Abstract
The airway epithelium is the primary site of the earliest pathologic changes induced by smoking, contributing to the development of chronic obstructive pulmonary disease (COPD). The normal human airway epithelium is composed of several major cell types, including differentiated ciliated and secretory cells, intermediate undifferentiated cells, and basal cells (BC). BC contain the stem/progenitor cell population responsible for maintenance of the normally differentiated airway epithelium. Although inflammatory and immune processes play a significant role in the pathogenesis of COPD, the earliest lesions include hyperplasia of the BC population, suggesting that the disease may start with this cell type. Apart from BC hyperplasia, smoking induces a number of COPD-relevant airway epithelial remodeling phenotypes that are likely initiated in the BC population, including mucous cell hyperplasia, squamous cell metaplasia, epithelial–mesenchymal transition, altered ciliated and nonmucous secretory cell differentiation, and suppression of junctional barrier integrity. Significant progress has been recently made in understanding the biology of human airway BC, including gene expression features, stem/progenitor, and other functions, including interaction with other airway cell types. Accumulating evidence suggests that human airway BC function as both sensors and cellular sources of various cytokines and growth factors relevant to smoking-associated airway injury, as well as the origin of various molecular and histological phenotypes relevant to the pathogenesis of COPD. In the context of these considerations, we suggest that early BC-specific smoking-induced molecular changes are critical to the pathogenesis of COPD, and these represent a candidate target for novel therapeutic approaches to prevent COPD progression in susceptible individuals.
Keywords: adult stem cells, epithelium, lung, gene expression, phenotype
Summary
-
1.
Airway basal cells (BC), the stem/progenitor cells of the airway epithelium, play a key role in the maintenance of the normal airway epithelial architecture through their capacity to self-renew, differentiate into ciliated and secretory cells, and establish interactions with mesenchymal cells.
-
2.
Based on recent advances in isolating and characterizing human airway BC, accumulating evidence suggests that smoking-mediated reprogramming of airway BC phenotype and function represents an early event in the pathogenesis of chronic obstructive pulmonary disease (COPD)-relevant airway remodeling.
-
3.
Targeting specific biologic pathways that mediate smoking-induced reprogramming of airway BC phenotype and function may represent a novel therapeutic approach to prevent development and progression of COPD in susceptible individuals.
The earliest smoking-induced pathologic changes relevant to the pathogenesis of COPD are observed in the airway epithelium, an essential tissue barrier that protects lung from the exposure to harmful environmental factors, including up to 4,000 toxic compounds present in the tobacco smoke (1, 2). The normal human airway epithelium is composed of various cell populations, including ciliated and secretory cells, which contribute to the mucociliary clearance system at the luminal airway surface, intermediate undifferentiated cells, and BC (Figure 1) (3). Airway BC reside in the basal epithelial layer, immediately above the basement membrane that separates airway epithelium from the underlying mesenchyme. Extensive evidence exists that airway BC contain the stem/progenitor cell population critical for the maintenance of normally differentiated airway epithelium through their unique capacity to self-renew and generate the differentiated ciliated and secretory cell populations (4–9).
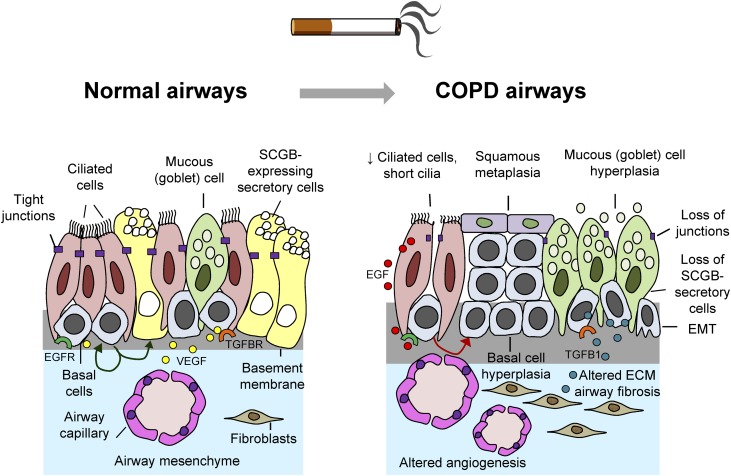
Figure 1.
Smoking-induced airway epithelial remodeling in chronic obstructive pulmonary disease (COPD). (Left panel) Normally differentiated airway epithelium is composed of differentiated cells, including ciliated, mucus-producing, and secretoglobin (SCGB1A1)-producing nonmucous secretory cells; intermediate undifferentiated cells (not shown); and basal cells (BC). BC contain stem/progenitor cell populations that can self-renew and differentiate into ciliated and secretory cells (green arrows). In addition, BC express receptors, such as epidermal growth factor (EGF) receptor (EGFR; green) and transforming growth factor β1 (TGF-β1) receptor (TGFBR; orange) to sense extracellular signals and produce growth factors such as vascular endothelial growth factor A (VEGF; yellow dots) necessary for interaction with underlying mesenchymal cells. Tight junctions between adjacent epithelial cells keep the airway barrier impermeable to inhaled xenobiotics, microbes, and other toxic molecules. (Right panel) Cigarette smoking induces COPD-relevant airway remodeling phenotypes, including BC hyperplasia, squamous metaplasia, shortening of cilia, loss of ciliated and SCGB1A1-expressing nonmucous secretory cells, mucous cell hyperplasia, loss of the junctional barrier, acquisition of epithelial–mesenchymal transition (EMT)-like features, airway fibrosis, and altered angiogenesis. Many, if not all, of these COPD-relevant abnormalities are induced as a result of reprogramming of airway BC phenotype and function through various smoking-dependent mechanisms, including activation of EGFR by smoking-induced ciliated cell–derived EGF (red dots) and up-regulation of TGFB1 (blue dots) that alters airway BC differentiation and epithelial–mesenchymal (ECM) interactions.
The cellular composition of the human airway epithelium is heterogenous along the tracheobronchial tree, with the ciliated cell being the major cell population (60–80%) throughout the airways except for the smallest bronchioli, where secretoglobin (SCGB1A1)-producing nonmucous secretory cells dominate (3, 6, 10). By contrast, mucus-producing goblet cells are present in the normal human lungs almost exclusively in the large airways (zero to fifth generation of bronchi) (6, 10). BC are present throughout the human airways (i.e., from trachea to the terminal bronchioles), decreasing in frequency along the proximal–distal axis, contributing to ∼15 to 30% and 5 to 10% cells of the large and small airway epithelia, respectively (6, 8). Existence of BC in the human small airway epithelium (SAE), a feature not shared by the mouse lung (6, 8, 11), suggests the possibility that this stem/progenitor cell–containing population of cells may have a unique role in the maintenance of the human small airways, a region where the earliest changes relevant to the pathogenesis of COPD are observed (1, 2, 12–14). In this article, recent advances in understanding the biology of human airway BC and the role of this cell population in the pathogenesis of smoking-induced COPD-relevant airway epithelial remodeling are discussed. Some of the results of these studies have been previously reported in the form of abstracts (15–17).
Airway Epithelium Remodeling in COPD
Although pathologic changes in the lungs of patients with COPD involve both airways and alveoli, collectively contributing to progressive irreversible airway obstruction, which is the hallmark of this disease (14), comprehensive morphologic studies by the Hogg laboratory (12, 13) provide compelling evidence that alterations in the small airways precede and likely lead to the emphysematous destruction of the alveolar structure. Morphologic abnormalities in the small airways of individuals with COPD are variable, ranging from the remodeling of the airway wall and airway epithelium, and infiltration of airways with inflammatory and immune cells, to physical loss of small bronchioles (12–14), indicative of a broad dysregulation of regulatory mechanisms responsible for the maintenance of the small airway architecture. The airway epithelium represents the primary site of dramatic molecular and histologic changes in COPD (14). The most typical airway epithelial remodeling phenotypes in the lungs of smokers with COPD include BC hyperplasia, mucous cell hyperplasia, squamous metaplasia, epithelial–mesenchymal transition (EMT), alterations of cilia, loss of SCGB1A1-producing secretory cells, and decreased integrity of the apical junctional barrier that controls airway epithelial permeability (Figure 1) (14, 18–24). These phenotypes collectively contribute to airway obstruction as well as decreased barrier and host defense function of the airway epithelium (14, 25–27).
Notably, COPD-relevant gene expression changes and histologic alterations are present in both large and small airway epithelia of a subset of clinically asymptomatic smokers with normal chest imaging and lung function tests (1, 19, 21, 23, 24, 28–30). These observations suggest that smoking-induced changes in the airway epithelium occur early during the pathogenesis of COPD and before decline in lung functon. Surprisingly, however, in contrast to the well-established role of inflammation in progression of already-established COPD (14, 25, 26, 31, 32), genome-wide studies revealed that the overall expression of genes related to inflammation and immunity are down-regulated in both the large and small airway epithelia of smokers (19, 27, 28, 33). These observations suggest that the early events in the pathogenesis of COPD in smokers may involve noninflammatory mechanisms. Indeed, a number of gene expression patterns relevant to regulation of epithelial homeostasis, including Notch and Wnt signaling pathways, as well as transcriptional programs that control the maintenance of the essential structural features of the normally differentiated airway epithelium, such as cilia and apical junctional complex, are broadly suppressed in the airway epithelium of healthy smokers and more so in smokers with COPD (19, 21, 23, 34, 35). Given that BC are stem/progenitor cells responsible for the maintenance of the normally differentiated airway epithelium (4–7), it is possible that the earliest COPD-relevant changes in the airway epithelium occur in the airway BC population and that abnormal phenotypes observed in the airways of patients with COPD result from smoking-induced reprogramming of the stem/progenitor and other functions of airway BC.
Human Airway BC Transcriptome
Although histological alterations in the airway epithelium of smokers with COPD involving the features of abnormal proliferation and differentiation of airway BC have been known since the 1950s (1), the specific molecular changes induced by smoking in human airway BC and contribution of these changes to the biologic phenotype of the airway epithelium in COPD have not been comprehensively analyzed until recently. With the development of a culture-based strategy to isolate pure BC population from human airway epithelium obtained by bronchoscopic brushings, the human airway BC signature, composed of 1,161 unique genes with greater than fivefold higher expression level compared with differentiated epithelium, was identified (36). Some of the identified human airway BC markers were well-known BC genes, such as intermediate filament constituents keratins KRT5 and KRT17, essential for the cytoskeleton assembly; integrins ITGA6, ITGB1, and ITGB4, which mediate interaction with the extracellular matrix (ECM) components of the basement membrane; and transcription factors TP63 and basonuclin, critical for the maintenance and renewal of the BC population. Notably, many similar gene expression features have been described for mouse tracheal BC isolated using the fluorescence-activated cell sorter–based (i.e., culture-independent) method, with more than 100 genes belonging to both mouse and human airway BC signatures (5). This suggests that, although culturing BC may change their gene expression phenotype, the essential molecular features associated with airway BC identity are preserved in human airway BC isolated using this method. In addition to known BC-related genes, the human airway BC transcriptome was remarkably enriched in molecular features and pathways relevant to maintenance of tissue homeostasis. Notably, more than 20% of genes belonging to the epidermal growth factor receptor (EGFR) signaling pathway, including EGFR and its ligands transforming growth factor (TGF)-α, heparin-binding EGF, and amphiregulin, were highly expressed in airway BC. Other enriched pathways were those related to the TGF-β, nuclear factor (NF)-κB, mitogen-activated protein kinases, Notch, and vascular endothelial growth factor (VEGF) signaling. Thus, human airway BC have a potential to sense and produce signals important for regulation of both BC homeostasis and interaction with other airway cell populations relevant to the pathogenesis of smoking-induced COPD-relevant airway lesions.
Smoking-induced COPD-Relevant Changes in Airway BC Transcriptome
An important step forward in understanding the global molecular changes in airway BC induced by smoking relevant to COPD pathogenesis has recently been made by comparing the transcriptomes of BC from the airway epithelium of healthy smokers and nonsmokers using ultrasensitive and highly specific massively parallel RNA-sequencing technology (37). Out of the 13,385 annotated genes expressed in airway BC, 676 were differentially expressed between the two groups, with 662 (∼98%) being significantly up-regulated in airway BC of smokers. Among the top 50 smoking-dysregulated genes were those related to oxidative stress and central components of the signaling pathways previously shown to be enriched in the normal airway BC transcriptome (36), including EGFR, NF-κB, VEGF, and TGF-β.
Strikingly, 25% of 676 differentially expressed genes were localized to chromosome 19, with 13 of 676 (2%) of these genes on locus 19q13.2 (37), a region where a number of genome-wide association studies and candidate gene studies have identified single-nucleotide polymorphisms associated with a risk for COPD in relation to smoking (38–40). Among the “COPD risk genes” up-regulated in airway BC of smokers were TGFB1, LTBP4, EGLN2, and NFKBIB, each potentially relevant to the pathogenesis of COPD. TGFB1 encodes TGF-β, a growth factor increased in the lungs of smokers with COPD in association with airway obstruction (41) and contributing to airway remodeling by promoting squamous metaplasia and airway fibrosis (18, 42). LTBP4, the latent TGFB1 binding protein 4 that binds TGF-β1 and targets it to the ECM, plays a role in maintaining of the distal lung architecture, as LTBP4-null mice develop emphysema (43). EGLN2 (Egl nine homolog 2) encodes a prolyl hydroxylase, which functions as a cellular oxygen sensor that, in response to hypoxia, targets the hypoxia inducible factor 1 α for degradation, a process implicated in the pathogenesis of emphysema (44, 45). Altered EGLN2 expression is associated with increased epithelial cell proliferation and impaired epithelial junctional barrier function (46, 47), which are characteristic features of the airway epithelium of healthy smokers and smokers with COPD. Finally, NFKBIB, which encodes NF-κB inhibitor β, has recently been linked to cigarette smoke–induced oxidative stress response mediated via nuclear factor erythroid 2–related factor (NRF2) relevant to the pathogenesis of smoking-induced COPD (48).
Intriguingly, in airway BC of nonsmokers, smoking-dysregulated genes localized to chromosomal band 19q13.2 were expressed at relatively low levels but in a highly coordinate manner, whereas in BC of smokers, coexpression of these genes was markedly lost, accompanied by their up-regulation (37). This suggests that some central mechanism may exist in airway BC that provides coordinate transcriptional regulation of these genes to ensure their physiological relatively low expression levels adequate to the normal airway homeostasis, and disturbance of this mechanism by smoking may lead to a disordered, noncoordinated up-regulation of these genes. If this model is correct, identification and restoration of the central smoking-responsive mechanism that controls expression of multiple COPD-relevant genes, rather than targeting individual genes, might represent a productive new approach to normalize the molecular phenotype airway BC in smokers. Finally, differential expression of these genes was not detectable when the transcriptomes of the complete airway epithelium samples from healthy smokers and nonsmokers, containing all airway epithelial cell types, were compared (37), suggesting that these early COPD-relevant smoking-induced molecular changes are specific to the BC population.
Airway BC as a Cellular Origin of COPD-Relevant Airway Phenotypes
Extensive evidence has been provided that airway BC contain the stem/progenitor cell population capable of self-renewal and generating differentiated cell types of the airway epithelium (i.e., ciliated and secretory cells), including both mucus-producing and SCGB1A1-producing nonmucous secretory cells (4–7, 36, 49). In addition, airway BC contribute to signal exchange between the airway epithelium and underlying mesenchyme, as they can both sense and produce cytokines and growth factors relevant to epithelial–mesenchymal interaction, which is disturbed in COPD (18, 50, 51). In humans, these BC functions can be studied using various in vitro models, including the air–liquid interface culture, which mimics in vivo airway epithelial organization and differentiation. In this model, BC are seeded onto semipermeable Transwell membranes, and, after the cells form a continuous layer, the growth factor–containing media or mesenchymal cells are added only to the basolateral compartment to mimic physiological BC–mesenchymal interactions, while the apical cell surface is exposed to air (7, 36, 52). This model has been helpful in understanding the specific contribution of airway BC to the pathogenesis of the earliest smoking-associated COPD-relevant disordering of the human airway epithelium. Some of these advances are summarized below.
One unique feature of the normally differentiated airway epithelium is its polarized organization, with apical and basolateral domains separated by the apical junctional complex composed of tight and adherens junctions between adjacent cells that segregate so-called “lateral intercellular space,” where BC equipped with a broad set of receptors (19, 36, 53) establish contacts with other cells and sense their secreted products (4). Virtually all these features are disordered in the airway epithelium of healthy smokers and more so smokers with COPD, with the loss of the apical, ciliated, and nonmucous secretory cell compartment, accompanied by expansion of BC and a broad suppression of junctional barrier integrity. Consistent with extensive evidence that links altered EGFR signaling by cigarette smoking to COPD-relevant airway phenotypes (54), genome-wide studies revealed that EGF, the major EGFR ligand, is significantly up-regulated in differentiated airway epithelial cells of smokers, whereas EGFR expression is normally enriched in airway BC (49). Further analysis has demonstrated that smoking induces EGF in ciliated cells in association with oxidative stress–related genome-wide changes, and increased amount of EGF, when added to BC in the air–liquid interface model, shifted BC phenotype toward an abnormal, squamous EMT-like phenotype with decreased differentiation to ciliated and SCGB1A1-producing nonmucous secretory cells and markedly suppressed junctional barrier integrity and function (49). Notably, the phenotype of the airway epithelium derived from EGF-treated airway BC was remarkably similar to that present in the airway epithelium of healthy smokers and smokers with COPD in vivo. Defective apical junctional barrier structure due to EGF-mediated reprogramming of airway BC function may facilitate further access of the luminal content, including EGF and cigarette smoke components, to the BC compartment leading to progressive self-amplifying disordering of airway epithelial differentiation and function. Indeed, nicotine, the major constituent of tobacco smoke, and nicotine-associated signaling have been shown to alter airway BC proliferation and differentiation (55–57), and exposure of differentiating human airway BC to cigarette smoke extract results in airway epithelium with squamous metaplasia and shortened cilia (15).
Interestingly, EGF, through its action on BC, induces expression of other smoking-associated genes, including the chemokine CXCL14, which is enriched within hyperplastic and squamous metaplastic lesions in the airways of smokers (58). Intriguingly, healthy smokers with high expression of CXCL14 in the SAE are indistinguishable from smokers with COPD in terms of global gene SAE expression (58). This suggests that up-regulation of CXCL14 in the airway epithelium resulting from smoking-induced EGF-mediated modification of airway BC may potentially mark a subset of clinically “healthy” smokers who, at the biologic level, have already developed a COPD-like phenotype. This also implies that a single mechanism, such as that mediated by altered EGFR signaling in airway BC, may potentially contribute to a broad set of histologic and molecular changes in the entire airway epithelium so that the latter acquires the whole diversity of disease-associated features (Table 1). In support of the latter concept, amphiregulin, another smoking-inducible EGFR ligand (59, 60), can also modify airway BC function by suppressing junctional barrier integrity and ciliated cell differentiation and by promoting BC and mucous cell hyperplasia (17). Furthermore, the Notch pathway, which cooperates with EGFR signaling and mediates generation of SCGB1A1-producing secretory cells (16, 61–63), is enriched in the normal airway BC transcriptome (36) and is broadly suppressed in the airway epithelium of smokers. The latter observation is potentially relevant to the loss of SCGB1A1-producing secretory cells in the small airways of smokers (35).
Table 1.
Chronic obstructive pulmonary disease–relevant abnormal airway basal cell distribution and differentiation phenotypes induced by cigarette smoking
| Phenotype | Description | Mediators | References |
|---|---|---|---|
| Squamous metaplasia | Appearance of squamous cells that replace ciliated cells in the luminal compartment | EGFR signaling (EGF), TGF-β, cigarette smoke (CSE) | (1, 15, 18, 20, 24, 42, 49) |
| BC hyperplasia | ↑ Number of BC that form more than 1 layer above the BM | EGFR signaling (amphiregulin), cigarette smoke (nicotine) | (1, 17, 55–57, 60, 66) |
| Mucous cell hyperplasia | ↑ Number of mucus-producing (mucin MUC5AC-expressing) cells; overproduction of mucus | EGFR signaling (amphiregulin) | (2, 10, 13, 17, 54) |
| Altered ciliated cell differentiation | ↓ Number of ciliated cells, shortened cilia | EGFR signaling (EGF, amphiregulin), cigarette smoke (CSE) | (15, 17, 21, 23, 49) |
| Loss of SCGB1A1- producing cells | ↓ Number of SCGB1A1-producing nonmucous secretory cells; ↓ SCGB1A1 protein secretion | EGFR signaling (EGF), decreased Notch signaling | (9, 16, 35, 49, 62) |
| Suppressed barrier function and increased epithelial permeability | ↓ Structural integrity of the apical junctional barrier, ↓ transepithelial electrical resistance | EGFR signaling (EGF, amphiregulin) | (17, 19, 22, 49) |
| EMT-like phenotype and airway fibrosis | Acquisition of the mesenchymal gene and protein expression (vimentin, N-cadherin), increased expression of EMT transcription factors (SNAI2/SLUG), loss of epithelium-specific features (E-cadherin, tight junctions), BM and ECM remodeling | EGFR signaling (EGF), TGF-β | (18–20, 49, 65) |
Definition of abbreviations: BC = basal cell; BM = basement membrane; CSE = cigarette smoke extract; ECM = extracellular matrix; EGF = epidermal growth factor; EGFR = EGF receptor; EMT = epithelial–mesenchymal transition; SCGB1A1 = secretoglobin 1A1; SNAI2/SLUG = Snail homolog 2; TGF-β = transforming growth factor-β.
Another function of airway BC that seems to be altered in smokers is interaction with the ECM of the basement membrane and with underlying mesenchymal cells relevant to maintenance of the normal airway wall architecture (18, 50). A number of studies imply TGF-β as a key mediator that mediates EMT and airway fibrosis relevant to airway remodeling in COPD (18, 20, 41). As mentioned above, components of the TGF-β signaling pathway are enriched in the human airway BC transcriptome and further up-regulated in airway BC of smokers, including TGF-β and LTBP4, which constitute TGF-β latency complex within the ECM and both represent “COPD risk” genes (36, 37). Relevent to this concept, stimulation of human airway BC with TGF-β leads to squamous metaplasia and EMT-like phenotype relevant to airway fibrosis (18, 20). Furthermore, high expression of vascular endothelial growth factor A (VEGFA) by airway BC may be relevant to vascular remodeling frequently present in the airways of patients with COPD (36, 51, 64–68). In support of this model, human airway BC activate endothelial cells via production of VEGFA, and endothelial cells activated through this mechanism, in turn, promote survival of airway BC (51). Consistent with this observation, the airway capillary endothelium is situated in close proximity to airway BC below the basement membrane, and smoking-induced COPD-relevant histologic lesions with altered numbers of airway BC are accompanied by abnormal distribution of blood vessels in the airway walls (64–68).
Conclusions and Therapeutic Perspectives
There is increasing evidence that airway BC represent a common origin of the earliest smoking-induced molecular changes that alter stem/progenitor and other functions of these cells, resulting in generation of COPD-relevant airway epithelium remodeling phenotypes. Although manifestations of smoking-disordered features in the airways of patients with COPD are complex and diverse, recent evidence summarized above suggests that the molecular and cellular origins of these manifestations might be remarkably common in nature and involve dysregulation of the central signaling pathways in a single cell population (i.e., airway BC). One mechanism whereby smoking may broadly alter airway BC biology could be reprogramming of these cells toward a more primitive, embryonic stem cell–like, state with increased proliferation, plasticity, and remarkably diverse yet highly controlled differentiation potential. In support of this concept, genome-wide analysis revealed selective up-regulation of ∼30% of the human embryonic stem cell signature genes in airway BC of smokers (69). Taken together, novel approaches aiming at normalization of the airway BC phenotype and function through targeting central smoking-dysregulated signaling pathways in this cell population should be considered as potential therapeutic strategies to prevent the pathogenesis of smoking-induced, COPD-relevant airway remodeling at the earliest stages of disease development.
Acknowledgments
Acknowledgments
The authors thank N. Mohamed and D. N. McCarthy for help in preparing this manuscript.
Footnotes
Supported, in part, by National Institutes of Health grants 1R01HL107882 and P50 HL084936 (R.G.C.) and by The Parker B. Francis Foundation (R.S.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961;265:253–267. doi: 10.1056/NEJM196108102650601. [DOI] [PubMed] [Google Scholar]
- 2.Dye JA, Adler KB. Effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax. 1994;49:825–834. doi: 10.1136/thx.49.8.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- 5.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- 8.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 9.Shaykhiev R, Crystal RG. Basal cell origins of smoking-induced airway epithelial disorders. Cell Cycle. 2014;13:341–342. doi: 10.4161/cc.27510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumsden AB, McLean A, Lamb D. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax. 1984;39:844–849. doi: 10.1136/thx.39.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 14.Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84:89–97. doi: 10.1159/000341382. [DOI] [PubMed] [Google Scholar]
- 15.Brekman A, Walters MS, Crystal RG.FOXJ1 overexpression prevents cigarette smoke mediated inhibition of cilia growth in human airway epithelium [abstract] Am J Respir Crit Care Med 2014;189:A2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomi K, Walters MS, Wang R, Salit J, Crysal RG.Notch dependent regulation of human airway basal cell differentiation [abstract] Am J Respir Crit Care Med 2012185:A5523 [Google Scholar]
- 17.Shaykhiev RS, Zuo W-L, Fukui T, Chao I, O'Beirne SL, Crystal RG, Airway-Resident T.Cell-derived amphiregulin regulates human airway basal stem/progenitor cell function [abstract] Am J Respir Crit Care Med 2013187:A3817 [Google Scholar]
- 18.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, Salit J, Harvey BG, Crystal RG. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milara J, Peiró T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 21.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS ONE. 2009;4:e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy SM, Elwood RK, Wiggs BJ, Paré PD, Hogg JC. Increased airway mucosal permeability of smokers: relationship to airway reactivity. Am Rev Respir Dis. 1984;129:143–148. doi: 10.1164/arrd.1984.129.1.143. [DOI] [PubMed] [Google Scholar]
- 23.Hessel J, Heldrich J, Fuller J, Staudt MR, Radisch S, Hollmann C, Harvey BG, Kaner RJ, Salit J, Yee-Levin J, et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS ONE. 2014;9:e85453. doi: 10.1371/journal.pone.0085453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters EJ, Morice R, Benner SE, Lippman S, Lukeman J, Lee JS, Ro JY, Hong WK. Squamous metaplasia of the bronchial mucosa and its relationship to smoking. Chest. 1993;103:1429–1432. doi: 10.1378/chest.103.5.1429. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 27.Shaykhiev R, Crystal RG. Innate immunity and chronic obstructive pulmonary disease: a mini-review. Gerontology. 2013;59:481–489. doi: 10.1159/000354173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilley AE, O’Connor TP, Hackett NR, Strulovici-Barel Y, Salit J, Amoroso N, Zhou XK, Raman T, Omberg L, Clark A, et al. Biologic phenotyping of the human small airway epithelial response to cigarette smoking. PLoS ONE. 2011;6:e22798. doi: 10.1371/journal.pone.0022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiling K, van den Berge M, Hijazi K, Florido R, Campbell J, Liu G, Xiao J, Zhang X, Duclos G, Drizik E, et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med. 2013;187:933–942. doi: 10.1164/rccm.201208-1449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey BG, Heguy A, Leopold PL, Carolan BJ, Ferris B, Crystal RG. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med (Berl) 2007;85:39–53. doi: 10.1007/s00109-006-0103-z. [DOI] [PubMed] [Google Scholar]
- 31.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med. 2012;125:1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Salit J, Mezey JG, Crystal RG. Airway epithelial expression of TLR5 is downregulated in healthy smokers and smokers with chronic obstructive pulmonary disease. J Immunol. 2012;189:2217–2225. doi: 10.4049/jimmunol.1101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Hackett NR, Crystal RG. Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS ONE. 2011;6:e14793. doi: 10.1371/journal.pone.0014793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O’Connor TP, Crystal RG. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:457–466. doi: 10.1164/rccm.200705-795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS ONE. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan DM, Vincent TL, Salit J, Walters MS, Agosto-Perez F, Shaykhiev R, Strulovici-Barel Y, Downey RJ, Buro-Auriemma LJ, Staudt MR, et al. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS ONE. 2014;9:e88051. doi: 10.1371/journal.pone.0088051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakke PS, Zhu G, Gulsvik A, Kong X, Agusti AG, Calverley PM, Donner CF, Levy RD, Make BJ, Paré PD, et al. Candidate genes for COPD in two large data sets. Eur Respir J. 2011;37:255–263. doi: 10.1183/09031936.00091709. [DOI] [PubMed] [Google Scholar]
- 39.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, et al. ICGN Investigators; ECLIPSE Investigators; COPDGene Investigators. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet. 2012;21:947–957. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castaldi PJ, Cho MH, Cohn M, Langerman F, Moran S, Tarragona N, Moukhachen H, Venugopal R, Hasimja D, Kao E, et al. The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Hum Mol Genet. 2010;19:526–534. doi: 10.1093/hmg/ddp519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1951–1957. doi: 10.1164/ajrccm.158.6.9803053. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe T, Kanoh S, Moskowitz WB, Rubin BK. Cardiac asthma: transforming growth factor-β from the failing heart leads to squamous metaplasia in human airway cells and in the murine lung. Chest. 2012;142:1274–1283. doi: 10.1378/chest.11-1710. [DOI] [PubMed] [Google Scholar]
- 43.Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 45.Yasuo M, Mizuno S, Kraskauskas D, Bogaard HJ, Natarajan R, Cool CD, Zamora M, Voelkel NF. Hypoxia inducible factor-1α in human emphysema lung tissue. Eur Respir J. 2011;37:775–783. doi: 10.1183/09031936.00022910. [DOI] [PubMed] [Google Scholar]
- 46.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Gu J, Li L, Liu J, Luo B, Cheung HW, Boehm JS, Ni M, Geisen C, Root DE, et al. Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer Cell. 2009;16:413–424. doi: 10.1016/j.ccr.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLOS Comput Biol. 2008;4:e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, Crystal RG. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behzad AR, McDonough JE, Seyednejad N, Hogg JC, Walker DC. The disruption of the epithelial mesenchymal trophic unit in COPD. COPD. 2009;6:421–431. doi: 10.3109/15412550903341471. [DOI] [PubMed] [Google Scholar]
- 51.Curradi G, Walters MS, Ding BS, Rafii S, Hackett NR, Crystal RG. Airway basal cell vascular endothelial growth factor-mediated cross-talk regulates endothelial cell-dependent growth support of human airway basal cells. Cell Mol Life Sci. 2012;69:2217–2231. doi: 10.1007/s00018-012-0922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. 2011;44:465–473. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 54.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho YS, Chen CH, Wang YJ, Pestell RG, Albanese C, Chen RJ, Chang MC, Jeng JH, Lin SY, Liang YC, et al. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol Appl Pharmacol. 2005;205:133–148. doi: 10.1016/j.taap.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat Res. 2008;659:221–231. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Maouche K, Polette M, Jolly T, Medjber K, Cloëz-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, et al. alpha7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol. 2009;175:1868–1882. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaykhiev R, Sackrowitz R, Fukui T, Zuo WL, Chao IW, Strulovici-Barel Y, Downey RJ, Crystal RG. Smoking-induced CXCL14 expression in the human airway epithelium links chronic obstructive pulmonary disease to lung cancer. Am J Respir Cell Mol Biol. 2013;49:418–425. doi: 10.1165/rcmb.2012-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter A, O’Donnell RA, Powell RM, Sanders MW, Holgate ST, Djukanović R, Davies DE. Autocrine ligands for the epidermal growth factor receptor mediate interleukin-8 release from bronchial epithelial cells in response to cigarette smoke. Am J Respir Cell Mol Biol. 2002;27:85–90. doi: 10.1165/ajrcmb.27.1.4789. [DOI] [PubMed] [Google Scholar]
- 60.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 61.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang JH, Lee EH, Park SW, Chung IY. MUC5AC expression through bidirectional communication of Notch and epidermal growth factor receptor pathways. J Immunol. 2011;187:222–229. doi: 10.4049/jimmunol.1003606. [DOI] [PubMed] [Google Scholar]
- 64.Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res. 2010;11:132. doi: 10.1186/1465-9921-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soltani A, Reid DW, Sohal SS, Wood-Baker R, Weston S, Muller HK, Walters EH. Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: a cross-sectional study. Respir Res. 2010;11:105. doi: 10.1186/1465-9921-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiroshima K, Iyoda A, Shibuya K, Hoshino H, Haga Y, Toyozaki T, Shiba M, Baba M, Fujisawa T, Ohwada H. Evidence of neoangiogenesis and an increase in the number of proliferating cells within the bronchial epithelium of smokers. Cancer. 2002;95:1539–1545. doi: 10.1002/cncr.10850. [DOI] [PubMed] [Google Scholar]
- 67.Kristan SS, Marc MM, Kern I, Flezar M, Suskovic S, Kosnik M, Korosec P. Airway angiogenesis in stable and exacerbated chronic obstructive pulmonary disease. Scand J Immunol. 2012;75:109–114. doi: 10.1111/j.1365-3083.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 68.Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax. 2009;64:444–450. doi: 10.1136/thx.2008.100032. [DOI] [PubMed] [Google Scholar]
- 69.Shaykhiev R, Wang R, Zwick RK, Hackett NR, Leung R, Moore MA, Sima CS, Chao IW, Downey RJ, Strulovici-Barel Y, et al. Airway basal cells of healthy smokers express an embryonic stem cell signature relevant to lung cancer. Stem Cells. 2013;31:1992–2002. doi: 10.1002/stem.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]