Abstract
Insult or injury to the lung epithelial cells from pathogens, pollutants, and allergens can initiate the process of apoptotic cell death. Although “Creola bodies,” which are clusters of uncleared, apoptotic, epithelial cells, have been seen in the sputum of patients with asthma, the clearance of these dying epithelial cells and the consequence of failed clearance in the airway have not been directly addressed. We have observed that bronchial epithelial cells efficiently engulf their apoptotic neighbors and produce antiinflammatory cytokines when engulfing apoptotic cells. Furthermore, when the phagocytic capacity of bronchial epithelial cells was impaired, mice developed severe, IL-33–dependent, allergic airway inflammation. This inflammation could be ameliorated by exogenous administration of the antiinflammatory cytokine IL-10. Our data suggest that the process of apoptotic cell engulfment is a mechanism by which bronchial epithelial cells regulate the inflammatory environment within the lung. Collectively, these studies suggest that impaired engulfment pathways in airway epithelial cells can contribute to allergic airway inflammation and that targeting these pathways may be of benefit in human airway inflammation.
Keywords: airway epithelium, apoptotic cells, phagocytosis, IL-10, IL-33
The epithelial cells of the airways serve not only as a barrier to pathogens and allergens they also regulate immune responses and play a crucial role in the pathogenesis of allergic asthma. When exposed to allergens, bronchial epithelial cells produce the inflammatory cytokines thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, which contribute to the innate inflammatory responses (1–3). In addition, allergen exposure can disrupt the integrity of the epithelial barrier by inducing apoptosis of bronchial epithelial cells (4). The maintenance of barrier integrity and function necessitates rapid and efficient clearance of these apoptotic cells as secondary necrosis leads to the release of additional IL-33 (5, 6). Interestingly, clusters of apoptotic bronchial epithelial cells known as “Creola bodies” are regularly seen in the sputum of patients with asthma, suggesting that the phagocytosis and clearance of apoptotic cells in the airways of these individuals is either impaired or insufficient (7–9). Apoptotic cells can be cleared by professional phagocytes, that is, macrophages, neutrophils, and dendritic cells, or by nonprofessional phagocytes, for example, fibroblasts and epithelial cells. In a publication from our laboratory, we investigated the role of bronchial epithelial cells in apoptotic cell clearance and the effect of impaired phagocytic activity in bronchial epithelial cells (10). As mammary epithelial cells were previously shown to play a key role in phagocytosis during the process of mammary involution postlactation, we hypothesized that bronchial epithelial cells could play a role in the clearance of their apoptotic neighbors (11). Furthermore, we postulated that disruption of the phagocytic activity of epithelial cells might contribute to or promote allergic airway inflammation.
The engulfment of apoptotic cells dampens inflammation locally as it drives the production of antiinflammatory cytokines (12). We hypothesized that bronchial epithelial cell engulfment of apoptotic cells might create an antiinflammatory milieu. We first observed that bronchial epithelial cells that engulf other apoptotic airway epithelial cells produce the antiinflammatory cytokines transforming growth factor-β and prostaglandin E2 (10). This finding supported our hypothesis that epithelial cells could likely engulf their apoptotic neighbors and that phagocytosis by bronchial epithelial cells might also help establish an antiinflammatory environment in the airways. Testing the importance of phagocytosis in an in vivo disease model is complicated by the existence of multiple and redundant engulfment pathways. Instead of targeting engulfment receptors or immediate downstream mediators we chose to target the small GTPase Rac1. Rac1 is involved in actin-dependent cytoskeletal rearrangement and functions downstream of multiple engulfment receptors (13). Therefore, to examine the importance of phagocytosis by bronchial epithelial cells in a model of allergic airway inflammation, we generated a mouse model of inducible and conditional deletion of Rac1 in airway epithelial cells. Our mouse model was generated by crossing Rac1fl/fl mice to mice with a Cre recombinase that is expressed in a tetracycline-inducible manner under the control of the promoter for club cell (Clara cell) secretory protein (denoted CCSP-Cre/Rac1fl/fl mice) (10, 14).
Disruption of the integrity of the epithelial barrier, such as disruption of apical junctional complexes, is associated with exacerbations of allergic airway inflammation (15, 16). We confirmed that a loss of Rac1 expression had no apparent effects on the integrity of the epithelial barrier at baseline: lung morphology, epithelial cell number, tight junction formation, alveolar–capillary membrane integrity, and uptake of antigen in CCSP-Cre/Rac1fl/fl mice were all comparable to control animals. We did, however, observe that deletion of Rac1 in airway epithelial cells caused a phagocytic defect in vitro and in vivo. Furthermore, CCSP-Cre/Rac1fl/fl mice had significantly lower levels of antiinflammatory cytokines in their bronchoalveolar lavage after intranasal instillation of apoptotic cells (10). Importantly, deletion of Rac1 in myeloid cells (including alveolar macrophages) did not affect the production of antiinflammatory cytokines after intranasal administration of apoptotic cells. These data further suggested that airway epithelial cells play a distinct role in the phagocytosis of apoptotic targets in the airways.
We then went on to evaluate our CCSP-Cre/Rac1fl/fl mice in a model of house dust mite (HDM)–induced allergic airway inflammation. HDM is a potent and pervasive allergen that is highly associated with asthma severity and morbidity (17, 18). We observed that CCSP-Cre/Rac1fl/fl mice developed more severe inflammation than littermate controls and had an inflammatory phenotype that mimicked several of the characteristics observed in allergic asthma (19, 20). Specifically, CCSP-Cre/Rac1fl/fl mice had increased eosinophilia, lymphocytic infiltration, and IgE levels (mice without Cre, mice given only buffer, or mice heterozygous for the Rac1 locus) (10). Further, the CCSP-Cre/Rac1fl/fl mice performed worse on pulmonary function tests and displayed increased hypersensitivity on methacholine challenge. In addition, bronchoalveolar lavage fluid of CCSP-Cre/Rac1fl/fl mice had higher levels of the classic helper T-cell type 2 cytokines IL-4, IL-5, and IL-13. Interestingly, CCSP-Cre/Rac1fl/fl mice also had increased levels of the cytokine IL-33.
IL-33 is released from epithelial cells on exposure to allergens and is crucial for the process of allergic sensitization (21, 22). IL-33 recruits type 2 innate lymphoid cells (ILC2s), which express the transcription factor GATA3 and produce IL-13 and IL-5, much like helper T type 2 cells (23, 24). However, these cells do not express a T-cell receptor and do not demonstrate any known antigen specificity. This cell type is rapidly emerging as a critical mediator of sensitization in the process of allergic airway inflammation (25). We noted that the severe inflammatory phenotype observed in our CCSP-Cre/Rac1fl/fl mice was associated with a dramatic increase in IL-33 levels in the airways and a concomitant increase in ILC2 recruitment. Given the previously reported importance of IL-33 and ILC2s in the pathogenesis of allergic airway inflammation, we then tested whether the inflammatory phenotype in our mice was IL-33 dependent. When CCSP-Cre/Rac1fl/fl mice were treated with an IL-33 neutralizing antibody during prime and challenge with HDM the inflammatory phenotype was attenuated. Of note, bronchial epithelial cell lines and primary cultures of human nasal epithelial cells produced high levels of IL-33 when exposed to HDM in the presence of a Rac1 inhibitor. These data suggest that IL-33 release is not simply the result of increased damage and necrosis within the airways but might be fundamentally regulated via a Rac1-dependent mechanism. In light of the major role that IL-33 plays in allergic airway inflammation and the early epithelial response to allergens, the specifics of this mechanism might warrant more investigation and study.
Although the inflammatory phenotype in our CCSP-Cre/Rac1fl/fl mice was associated with an increase in the levels of many inflammatory cytokines, these mice did not display a general increase in all cytokine levels. Notably, the level of the epithelial cell cytokine TSLP did not differ between CCSP-Cre/Rac1fl/fl mice and littermate controls (10). In addition, CCSP-Cre/Rac1fl/fl mice had lower levels of the antiinflammatory cytokine IL-10 when compared with littermate controls (10). This finding in our CCSP-Cre/Rac1fl/fl mice recapitulates an earlier observation in patients with asthma, who were found to have lower levels of IL-10 in their bronchoalveolar lavage fluid compared with healthy control subjects (20). IL-10 is crucial for controlling inflammatory responses at environmental interfaces (26). Interestingly, intranasal administration of exogenous IL-10 during prime and challenge with HDM ameliorated the inflammatory phenotype in our CCSP-Cre/Rac1fl/fl mice. Administration of IL-10 also decreased IL-33 levels, suggesting that this IL-10 was either directly or indirectly dampening inflammatory responses in epithelial cells and that these cytokines might be reciprocally regulated in the airways.
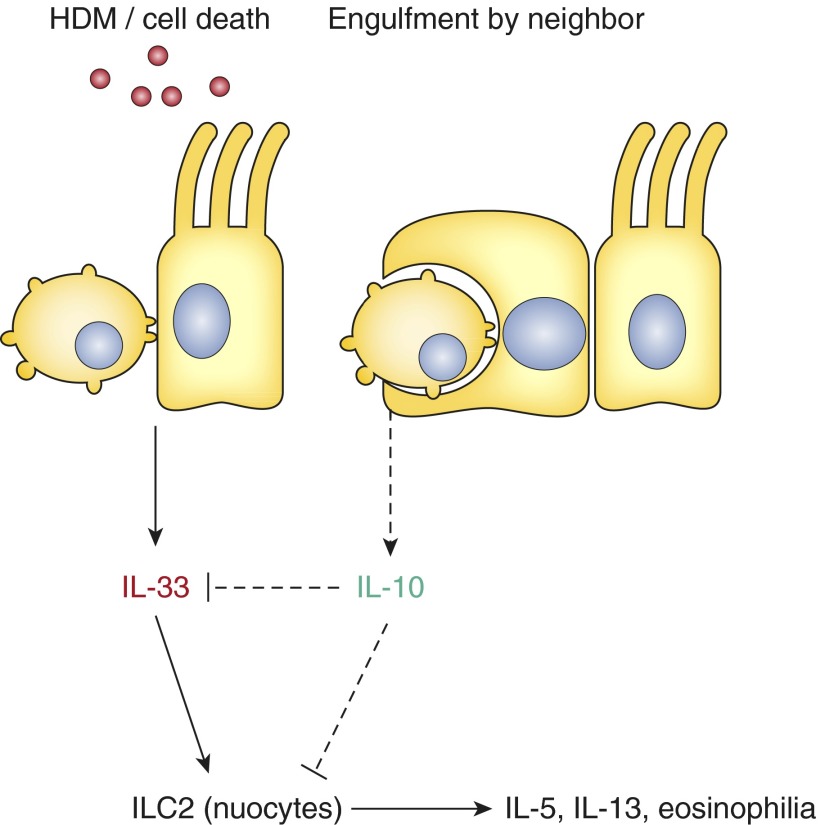
The epithelial barrier is being increasingly recognized as a major player in the pathogenesis of allergic airway inflammation. Epithelial cells respond to allergens by producing inflammatory cytokines and can undergo apoptotic cell death in response to allergen exposure. The early epithelial response to allergens drives the inflammatory cascade that underlies allergic airway inflammation. Our findings suggest that airway epithelial cells are not only capable of engulfing apoptotic cells but that apoptotic cell engulfment is an important mechanism by which airway epithelial cells regulate pulmonary immunity. Disruption of phagocytic activity in airway epithelial cells by deletion of the small GTPase Rac1 increases the severity of allergic airway inflammation in an IL-33–dependent manner. In turn, administration of exogenous IL-10 can abrogate the increase in IL-33 and ameliorate inflammation (Figure 1). Collectively, these data suggest that airway epithelial cells dampen the inflammatory response in the airways via a Rac1-dependent mechanism and that dysregulation of Rac1, and potentially other components of engulfment pathways, might contribute to allergic airway inflammation. In support of this hypothesis, Creola bodies are already known to be a positive predictor of future asthmatic disease in pediatric patients with both wheeze and respiratory syncytial virus (8, 9). It is possible that enhancing phagocytic activity or increasing the antiinflammatory function of engulfment pathways may represent an unexplored therapeutic strategy in the treatment of allergic airway inflammation.
Figure 1.
Proposed model for the role of epithelial cell death and engulfment in allergic airway inflammation. HDM = house dust mite allergen; ILC2 = type 2 innate lymphoid cells.
Acknowledgments
Acknowledgment
The authors thank J. Whitsett for the rtTA-CCSP/Cre mice, X. Liu for the TGF-β–responsive cell line PE25, and J. Steinke and J. Kennedy for providing human nasal epithelial cells.
Footnotes
Supported by an Immunology Training Grant (I.J.J. and K.K.P.), an F32 postdoctoral fellowship from the NHLBI (I.J.J.), and grants from the American Asthma Foundation and the NIGMS/National Institutes of Health (GM064709 and GM107848 to K.S.R.). K.S.R. has been a William Benter Senior Fellow of the American Asthma Foundation.
Author Contributions: K.K.P. and K.S.R. wrote the report. I.J.J. designed, performed, and analyzed most of the experiments in this study, with input from K.S.R.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willart MAM, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BRP, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, et al. Endoplasmic reticulum stress mediates house dust mite–induced airway epithelial apoptosis and fibrosis. Respir Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cayrol C, Girard J-P. The IL-1–like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Naylor B. The shedding of the mucosa of the bronchial tree in asthma. Thorax. 1962;17:69–72. doi: 10.1136/thx.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada Y, Yoshihara S, Arisaka O. Creola bodies in wheezing infants predict the development of asthma. Pediatr Allergy Immunol. 2004;15:159–162. doi: 10.1111/j.1399-3038.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Yoshihara S. Creola bodies in infancy with respiratory syncytial virus bronchiolitis predict the development of asthma. Allergol Int. 2010;59:375–380. doi: 10.2332/allergolint.09-OA-0165. [DOI] [PubMed] [Google Scholar]
- 10.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, Ravichandran KS. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12:107–114. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 12.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumienny TL, Brugnera E, Tosello-Trampont A-C, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 14.Perl A-KT, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 15.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556.e12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Saatian B, Rezaee F, Desando S, Emo J, Chapman T, Knowlden S, Georas SN. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1:e24333. doi: 10.4161/tisb.24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9:128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, Serralha M, Holt BJ, Hollams E, Yerkovich S, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–149. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 20.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 21.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–188, e1–e8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halim TYF, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227.e1–6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell–derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]