Abstract
Rationale: Oxygen consumption may be impaired in critically ill patients.
Objectives: To evaluate the effect of intravenous thiamine on oxygen consumption (o2) in critically ill patients.
Methods: This was a small, exploratory open-label pilot study conducted in the intensive care units at a tertiary care medical center. Critically ill adults requiring mechanical ventilation were screened for enrollment. Oxygen consumption (o2) and cardiac index (CI) were recorded continuously for 9 hours. After 3 hours of baseline data collection, 200 mg of intravenous thiamine was administered. The outcome was change in o2 after thiamine administration.
Measurements and Main Results: Twenty patients were enrolled and 3 were excluded because of incomplete o2 data, leaving 17 patients for analysis. There was a trend toward increase in o2 after thiamine administration (16.3 ml/min, SE 8.5; P = 0.052). After preplanned adjustment for changes in CI in case of a delivery-dependent state in some patients (with exclusion of one additional patient because of missing CI data), this became statistically significant (16.9 ml/min, SE 8.6; P = 0.047). In patients with average CI greater than our cohort’s mean value of 3 L/min/m2, o2 increased by 70.9 ml/min (±16; P < 0.0001) after thiamine. Thiamine had no effect in patients with reduced CI (< 2.4 L/min/m2). There was no association between initial thiamine level and change in o2 after thiamine administration.
Conclusions: The administration of a single dose of thiamine was associated with a trend toward increase in o2 in critically ill patients. There was a significant increase in o2 in those patients with preserved or elevated CI. Further study is needed to better characterize the role of thiamine in oxygen extraction.
Clinical trial registered with www.clinicaltrials.gov (NCT01462279).
Keywords: oxygen consumption, o2, thiamine, o2, oxygen extraction
Oxygen consumption (o2) is determined by oxygen delivery (o2), a product of cardiac output and the oxygen content in the blood, and oxygen extraction. Normally, o2 far exceeds the body’s needs. o2 is not dependent on o2 until delivery is so low that everything delivered is being used, a point referred to as the critical o2.
In the critically ill, lower o2 is associated with higher mortality (1). Previous investigators have sought to increase o2 in the critically ill with the goal of improving outcome (2, 3). Prior efforts focused on increasing o2, based on the theory that in the critically ill o2 is pathologically dependent on o2, but this ultimately failed to show efficacy (2, 4). In fact, those patients whose o2 did not increase when o2 was increased were found to have exceedingly high mortality, suggesting the importance of impaired oxygen extraction (4). Cytopathic hypoxia, or the pathologic breakdown of aerobic metabolism and oxygen extraction, is now known to be an important factor in critical illness (5). In spite of the wide acceptance of this concept, however, no effective intervention for increasing the extraction component of o2 has been described.
Thiamine is a cofactor for pyruvate dehydrogenase, an essential enzyme for aerobic metabolism. In thiamine deficiency, pyruvate cannot enter the Krebs cycle, and anaerobic metabolism takes over. Decreased ATP production, vasodilatory shock, and lactic acidosis ensue. Thiamine administration rapidly reverses these effects in patients with thiamine deficiency. Investigators have shown previously that thiamine deficiency is more common in the critically ill (6, 7). Thiamine administration has also been shown to improve o2 and lower lactate in an animal model of sepsis and in healthy humans, regardless of initial thiamine level (8, 9). This suggests that thiamine may augment aerobic metabolism in the critically ill, even in the absence of absolute deficiency. We hypothesized that the administration of intravenous thiamine to critically ill patients would cause an increase in oxygen extraction and o2. As there are no prior data in critically ill patients, we conducted the following pilot, open label study investigating the effect of a single dose of intravenous thiamine on o2 in critically ill adults.
Methods
Study Design
This was a pilot, open label, prospective study of critically ill adults presenting to an urban tertiary care center. All adult patients admitted to an intensive care unit at our institution and requiring mechanical ventilation were screened for enrollment. This study was approved by the institutional review board, and written informed consent was obtained from each patient or their legally authorized surrogate before enrollment. This trial was registered on ClinicalTrials.gov (study ID: NCT01462279).
Patient Selection
Inclusion criteria were age at least 18 years, admission to an intensive care unit (ICU), and need for mechanical ventilation. We included only patients requiring mechanical ventilation because of our method of measuring o2. Regardless of the method used, o2 measurement is less accurate at a high fraction of inspired oxygen (FiO2) or in the presence of an air leak, and changes in body temperature are known to alter o2 (10, 11). Exclusion criteria therefore included the following: rapidly escalating ventilator settings, FiO2 greater than 60%, evidence of significant air leak, or temperature greater than 100°F. We excluded protected populations (pregnant patients and incarcerated patients), and patients taking more than 6 mg daily of thiamine before presentation or being given thiamine for a clinical indication in the hospital.
Data Collection
Baseline data collected included demographics, admission diagnosis, comorbidities, routine laboratory values, ventilator settings, vasopressors or sedatives being used, and vital signs. An initial venous blood sample was collected to determine thiamine level, lactate, and central venous oxygen saturation, if a central venous line was in place. Each patient was connected to both the Cheetah noninvasive cardiac output monitor (NICOM; Cheetah Medical, Newton Center, MA) and to the GE compact anesthesia monitor (General Electric, Fairfield, CT) for the duration of the study. The NICOM uses bioreactance to estimate cardiac index by interpreting signal from adhesive sensors attached to the patient’s torso, and has been validated in prior studies (12). The GE compact anesthesia monitor measures o2 using a pneumotachograph and a rapid paramagnetic analyzer attached in-line with the ventilator tubing, and has been validated in critically ill, mechanically ventilated patients (10, 11, 13). o2 and CI were recorded every 5 minutes for the duration of the 9-hour protocol. CI was measured due to the potential partial dependence of o2 on o2 in lower cardiac output states. We planned to measure change in o2 both in unadjusted analysis and adjusted for changes in CI based on this possibility.
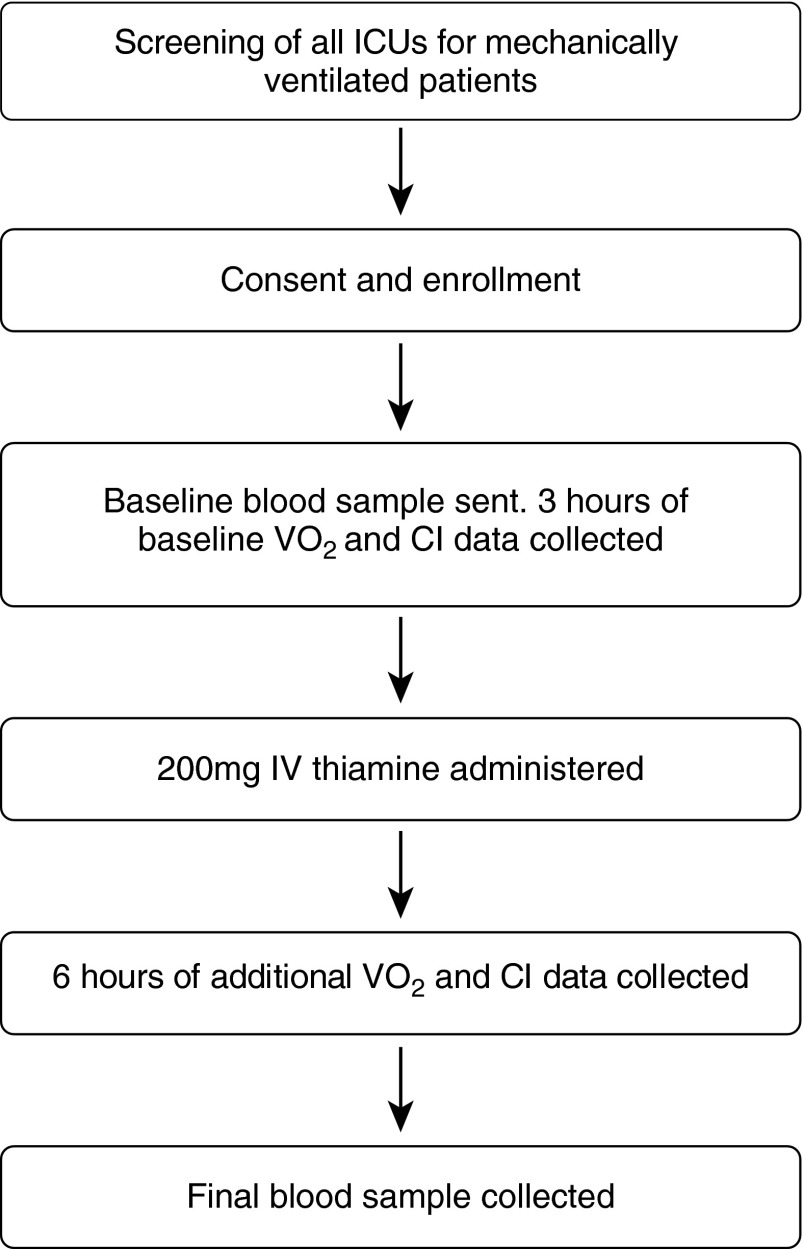
After 3 hours, a single dose of 200 mg of intravenous thiamine was administered to each patient. Six hours of additional data were then collected, and a blood sample was then collected to determine thiamine level, lactate, and, if a central venous line was in place, central venous oxygen saturation. See Figure 1 for a flow diagram of the study protocol.
Figure 1.
Flow chart of study protocol. CI = cardiac index; IV = intravenous; o2 = oxygen consumption.
Data Analysis
Baseline characteristics were delineated using descriptive statistics. Continuous data were summarized with means and standard deviations or medians and interquartile ranges depending on the distribution of the data, discrete data with frequencies and percentages. We used linear mixed modeling for repeated measurements to assess the effect over time of the primary exposure of thiamine on o2. We reported this result both in a univariate analysis, and adjusted for changes in CI. Missing data for CI were imputed, using the last-observation-carried-forward method. For linear mixed-effect models we assessed the following variance–covariance structures: independent, compound symmetry, first-order autoregressive [AR(1)], and unstructured. We used the Akaike information criterion (AIC) for final model selection (14). All tests of the data were two-sided and statistical analyses were performed in SAS version 9.2 (SAS, Cary, NC).
We then stratified patients by CI. We first separated patients into groups of those with preserved or depressed cardiac function (mean CI, >2.4 or ≤ 2.4 L/m2/min). We then analyzed the distribution of CI in our patient group, and stratified patients into those with mean CI greater than or less than the mean. A Pearson correlation was used to analyze the relationship between CI and o2 to evaluate for any dependency of o2 on CI.
Results
Twenty patients were enrolled in the study. Three were excluded for incomplete data (due to emergency procedures requiring interruption of study protocol or equipment malfunction leading to large blocks of missing o2 data), leaving 17 for analysis. For one additional patient the NICOM malfunctioned, so that patient was excluded from the analysis in which we adjusted for changes in CI, and from the analysis stratified by mean CI. Baseline characteristics are described in Table 1. The average age was 66 (±17) years, and 65% of the patients were men. Overall in-hospital mortality was 35%. Baseline thiamine levels ranged from below the detectable limit to 73 nmol/L (reference range, 9–44 nmol/L). Two patients (11.7%) had levels below the reference range. Baseline o2 ranged from 175 to 450 ml/min.
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Age, yr, mean (±SD) | 66.1 (±17.4) |
| Male, % | 64.71 |
| Race, % | |
| White | 88.24 |
| Black | 5.88 |
| Asian | 5.88 |
| Past medical history, % | |
| None | 17.60 |
| CAD | 5.90 |
| Stroke | 5.90 |
| COPD | 11.80 |
| DM | 11.80 |
| IVDA | 11.80 |
| Liver disease | 11.80 |
| Hyperlipidemia | 29.41 |
| Alcohol dependence | 11.76 |
| Obesity | 23.53 |
| Admitting diagnosis, % | |
| Pneumonia | 17.65 |
| Sepsis | 29.41 |
| Acute renal failure | 5.88 |
| Aortic aneurysm | 23.53 |
| Other (including pancreatitis, endocarditis, pleural effusion, cardiac arrest) | 41.18 |
| Heart rate, mean (±SD) | 84.4 (±13.9) |
| SBP, mm Hg, mean (±SD) | 112.2 (±19.7) |
| FiO2, %, mean (±SD) | 48.6 (±8.8) |
| PEEP, cm H2O, mean (±SD) | 7.2 (±3.1) |
| Lactate, mmol/L, median (IQR) | 2.9 (1–3.1) |
| Lactate > 2 mmol/L, % | 23.53 |
| Initial thiamine, nmol/L, median (IQR) | 31.9 (16–53) |
| Vasopressor use, % | 47.06 |
| Norepinephrine, n | 5 |
| Phenylephrine, n | 2 |
| Vasopressin, n | 3 |
| Sedation use, % | 82.35 |
| SOFA score, median (IQR) | 5 (4–9) |
| Mortality, n (%) | 6 (35) |
Definition of abbreviations: CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; FiO2 = fraction of inspired oxygen; IQR = interquartile range; IVDA = intravenous drug abuse; PEEP = positive end expiratory pressure; SBP = systolic blood pressure; SOFA = Sequential Organ Failure Assessment.
n = 17.
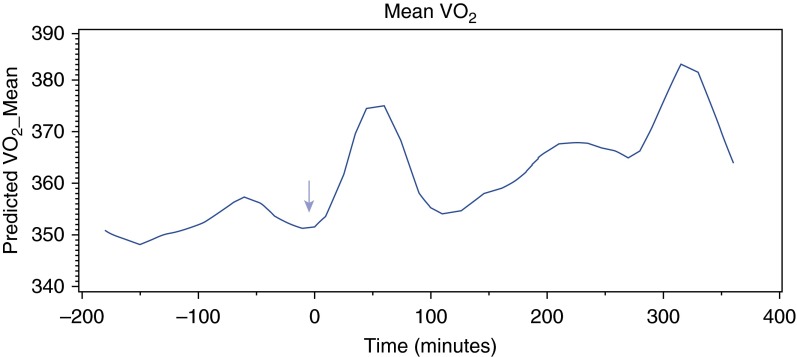
There was a trend toward increase in o2 after thiamine administration in all patients (16.3 ml/min, SE 8.5; P = 0.052), graphically represented in Figure 2. There was a very weak correlation between CI and o2 (r = 0.12; P < 0.0001), and the increase in o2 reached statistical significance after the planned adjustment for changes in CI (16.9 ml/min, SE 8.6; P = 0.047). In patients with preserved CI (>2.4 L/min/m2) there was an increase of 21.4 ml/min (P = 0.027) (Table 2). There was no increase in o2 in patients with mean CI below the group mean. In contrast, patients with a mean CI above the group mean of 3 L/min/m2 showed an average increase of 70.9 ml/min in o2 (P < 0.0001). There was a nonsignificant trend toward higher mortality in the group with CI less than or equal to 3, although median Sequential Organ Failure Assessment (SOFA) scores were largely similar (Table 3). There was no association between initial thiamine level and change in o2. There was no change in CI after thiamine administration.
Figure 2.
Smoothed curve of oxygen consumption (o2) over time in all patients. Arrow at time zero indicates time of thiamine administration.
Table 2.
Change in oxygen consumption after thiamine administration, stratified by cardiac index group
| Average o2 Change (ml/min) after Thiamine | P Value | |
|---|---|---|
| All patients (n = 16) | 16.3 | 0.052 |
| Mean CI ≤ 3* (n = 9) | −1.2 | 0.89 |
| Mean CI > 2.4* (n = 10) | 21.4 | 0.027 |
| Mean CI > 3* (n = 7) | 70.9 | <0.0001 |
Definition of abbreviations: CI = cardiac index; o2 = oxygen consumption.
Units of CI, L/min/m2.
Table 3.
Age, admitting diagnoses, time of enrollment, and mortality stratified by cardiac index group
| CI (n) |
||
|---|---|---|
| >3 L/min/m2 (7) | ≤3 L/min/m2 (9) | |
| Age, yr, median (IQR) | 61 (41–71) | 69 (62–82) |
| Admitting diagnosis, n (%) | ||
| Pneumonia | 2 (28.57) | 0 |
| Pleural effusion | 1 (14.29) | 0 |
| Valve surgery | 0 | 1 (11.11) |
| Sepsis | 2 (28.57) | 3 (33.33) |
| Acute renal failure | 1 (14.29) | 0 |
| Aortic aneurysm | 2 (28.57) | 2 (22.22) |
| Endocarditis | 1 (14.29) | 0 |
| Pancreatitis | 0 | 1 (11.11) |
| Bowel ischemia | 0 | 2 (22.22) |
| Spinal hematoma | 0 | 1 (11.11) |
| Days from ICU admission-enrollment, median (IQR) | 4 (2–5) | 2 (1–14.5) |
| SOFA score | 5 (2.5–8) | 5 (5–10) |
| Mortality, n (%) | 1 (14) | 5 (55) |
Definition of abbreviations: CI = cardiac index; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Discussion
We found a trend toward an increase in o2 after the administration of a single dose of intravenous thiamine in critically ill patients, which achieved statistical significance after preplanned adjustment for CI. The increase in o2 was considerably greater in patients with preserved CI, and the change was independent of baseline thiamine level.
o2 depends on o2 and the body’s ability to extract oxygen effectively from the blood. In low CI states, o2 is delivery dependent, but above a certain threshold, o2 is more dependent on extraction. Cytopathic hypoxia refers to the defect in oxygen extraction that appears to develop later in sepsis and perhaps other forms of critical illness (5). If cellular metabolism is working correctly, as oxygen delivery decreases the extraction will continue at the normal rate. A greater fraction of the available oxygen will therefore be extracted by the cells, leading to a decrease in tissue Po2. If delivery is not impaired and there is a breakdown in cellular metabolism, less oxygen will be extracted and tissue Po2 may actually increase. This finding has been reported in multiple animal and human studies, with skeletal muscle, bladder, and bowel mucosa Po2 found to be higher than normal in septic shock, and lower in cardiogenic shock (15–17). This research, which suggests that oxygen extraction is deficient in sepsis, but not in cardiogenic shock, could explain why we saw a rise in o2 after thiamine in patients with preserved CI (septic physiology), but not in those with low CI (cardiogenic shock physiology). Lower o2 in spite of adequate delivery has been associated with higher lactate levels and poorer outcomes in the critically ill (4), and our data raise the possibility that thiamine could increase o2 in these patients. The degree of rise in o2 that would be clinically significant is unknown. In a study by Hayes and colleagues, in which they found that patients who were unable to mount an increase in o2 when o2 was increased had much higher mortality, the increase in o2 in the group that did well averaged about 30%, but further study is needed (2).
Of the patients in our study, 11.7% were thiamine deficient, but thiamine level was not predictive of the change in o2. This suggests that thiamine may be useful for the augmentation of oxygen extraction even in the absence of absolute deficiency, a response that we hypothesize is due to stimulation of pyruvate dehydrogenase and consequent augmentation of aerobic metabolism. There is scant prior literature on the effect of thiamine on o2, but in a dog model of septic shock, Lindenbaum and colleagues demonstrated that thiamine improved lactate clearance, mean arterial pressure, and o2, regardless of whether thiamine deficiency was present (9). Although there have been no prior studies in critically ill patients, an increase in maximal o2 after administration of thiamine to healthy male athletes has been described (8). Our study is the first to our knowledge to investigate this effect in critically ill patients.
Our study was limited by its small size and pilot nature. The lack of a control arm prevents any conclusions about causality. Because of the small number of patients we were unable to control for other factors such as level of sedation, which could affect o2. The patients enrolled had widely variable CIs, and patients with low CI did not mount an increase in o2 after thiamine. Thus we might have seen a stronger effect if enrolling only patients with preserved CI. We were unable to evaluate the effect of thiamine on lactate clearance as has been done in prior studies because 75% of our patients had normal lactate levels at enrollment. With the preliminary data supplied by this pilot study, we are now enrolling patients in a randomized controlled trial (NCT01985685) comparing the effect of thiamine versus placebo on o2 in critically ill patients to investigate this question further. Because of the differences we found in response between patients with preserved versus low CI, we are enrolling only patients with preserved CI in this follow-up study.
Conclusions
In this small, preliminary study, the administration of a single dose of thiamine was associated with a trend toward increase in o2 in critically ill patients, which reached significance after planned adjustment for changes in CI. There was a larger and significant increase in o2 in those patients with preserved or elevated CI.
Acknowledgments
Acknowledgment
The authors acknowledge the assistance of Francesca Montillo in preparation of the manuscript.
Footnotes
Supported, in part, by grant UL1 RR025758-Harvard Clinical and Translational Science Center, from the National Center for Research Resources. M.W.D. is supported by the NHLBI (1K02HL107447-01A1) and NIH (R21AT005119-01). This study was additionally funded in part by a SEED grant from the American Medical Association, and K.M.B. is supported by American Heart Association grant 13CRP16930000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health, or the American Heart Association.
Author Contributions: K.M.B. helped conceive of and design the study, oversaw patient enrollment and data collection, was involved in data analysis, and was the primary writer of the manuscript. S.G. helped with study design and statistical analysis and critically reviewed and edited the manuscript. J.D.S. contributed to data collection and patient enrollment, as well as data analysis and manuscript writing. T.G. was involved in trial design, data analysis, and manuscript writing and revision. B.S. assisted with data analysis and contributed to the writing of the manuscript. M.W.D. oversaw the design of the study, patient enrollments, data analysis, and writing of the manuscript. All authors have approved the final content of this manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wilson RF, Christensen C, LeBlanc LP. Oxygen consumption in critically-ill surgical patients. Ann Surg. 1972;176:801–804. doi: 10.1097/00000658-197212000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes MA, Yau EH, Timmins AC, Hinds CJ, Watson D. Response of critically ill patients to treatment aimed at achieving supranormal oxygen delivery and consumption: relationship to outcome. Chest. 1993;103:886–895. doi: 10.1378/chest.103.3.886. [DOI] [PubMed] [Google Scholar]
- 3.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 4.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 5.Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnino MW, Cocchi MN, Smithline H, Carney E, Chou PP, Salciccioli J. Coronary artery bypass graft surgery depletes plasma thiamine levels. Nutrition. 2010;26:133–136. doi: 10.1016/j.nut.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, Chou PP, Ngo L. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25:576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bautista-Hernández VM, López-Ascencio R, Del Toro-Equihua M, Vásquez C. Effect of thiamine pyrophosphate on levels of serum lactate, maximum oxygen consumption and heart rate in athletes performing aerobic activity. J Int Med Res. 2008;36:1220–1226. doi: 10.1177/147323000803600608. [DOI] [PubMed] [Google Scholar]
- 9.Lindenbaum GA, Larrieu AJ, Carroll SF, Kapusnick RA. Effect of cocarboxylase in dogs subjected to experimental septic shock. Crit Care Med. 1989;17:1036–1040. doi: 10.1097/00003246-198910000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TS. Recent advances in gas exchange measurement in intensive care patients. Br J Anaesth. 2003;91:120–131. doi: 10.1093/bja/aeg128. [DOI] [PubMed] [Google Scholar]
- 11.McLellan S, Walsh T, Burdess A, Lee A. Comparison between the Datex-Ohmeda M-COVX metabolic monitor and the Deltatrac II in mechanically ventilated patients. Intensive Care Med. 2002;28:870–876. doi: 10.1007/s00134-002-1323-5. [DOI] [PubMed] [Google Scholar]
- 12.Marqué S, Cariou A, Chiche JD, Squara P. Comparison between Flotrac-Vigileo and Bioreactance, a totally noninvasive method for cardiac output monitoring. Crit Care. 2009;13:R73. doi: 10.1186/cc7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson L, Dodds S, Walsh TS. Clinical evaluation of a continuous oxygen consumption monitor in mechanically ventilated patients. Anaesthesia. 2003;58:455–460. doi: 10.1046/j.1365-2044.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 14.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17:228–243. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boekstegers P, Weidenhöfer S, Pilz G, Werdan K. Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection. 1991;19:317–323. doi: 10.1007/BF01645355. [DOI] [PubMed] [Google Scholar]
- 16.VanderMeer TJ, Wang H, Fink MP. Endotoxemia causes ileal mucosal acidosis in the absence of mucosal hypoxia in a normodynamic porcine model of septic shock. Crit Care Med. 1995;23:1217–1226. doi: 10.1097/00003246-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Rosser DM, Stidwill RP, Jacobson D, Singer M. Oxygen tension in the bladder epithelium rises in both high and low cardiac output endotoxemic sepsis. J Appl Physiol (1985) 1995;79:1878–1882. doi: 10.1152/jappl.1995.79.6.1878. [DOI] [PubMed] [Google Scholar]