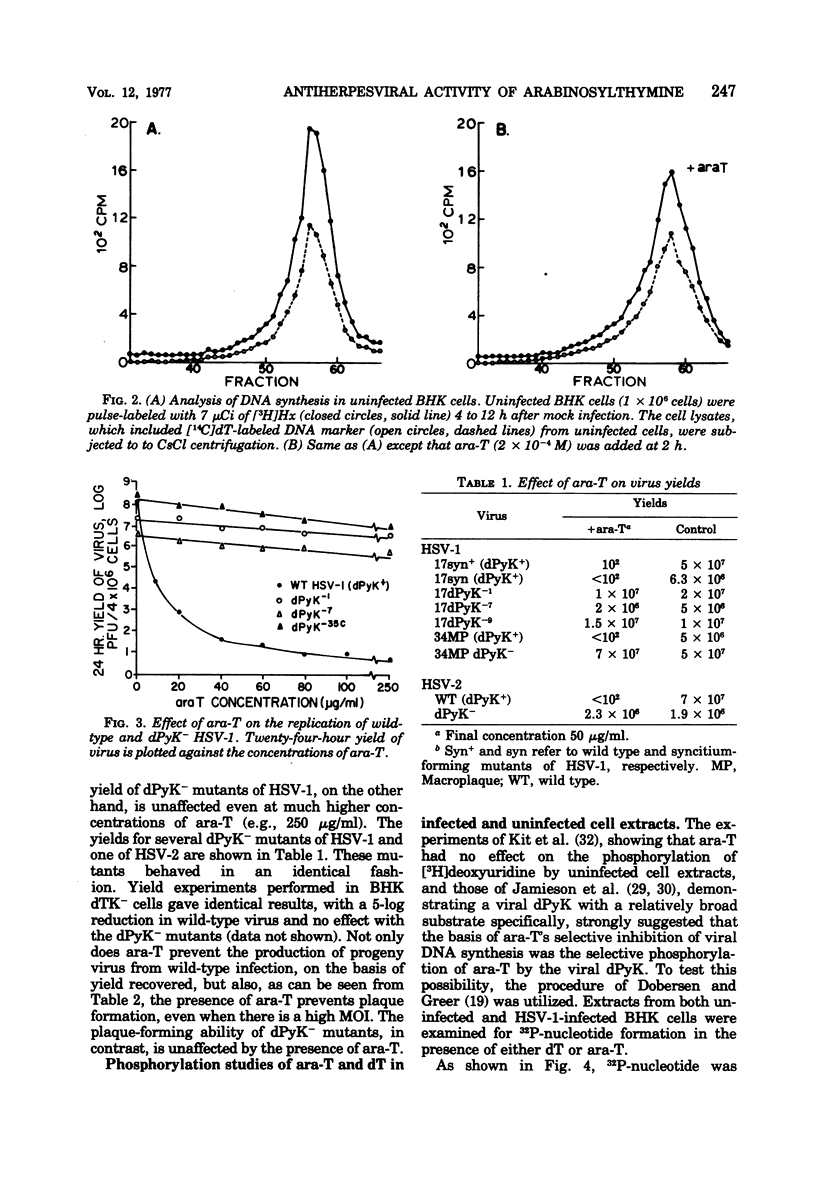
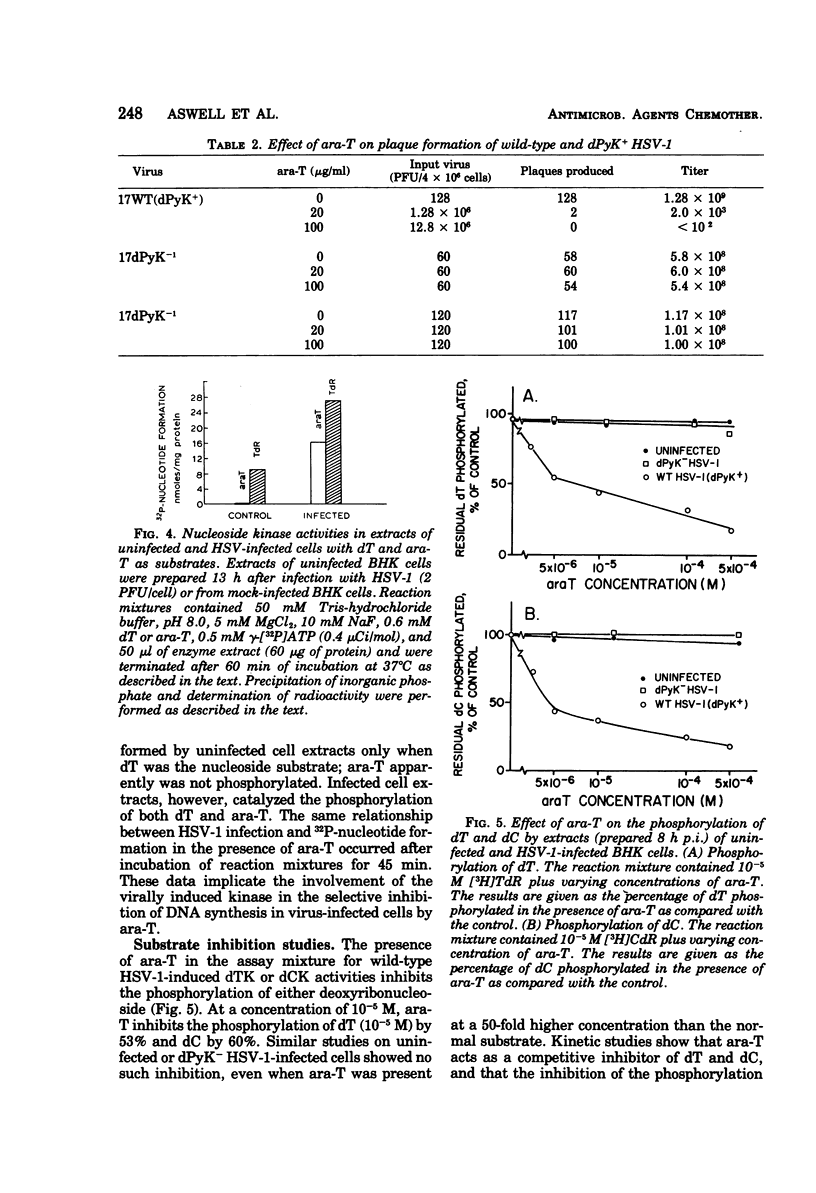
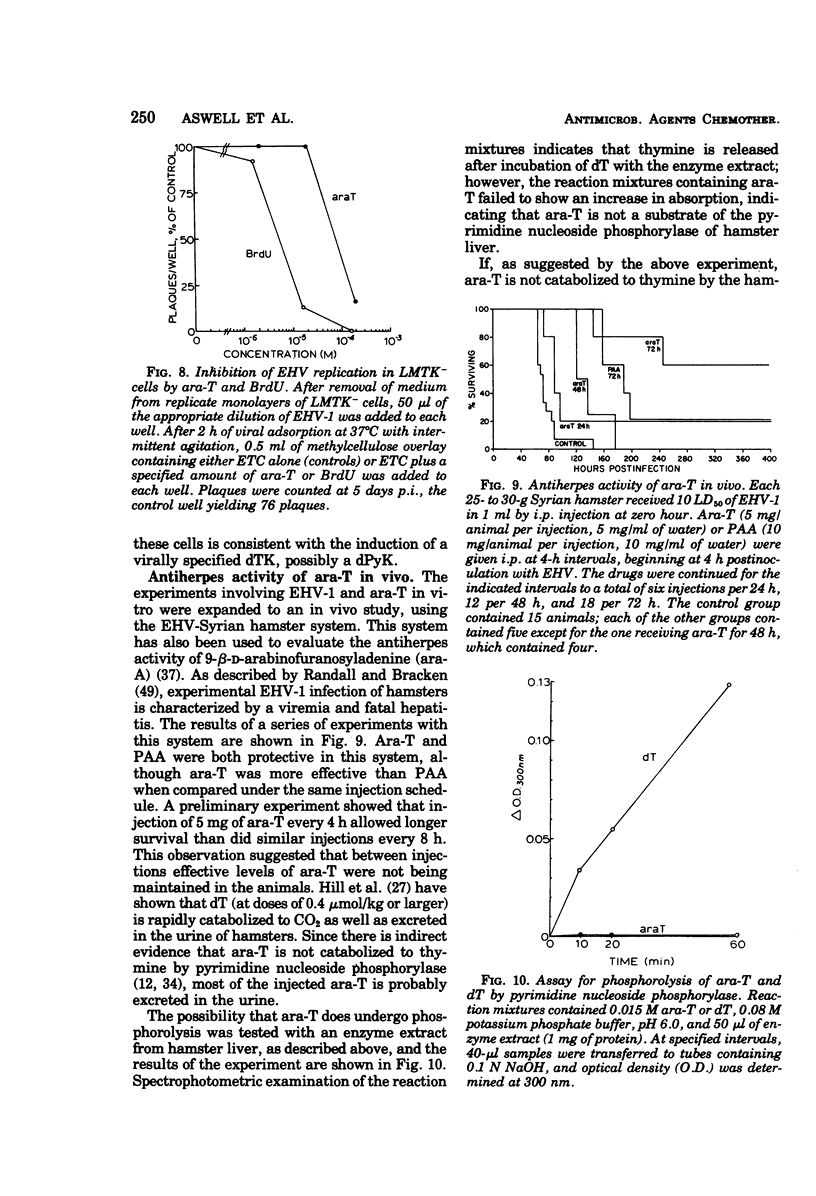
1-β-d-Arabinofuranosylthymine (ara-T), a metabolite of the sponge Tethya crypta, has shown selective activity against herpes simplex virus (HSV) replication (G. A. Gentry and J. F. Aswell, Virology 65:294–296, 1975). Analysis of HSV-infected and uninfected cell lysates by CsCl isopycnic centrifugation showed that ara-T blocked the incorporation of [3H]hypoxanthine into viral deoxyribonucleic acid and, to a large extent, into host deoxyribonucleic acid of infected (but not uninfected) cells. Additional experiments with [γ-32P]adenosine 5′-triphosphate as a radiophosphate donor demonstrated that ara-T is phosphorylated by extracts of HSV-infected BHK cells and not by those of uninfected cells. At an ara-T concentration that almost completely inhibited the growth of LM cells, which had been transformed to a pyrimidine deoxyribonucleoside kinase+ (dPyK+) phenotype by ultraviolet-inactivated HSV-1, the growth of uninfected LM cells was not affected. These results indicate that the viral dPyK is responsible for the selective antiviral activity of ara-T. This conclusion was further supported by experiments that showed that the replication of a variety of dPyK− mutants of HSV-1 and HSV-2 were not affected by ara-T and that ara-T inhibited the phosphorylation of deoxycytidine and deoxythymidine by HSV-1 dPyK, but not by host deoxycytidine and deoxythymidine kinases, respectively. Ara-T also selectively inhibited the replication of equine herpesvirus type 1 (EHV-1) in vitro and was effective against EHV-1 infection in vivo in hamsters. Further, EHV-1 was inhibited by ara-T and by bromodeoxyuridine in LM cells lacking a cytosol thymidine kinase, suggesting that EHV-1 induces a dPyK. Finally, spectrophotometric assay for thymine suggested that ara-T is not a substrate for nucleoside phosphorylase of hamster liver, and a microbiological assay indicated that substantial amounts of ara-T were excreted in the urine of uninfected hamsters that had received a single injection of 5 mg of ara-T, the amount given in each injection in the in vivo experiments with EHV-1.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswell J. F., Gentry G. A. Cell-dependent antiherpesviral activity of 5-methylarabinosylcytosine, an intracellular ara-T donor. Ann N Y Acad Sci. 1977 Mar 4;284:342–350. doi: 10.1111/j.1749-6632.1977.tb21969.x. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Meldrum B., Gupta V. S., Rouse B. T. Comparison of the antiviral effects of 5-methoxymethyl-deoxyuridine with 5-iododeoxyuridine, cytosine arabinoside, and adenine arabinoside. Antimicrob Agents Chemother. 1975 Dec;8(6):643–650. doi: 10.1128/aac.8.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Effect of anti-herpesvirus drugs on human and bovine lymphoid function in vitro. Infect Immun. 1975 Dec;12(6):1281–1289. doi: 10.1128/iai.12.6.1281-1289.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2722–2729. [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. Thymidylate nucleotide supply for mitochondrial DNA synthesis in mouse L-cells. Effect of 5-fluorodeoxyuridine and methotrexate in thymidine kinase plus and thymidine kinase minus cells. J Biol Chem. 1976 May 25;251(10):2938–2944. [PubMed] [Google Scholar]
- CHU M. Y., FISCHER G. A. A proposed mechanism of action of 1-beta-D-arabinofuranosyl-cytosine as an inhibitor of the growth of leukemic cells. Biochem Pharmacol. 1962 Jun;11:423–430. doi: 10.1016/0006-2952(62)90225-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. A rational approach to the development of antiviral chemotherapy: alternative substrates of herpes simplex virus Type 1 (HSV-1) and Type 2 (HSV-2) thymidine kinase (TK). Ann N Y Acad Sci. 1977 Mar 4;284:594–598. doi: 10.1111/j.1749-6632.1977.tb21992.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. Deoxythymidine kinase induced in the HELA TK- cells by herpes simplex virus type I and type II. Substrate specificity and kinetic behavior. Biochim Biophys Acta. 1976 Dec 8;452(2):370–381. doi: 10.1016/0005-2744(76)90186-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Neenan J. P., Ward D. C., Prusoff W. H. Selective inhibition of herpes simplex virus by 5-amino-2,5-dideoxy-5-iodouridine. J Virol. 1975 May;15(5):1284–1285. doi: 10.1128/jvi.15.5.1284-1285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Phosphorylation of 5-bromodeoxycytidine in cells infected with herpes simplex virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3788–3792. doi: 10.1073/pnas.70.12.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLINGTON R. W., RANDALL C. C. The nucleic acid content of equine abortion virus. Virology. 1963 Mar;19:322–327. doi: 10.1016/0042-6822(63)90071-0. [DOI] [PubMed] [Google Scholar]
- DOLL E. R., RICHARDS M. G., WALLACE M. E. Adaptation of the equine abortion virus to suckling Syrian hamsters. Cornell Vet. 1953 Oct;43(4):551–558. [PubMed] [Google Scholar]
- Dobersen M. J., Greer S. An assay for pyrimidine deoxyribonucleoside kinase using gamma-32P-labeled ATP. Anal Biochem. 1975 Aug;67(2):602–610. doi: 10.1016/0003-2697(75)90335-8. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Hill E. A., Russell G. J. Identification of strains of herpes simplex virus by comparison of the density of their DNA using the preparative ultracentrifuge. Arch Virol. 1975;48(2):157–168. doi: 10.1007/BF01318148. [DOI] [PubMed] [Google Scholar]
- Hay J., Perera P. A., Morrison J. M., Gentry G. A., Subak-Sharpe J. H. Herpes virus-specified proteins. In: strategy of the viral genome. Ciba Found Symp. 1971:355–376. [PubMed] [Google Scholar]
- Hill J. M., Morse P. A., Jr, Gentry G. A. Metabolism of deoxycytidine, thymine, and deoxythymidine in the hamster. Cancer Res. 1975 May;35(5):1314–1319. [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M. A., Dobersen M. J., Greer S. Selective inhibition of the replication of varicella-zoster virus by 5-halogenated analogs of deoxycytidine. Ann N Y Acad Sci. 1977 Mar 4;284:389–395. doi: 10.1111/j.1749-6632.1977.tb21975.x. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KRISS J. P., BOND S. B. THE EFFECT OF NATURAL AND UNNNATURAL PYRIMIDINES AND PYRIMIDINE NUCLEOSIDES ON THE PHOSPHOROLYSIS OF 5-IODODEOXYURIDINE BY MOUSE LIVER EXTRACT. Biochem Pharmacol. 1964 Mar;13:365–370. doi: 10.1016/0006-2952(64)90153-4. [DOI] [PubMed] [Google Scholar]
- KUCHLER R. J., MERCHANT D. J. Propagation of strain L (Earle) cells in agitated fluid suspension cultures. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):803–806. doi: 10.3181/00379727-92-22620. [DOI] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R. Arabinofuranosylcytosine-induced stimulation of thymidine kinase and deoxycytidylic deaminase activities of mammalian cultures. Cancer Res. 1966 Sep;26(9):1859–1866. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Pascale A., Schafer T. W., Came P. E. Effect of antiviral agents in equine abortion virus-infected hamsters. Antimicrob Agents Chemother. 1972 Feb;1(2):143–147. doi: 10.1128/aac.1.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Cheevers W. P., Gentry G. A., Randall C. C. Kinetics of cellular and viral DNA synthesis in equine abortion (herpes) virus infection of L-M cells. Virology. 1968 Sep;36(1):104–114. doi: 10.1016/0042-6822(68)90120-7. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D. J., Hyde J. M., Gentry G. A., Randall C. C. Kinetics of viral deoxyribonucleic acid, protein, and infectious particle production and alterations in host macromolecular syntheses in equine abortion (herpes) virus-infected cells. J Virol. 1968 Aug;2(8):793–804. doi: 10.1128/jvi.2.8.793-804.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Ward D. C., Lin T. S., Chen M. S., Shaiu G. T., Chai C., Lentz E., Capizzi R., Idriss J., Ruddle N. H. Recent studies on the antiviral and biochemical properties of 5-halo-5'-amino-deoxyribonucleosides. Ann N Y Acad Sci. 1977 Mar 4;284:335–341. doi: 10.1111/j.1749-6632.1977.tb21968.x. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., BRACKEN E. C. Studies on hepatitis in hamsters infected with equine abortion virus. I. Sequential development of inclusions and the growth cycle. Am J Pathol. 1957 Jul-Aug;33(4):709–727. [PMC free article] [PubMed] [Google Scholar]
- RANDALL C. C., LAWSON L. A. Adaptation of equine abortion virus to Earle's L cells in serum-free medium with plaque formation. Proc Soc Exp Biol Med. 1962 Jul;110:487–489. doi: 10.3181/00379727-110-27558. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C. A sensitive and precise plaque assay for herpes virus. Nature. 1962 Sep 8;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Cashon G., Adam E., Ogino T., Duff R., Rapp F. Herpesvirus type 2-induced thymidine kinase and carcinoma of the cervix. Cancer Res. 1974 Feb;34(2):362–366. [PubMed] [Google Scholar]
- Renis H. E., Buthala D. A. Development of resistance to antiviral drugs. Ann N Y Acad Sci. 1965 Jul 30;130(1):343–354. doi: 10.1111/j.1749-6632.1965.tb12568.x. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD G. E., WISNER C. A., WEED S. D. CYTOSINE ARABINOSIDE (CA) AND OTHER NUCLEOSIDES IN HERPES VIRUS INFECTIONS. Arch Ophthalmol. 1964 Oct;72:505–512. doi: 10.1001/archopht.1964.00970020505014. [DOI] [PubMed] [Google Scholar]



