Abstract
Rationale: Endotracheal intubation is associated with postextubation swallowing dysfunction, but no guidelines exist for postextubation swallowing assessments.
Objectives: We evaluated the prevalence, patient demographic and clinical factors, and intensive care unit (ICU) and hospital organizational factors associated with swallowing assessment after oral endotracheal intubation and mechanical ventilation in patients with acute lung injury (ALI).
Methods: We performed a secondary analysis of a prospective cohort study in which investigators evaluated 178 eligible patients with ALI who were mechanically ventilated via oral endotracheal tube. The patients were recruited from 13 ICUs at four teaching hospitals in Baltimore, Maryland. Patient demographic and clinical factors, types of ICU, and hospital study sites were evaluated for their association with completion of a swallowing assessment both in the ICU and after the ICU stay before hospital discharge. Factors significantly associated with a swallow assessment were evaluated in a multivariable logistic regression model.
Measurements and Main Results: Before hospital discharge, 79 (44%) patients completed a swallowing assessment, among whom 59 (75%) had their assessments initiated in ICU and 20 (25%) had their assessments initiated on the hospital ward. Female sex (odds ratio [OR] = 2.01; 95% confidence interval [95% CI] = 1.03–3.97), orotracheal intubation duration (OR = 1.13 per day; 95% CI = 1.05–1.22), and hospital study site (Site 3: OR = 2.41; 95% CI = 1.00–5.78) were independently associated with swallowing assessment. Although Site 3 had a twofold increase in swallowing assessments in the ICU, there was no significant difference between hospitals in the frequency of swallowing assessments completed after ICU discharge (P = 0.287) or in the proportion of patients who failed a swallowing assessment conducted in the ICU (P = 0.468) or on the ward (P = 0.746).
Conclusions: In this multisite prospective study, female sex, intubation duration, and hospital site were associated with postextubation swallowing assessment. These results demonstrate variability in practice patterns between institutions and highlight the need to determine the appropriate timing and indications for swallowing assessment and to more fully understand swallowing dysfunction after intubation.
Keywords: deglutition, deglutition disorders, intubation, referral and consultation, acute lung injury
Invasive airways are necessary for respiratory support in most patients with acute lung injury (ALI). However, one potential iatrogenic effect of an oral endotracheal intubation in mechanically ventilated patients is impairments in the anatomy and physiology of the pharynx and larynx that may be associated with subsequent swallowing disorders (1–7). Aspiration, a consequence of disordered swallowing (i.e., dysphagia), occurs in 14–56% of patients who have been mechanically ventilated for at least 48 hours (5–8). Moreover, dysphagia is associated with poor patient outcomes, such as feeding tube placement, increased hospital length of stay, nursing home placement, and increased risk of death (9).
Recognition of dysphagia and referrals to speech-language pathologists (SLPs) for swallowing assessment have increased dramatically over the past 20 years in neurology and otolaryngology patient populations (10–12). During this same period of time, nurses have become more aware of the risks of dysphagia and aspiration and have been trained to complete dysphagia screenings, particularly in neurological intensive care units (ICUs) (13–17). A national survey found that 41% of hospitals reported using clinical screening protocols after extubation, most often administered by nursing staff, and that in 3% of hospitals SLPs care for all recently extubated patients (18). Moreover, the survey authors reported that guidelines for SLP referral after extubation were present in 29% of hospitals, with the majority (60%) of SLPs using a clinical bedside swallowing evaluation and 40% completing a videofluoroscopic swallow study (VFSS). In addition to these self-reported, survey-based results, prospective evaluation of clinical practice, as part of routine care, is needed to more fully understand the epidemiology of swallowing assessment for ALI and other nonneurological populations of critically ill patients (19, 20).
Patients with ALI frequently have high severity of illness and an extended duration of mechanical ventilation, with concomitant impaired pulmonary function and muscle weakness after extubation (21, 22). Patients with ALI may be at especially high risk for dysphagia and aspiration after extubation. Consequently, we further analyzed a multisite prospective cohort of patients with ALI to evaluate (1) the prevalence of swallowing assessments after oral endotracheal intubation and (2) patient and organizational factors associated with these swallowing assessments.
Methods
Study Population
This assessment was conducted as a secondary analysis of a prospective cohort study in which researchers evaluated ICU care (provided as part of routine clinical practice) and associations with short- and long-term patient outcomes (23). Patients were eligible for enrollment in the cohort study if they were adults 18 years of age or older, mechanically ventilated, and diagnosed with ALI as per the American-European Consensus Conference criteria (24). Patients were recruited from 13 ICUs at 3 university-affiliated teaching hospitals and 1 Veterans Administration hospital in Baltimore, Maryland. Patients with ALI with primary neurological disease or trauma and neurological specialty ICUs at participating hospitals were excluded from the study. The following were the key patient exclusion criteria: (1) more than 5 days of mechanical ventilation before ALI, (2) preexisting cognitive impairment or a communication/language barrier, (3) transfer into a study site ICU with preexisting ALI of more than 24 hours’ duration, (4) limitations in ICU care at the time of study eligibility (e.g., no use of vasopressors), and (5) preexisting illness with a life expectancy shorter than 6 months.
To increase the homogeneity of the patient population with respect to dysphagia risk factors, we also excluded study patients who (1) had undergone a tracheostomy before or during their ICU stay and/or (2) had ever had a nasal endotracheal tube inserted during their ICU stay. Moreover, patients who died prior to hospital discharge were excluded, because this analysis focused on the epidemiology of swallowing assessment up to the time of hospital discharge among ALI survivors.
Primary Outcome Variable
The primary outcome measure was prevalence of a swallowing assessment in the hospital after extubation as documented in the medical record. For purposes of this evaluation, an assessment included either (1) a swallow screening completed by a nurse or SLP or (2) a VFSS completed by an SLP. The swallow screening is a noninstrumental procedure that includes assessment of the patient’s signs and symptoms of dysphagia with attempted swallowing of oral secretions and/or sips of water. The VFSS is a validated instrumental assessment in which fluoroscopy is used to determine (1) the presence and severity of physiologic swallowing impairments, (2) the effect of compensatory strategies, (3) the manner (e.g., oral versus nonoral) of receiving safe nutrition, and (4) the consistencies of foods and liquids a patient can safely consume on an oral diet (if applicable) (25, 26). Although the fiberoptic endoscopic evaluation of swallowing (FEES) is routinely conducted in some settings, the hospital sites included in this study do not routinely perform FEES; thus, FEES was not included in our primary outcome. Hospital Site 3 was the only site with an ICU-based, nurse-administered swallowing assessment algorithm that could prompt physicians for SLP referrals (14). This algorithm was based on the patient’s level of alertness, speech-language skills, oral motor skills, maintenance of oral secretions, and clinical signs of aspiration (e.g., coughing, choking) with the introduction of various volumes of water. There were no important differences in SLP availability between hospital sites, with all sites requiring SLP assessments to be completed within 24 hours after physician referral.
Patient and Organizational Variables
Relevant patient and organizational variables potentially associated with a swallowing assessment were selected on the basis of a review of the prior literature and investigators’ knowledge in this field. The following were the patient demographic and clinical factors evaluated in this study: age, sex, race, comorbidity status (Charlson Index [27] and Functional Comorbidity Index [28]), the presence of neurologic and upper gastrointestinal comorbidities, ICU admitting diagnostic category, primary risk factors for ALI, severity of illness (based on Acute Physiology and Chronic Health Evaluation II score [29] and Sequential Organ Failure Assessment (SOFA) score [30] at ICU admission), intubation duration, any reintubation required, and ICU length of stay. Organizational factors consisted of type of ICU (i.e., surgical or medical) and hospital study site. The duration of oral endotracheal intubation was measured in days since incident intubation. Patients who had been extubated for less than 48 hours before being reintubated were considered to have been intubated continuously from the initial placement of the oral endotracheal tube until extubation for 48 continuous hours or longer (31).
Statistical Analysis
We compared the prevalence of swallowing assessment for all patient and organizational variables using the Wilcoxon rank-sum test, Fisher’s exact test, or χ2 test, as appropriate. Any individual patient or organizational variable having a bivariable association with swallowing assessment in the ICU at P ≤ 0.20 was included in a multivariable logistic regression model to evaluate independent associations of variables with occurrence of an in-hospital swallowing assessment. The linearity of continuous variables’ association with the primary outcome was confirmed prior to their inclusion in the analyses. Variance inflation factors were used to evaluate multicollinearity in the multivariable regression model. Because of multicollinearity, intubation duration, ICU length of stay, and hospital length of stay could not be included in the multivariable regression model. We included intubation duration in the final multivariable model. An interaction between intubation duration and study site was evaluated, but it was not statistically significant and therefore was not included in the final model. To avoid overfitting the multivariable regression model, we collapsed ICU admission diagnoses from six categories into a binary variable (i.e., presence or absence of upper gastrointestinal disease) based on the results of the bivariable analysis. A single post hoc sensitivity analysis was conducted in which patients with neurological comorbidities were excluded from the final regression model, and we found that there were no material differences in the final results. Goodness of fit of the final multivariable logistic model was assessed using the Hosmer-Lemeshow test (32). No data were missing for the outcome or exposure variables in these analyses. Statistical analyses were conducted using Intercooled Stata version 12.1 software (StataCorp, College Station, TX). All significance tests were two-sided, with statistical significance defined as P < 0.05. This study was approved by the institutional review boards at all participating study sites.
Results
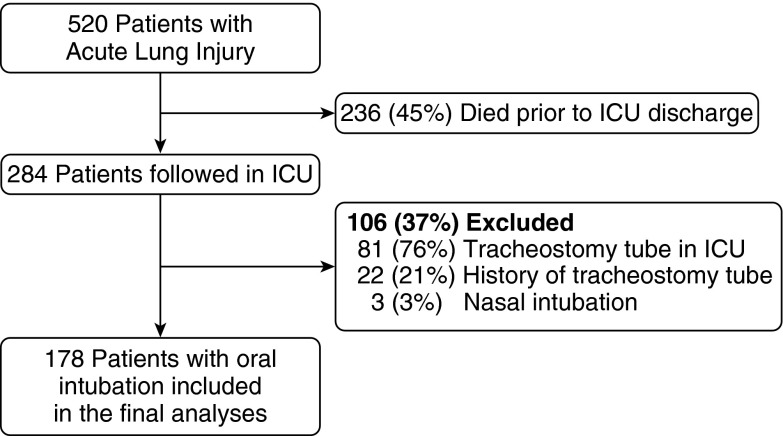
A total of 520 patients with ALI, with 63% (n = 178) of the 284 hospital survivors eligible for this analysis, were enrolled in the prospective cohort study (Figure 1). Only two patients were eligible from Hospital Site 4. Hence, for purposes of analyzing the association of hospital site with swallowing assessment, these two patients were included with Site 3 because Sites 3 and 4 shared the same ICU attending physicians, giving rise to similar physician-specific practice patterns regarding swallowing assessment. The patients’ median (interquartile range [IQR]) age was 49 (40, 59) years, and 47% were female and 60% were white (Table 1). The median (IQR) durations of endotracheal intubation and of ICU stay were 8 (5, 11) days and 12 (8, 16) days, respectively. Of all patients, 80% were admitted to a medical (versus surgical) ICU, and patients were approximately evenly distributed among the three study site hospitals.
Figure 1.
Flow diagram of subject selection with applied inclusion and exclusion criteria.
Table 1.
Patient and organizational characteristics by swallowing assessment completion
| Characteristics | Total (N = 178) | Assessment (n = 79) | No Assessment (n = 99) | P Value* |
|---|---|---|---|---|
| Patient demographics | ||||
| Age group, yr, median (IQR) | 49 (40, 59) | 50 (44, 61) | 46 (37, 57) | 0.065 |
| Female, n (%) | 83 (47%) | 42 (53%) | 41 (41%) | 0.13 |
| Race, n (%)† | 0.93 | |||
| Black | 71 (40%) | 31 (39%) | 40 (40%) | |
| White | 106 (60%) | 48 (61%) | 58 (59%) | |
| Other | 1 (1%) | 0 (0%) | 1 (1%) | |
| Baseline health status | ||||
| Comorbidity scores, median (IQR) | ||||
| Charlson Comorbidity Index at ICU admission | 1 (0, 4) | 1 (0, 4) | 1 (0, 3) | 0.23 |
| Functional Comorbidity Index at ICU admission | 1 (0, 3) | 1 (0, 3) | 2 (0, 3) | 0.27 |
| Neurologic disease, n (%) | 32 (49%) | 14 (50%) | 18 (49%) | 1.00 |
| Upper gastrointestinal disease, n (%) | 40 (62%) | 16 (57%) | 24 (65%) | 0.61 |
| ICU characteristics | ||||
| ICU admission diagnosis, n (%)† | 0.40 | |||
| Respiratory (including pneumonia) | 103 (58%) | 43 (54%) | 60 (61%) | |
| Nonpulmonary sepsis and infectious disease | 25 (14%) | 13 (16%) | 12 (12%) | |
| Upper gastrointestinal disease | 21 (12%) | 13 (16%) | 8 (8%) | |
| Cardiovascular disease | 8 (4%) | 2 (3%) | 6 (6%) | |
| Trauma | 7 (4%) | 3 (4%) | 4 (4%) | |
| Other | 14 (8%) | 5 (6%) | 9 (10%) | |
| Primary risk factors for ALI | ||||
| Aspiration, n (%) | 23 (13%) | 8 (10%) | 15 (15%) | 0.37 |
| Pneumonia, n (%) | 88 (49%) | 39 (49%) | 49 (49%) | 1.00 |
| APACHE II score at ICU admission, median (IQR) | 23 (19, 28) | 23 (19, 28) | 23 (17, 29) | 0.90 |
| SOFA score at ALI onset, median (IQR) | 8 (5, 10) | 8 (5, 10) | 8 (6, 10) | 0.22 |
| Ever reintubated, n (%) | 29 (16%) | 11 (14%) | 18 (18%) | 0.54 |
| Intubation duration, d, median (IQR) | 8 (5, 11) | 9 (7, 13) | 7 (4, 10) | <0.001 |
| Organizational characteristics | ||||
| Medical (vs. surgical) ICU, n (%) | 142 (80%) | 58 (73%) | 84 (85%) | 0.064 |
| Hospital study site, n (%)† | 0.014 | |||
| 1 | 52 (29%) | 21 (27%) | 31 (31%) | |
| 2 | 65 (37%) | 22 (28%) | 43 (43%) | |
| 3‡ | 61 (34%) | 36 (46%) | 25 (25%) |
Definition of abbreviations: ALI = acute lung injury; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Wilcoxon rank-sum test, Fisher’s exact test, or χ2 test.
Percentages may not add to 100% due to rounding.
Hospital uses a referral algorithm.
A total of 79 (44% of the 178 eligible) patients had at least one swallowing assessment completed by the time of hospital discharge. For patients in the ICU, a bedside swallowing assessment was completed for 58 (33%) patients, and a VFSS was completed for 6 (3%) patients (5 of these patients also had undergone previous bedside assessments). Of the 59 unique patients with initial assessments (either bedside assessment or VFSS) completed in the ICU, 24 (41%) were reevaluated on the ward after ICU discharge. An additional 20 (11%) patients had a swallowing assessment initially completed on the ward after ICU discharge. There were no important differences between patients who were initially assessed in the ICU versus those initially assessed on the wards (Table 2).
Table 2.
Patient characteristics by hospital unit type for initial swallowing assessment
| Characteristics | ICU (n = 59) | Ward (n = 20) | P value* |
|---|---|---|---|
| Patient demographics | |||
| Age group, yr, median (IQR) | 51 (45, 60) | 49 (42, 63) | 0.96 |
| Female, n (%) | 28 (47%) | 14 (70%) | 0.12 |
| White, n (%) | 34 (58%) | 14 (70%) | 0.43 |
| Baseline health status | |||
| Comorbidity scores, median (IQR) | |||
| Charlson Comorbidity Index at ICU admission | 1 (0, 4) | 2 (1, 6) | 0.22 |
| Functional Comorbidity Index at ICU admission | 1 (0, 3) | 2 (1, 3) | 0.65 |
| Neurologic disease, n (%) | 9 (47%) | 5 (56%) | 1.00 |
| Upper gastrointestinal disease, n (%) | 11 (58%) | 5 (56%) | 1.00 |
| ICU characteristics | |||
| ICU admission diagnosis, n (%) | 0.85 | ||
| Respiratory (including pneumonia) | 32 (54%) | 11 (55%) | |
| Nonpulmonary sepsis and infectious disease | 9 (15%) | 4 (20%) | |
| Upper gastrointestinal disease | 10 (17%) | 3 (15%) | |
| Cardiovascular disease | 1 (2%) | 1 (5%) | |
| Trauma | 3 (5%) | 0 (0%) | |
| Other | 4 (7%) | 1 (5%) | |
| Primary risk factors for ALI | |||
| Aspiration, n (%) | 5 (8%) | 3 (15%) | 0.41 |
| Pneumonia, n (%) | 30 (51%) | 9 (45%) | 0.80 |
| APACHE II score at ICU admission, median (IQR) | 22 (17, 29) | 24 (18, 30) | 0.79 |
| SOFA score at ALI onset, median (IQR) | 8 (6, 11) | 8 (5, 10) | 0.24 |
| Ever reintubated, n (%) | 10 (17%) | 1 (5%) | 0.27 |
| Intubation duration, d, median (IQR) | 8 (7, 11) | 10 (8, 16) | 0.07 |
| Organizational characteristics | |||
| Medical (vs. surgical) ICU, n (%) | 41 (69%) | 17 (85%) | 0.25 |
| Hospital study site, n (%)† | 0.10 | ||
| 1 | 14 (24%) | 7 (35%) | |
| 2 | 14 (24%) | 8 (40%) | |
| 3‡ | 31 (53%) | 5 (25%) |
Definition of abbreviations: ALI = acute lung injury; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Wilcoxon rank-sum test, Fisher’s exact test, or χ2 test.
Percentages may not add to 100% due to rounding.
Hospital uses a referral algorithm.
The frequency of swallowing assessment in the ICU was twofold greater at Hospital Site 3 compared with the other two sites (P = 0.001), but not significantly different for assessments completed after ICU discharge (P = 0.287). Despite this variability in the frequency of assessments, there were no significant differences between hospital sites in the proportion of patients who failed swallowing assessments conducted in the ICU (P = 0.468) or on the ward (P = 0.746). The frequency of swallowing assessments overall was 60% greater at Hospital Site 3 compared with the other sites (P = 0.043) (Table 3).
Table 3.
Frequency of swallowing assessments
| Totals, n (%) | Any Assessment, n (%)* | Assessment Completed in ICU, n (%)* | Assessment Completed on Ward, n (%)* | |
|---|---|---|---|---|
| Hospital 1 | 52 (29) | 21 (27) | 14 (24) | 17 (39) |
| Hospital 2 | 65 (37) | 22 (28) | 14 (24) | 14 (32) |
| Hospital 3 | 61 (34) | 36 (46) | 31 (53) | 13 (30) |
| Total | 178 (100) | 79 (44) | 59 (33) | 44 (25) |
Percentages do not add to 100% due to rounding.
In bivariable analyses, the variables potentially associated (at P < 0.2) with swallowing assessment by the time of hospital discharge were age, sex, ICU admission diagnosis of upper gastrointestinal disease, SOFA score at the time of ALI onset, intubation duration, being admitted to a medical ICU, and hospital study site (Table 4). In the multivariable logistic regression analysis, three of these seven variables were significantly associated with swallowing assessment (odds ratio; 95% confidence interval): females (2.01; 1.03–3.97; P = 0.041), intubation duration (1.13; 1.05–1.22; P = 0.001), and hospital site 3 (2.41; 1.00–5.78; P = 0.049).
Table 4.
Factors associated with swallowing assessment completion during hospital admission in subjects with acute lung injury
| Bivariable Association |
Multivariable Association |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value* | Odds Ratio (95% CI) | P Value* | |
| Patient demographics | ||||
| Age | 1.02 (1.00, 1.04) | 0.083 | 1.02 (0.99, 1.04) | 0.069 |
| Female | 1.61 (0.88, 2.91) | 0.119 | 2.01 (1.03, 3.97) | 0.041 |
| White | 1.07 (0.58, 1.96) | 0.832 | ||
| Baseline health status | ||||
| Comorbidity scores, median (IQR) | ||||
| Charlson Comorbidity Index at ICU admission | 1.06 (0.94, 1.20) | 0.318 | ||
| Functional Comorbidity Index at ICU admission | 0.88 (0.72, 1.07) | 0.204 | ||
| Neurologic disease | 1.06 (0.40, 2.82) | 0.914 | ||
| Upper GI disease | 0.72 (0.26, 1.98) | 0.527 | ||
| ICU characteristics | ||||
| Upper gastrointestinal disease as ICU admission diagnosis | 2.24 (0.88, 5.71) | 0.091 | 2.54 (0.84, 7.70) | 0.100 |
| Primary risk factors for ALI | ||||
| Aspiration | 0.63 (0.25, 1.57) | 0.324 | ||
| Pneumonia | 0.99 (0.55, 1.80) | 0.986 | ||
| APACHE II score at ICU admission | 1.00 (0.97, 1.04) | 0.844 | ||
| SOFA score at ALI onset | 1.07 (0.98, 1.17) | 0.129 | 1.03 (0.93, 1.14) | 0.610 |
| Ever reintubated | 0.73 (0.32, 1.65) | 0.446 | ||
| Intubation duration | 1.10 (1.03, 1.18) | 0.003 | 1.13 (1.05, 1.22) | 0.001 |
| Organizational characteristics | ||||
| Medical (vs. surgical) ICU | 0.49 (0.23, 1.04) | 0.062 | 0.66 (0.28, 1.56) | 0.347 |
| Hospital study site | ||||
| 1 | Reference | Reference | ||
| 2 | 0.76 (0.35, 1.61) | 0.467 | 0.68 (0.29, 1.62) | 0.387 |
| 3† | 2.13 (1.00, 4.51) | 0.050 | 2.41 (1.00, 5.79) | 0.049 |
Definition of abbreviations: ALI = acute lung injury; APACHE II = Acute Physiology and Chronic Health Evaluation II; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
P values were calculated using simple and multiple logistic regression analysis for bivariable and multivariable results, respectively. Covariates were included in the multivariable logistic model based on a bivariable association at P<0.10.
Hospital uses a referral algorithm.
Discussion
In our multisite study of 178 orotracheally intubated, mechanically ventilated patients with ALI, 44% of patients across all hospital sites had undergone a swallowing assessment prior to hospital discharge. The variables independently associated with swallowing assessments were female sex, longer intubation duration, and hospital study site. The hospital site with an algorithm for swallowing assessment after extubation had more than a twofold increased odds of swallowing assessment compared with the other two hospital sites.
There are a number of screenings for swallowing dysfunction (14, 16, 33–40), with several designed for specific patient populations, such as stroke, Parkinson’s disease, and head and neck cancer. A number of self-assessments of swallowing difficulties are used to augment these screening tests (41–45). Additionally, several screening models for referrals for swallowing assessments by SLPs have been suggested by the American Speech-Language-Hearing Association (46). To our knowledge, however, there are no published studies in which researchers empirically evaluated screening tests or models for SLP referral after extubation of critically ill patients.
In a systematic review of dysphagia following endotracheal intubation (19), the authors reported that four studies (4, 5, 7, 8) evaluated medical and surgical ICU patients, with the prevalence of dysphagia ranging from 44% to 62%. Of the 178 patients in our cohort, 44% completed swallowing assessments while in the hospital, with 75% of these initial assessments occurring in the ICU. However, because remarkably few high-quality studies of swallowing and dysphagia in critically ill patients have been conducted, it is uncertain when SLP assessments should be performed (19, 20). Despite our evaluation of potentially relevant demographic, clinical, and ICU/hospital organizational factors, it appears that completion of swallowing assessments in nonneurological populations of critically ill patients is highly variable and that the prevalence varies substantially between hospitals.
This wide variability in the frequency of performing swallowing assessments as part of routine medical care (27%, 28%, and 46% of patients in the present study at Hospital Sites 1, 2, and 3, respectively) may be disproportionately low compared with the estimated 44–62% prevalence of postextubation dysphagia in critically ill patients (4, 5, 7, 8). Moreover, the use of swallowing assessments at Sites 1 and 2 was disproportionately lower than at Site 3, suggesting that Sites 1 and 2 may be missing patients who need to be screened (especially because the rate of failed assessments was not lower at the site that had a higher frequency of assessments). Our data underscore previous findings (18) and provide some empirical evidence regarding variability in clinical practice, and they highlight the need for more rigorous clinical research designed to assess the frequency of postextubation dysphagia, including diagnostic evaluation focused on swallowing physiology and application in treatment strategies to help guide clinical practice and hospital policies.
In the present study, we did not address the reasons for differences in the frequency of swallowing assessments between hospitals. The hospital-wide, ICU-based, nurse-administered swallowing assessment used algorithm at Hospital Site 3 may explain the significantly higher rate of swallowing assessments completed in the ICU. The use of an algorithm such as that used at Hospital Site 3 (14), or as suggested by others (20), as well as screening tools in general, could give rise to greater opportunities for early identification (and subsequent intervention, if necessary) of patients with dysphagia with resultant aspiration. The clinical utility of the algorithm use at Hospital Site 3 is based on a small consensus validation panel in which a convenience sample of 25 patients with stroke was used, most of which was not compared against a reference standard. The clinical utility of either of these algorithms has not been empirically evaluated in this patient population, and we are not aware of any rigorous, evidence-based guidelines for when screening and/or instrumental swallowing assessment should take place after orotracheal extubation. Validation of clinical screening methods is needed in this patient population to ensure benefits, without unintentional harms, to patients and to avoid nonbeneficial increases in clinicians’ workloads.
The duration of oral endotracheal intubation with mechanical ventilation was independently associated with swallowing assessment in the present study. This may reflect clinicians’ beliefs that the likelihood or the potential danger of dysphagia increases with duration of intubation. However, we did not address the association of swallowing physiology with the duration of intubation in the present study, so the validity of these beliefs remains unclear (19).
A final factor that was significantly associated with completion of a swallowing assessment was sex. We found that females with ALI had a twofold increase in the odds of completing a swallowing assessment. This finding may be consistent with the intubation literature which suggests that females are at greater risk for laryngeal injury and related swallowing difficulties, possibly due to the placement of oversized endotracheal tubes (1, 47–49). Additional prospective, physiologically based studies with well-controlled, nonneurological populations of critically ill patients are needed to more fully address potential sex differences in swallowing assessments and dysphagia.
By offering this prospective, multisite research in which we determined the frequency of swallowing assessments completed by both nursing staff and SLPs as part of routine clinical practice, we add to data gathered in a previously published national survey of SLPs’ self-reported practices (18). Therefore, in the present study, we provide additional insight into variability between institutions with regard to swallowing assessments administered after extubation. This variability may arise, in part, because of limitations of the existing literature on the prevalence and risks of postextubation dysphagia, the validation of swallowing assessments in this patient population, and the clinical benefits of using screening algorithms.
Study Limitations
This research has potential limitations. First, we did not address the methods by which physicians made referrals to SLPs for swallowing assessments at Hospital Sites 1 and 2, and, likewise, we did not determine compliance with the nurse assessment and SLP referral algorithm at Site 3. However, we believe that our data still provide an initial foundation for future researchers to address clinical practice and its variability in administering swallowing assessments. Second, we were not able to determine the frequency of a true dysphagia diagnosis, because instrumental evaluation, such as videofluoroscopy, is not a standard of care and was not completed for all patients. Third, all participating sites are teaching hospitals in the same city (Baltimore, Maryland), possibly limiting the generalizability of the findings. However, our results do have some similarity to those derived from a prior national survey conducted in the United States (18). We encourage researchers to conduct additional multicenter studies to further evaluate swallowing assessment practices and dysphagia in critically ill patients. Finally, although study enrollment was completed in 2007, there have been no compelling data published since that time which address swallowing physiology in postextubated patients. Clinical practice remains similar at all three hospital sites that we studied.
Conclusions
In this multisite study of orally intubated patients with ALI, we have demonstrated that female sex, the duration of oral endotracheal intubation, and hospital study site were independently associated with the occurrence of in-hospital swallowing assessments. There is variability in clinical practice among hospitals, possibly reflecting limitations in existing knowledge as to which patients are at the greatest risk for dysphagia after extubation and when swallowing assessments are indicated. Further clinical research is needed to create a solid evidence base to guide the use of swallowing assessments after extubation in critically ill patients.
Acknowledgments
Acknowledgment
The authors thank Victor Dinglas for his assistance with data management for this study.
Footnotes
Supported by the following grants from the National Institutes of Health: P050HL73994, 5KL2RR025006, and 1K23DC011056.
Author Contributions: All authors reviewed, offered critical revision, and gave approval for final submission. M.B.B.: participated in study design, completed analyses, provided interpretation of data, completed manuscript writing; M.G.-F.: participated in study design, completed analyses, provided interpreation of data; P.A.M.-T.: participated in study design, completed data acquisition, provided interpretation of data; C.S.: participated in study design, completed data acquisition, provided interpretation of data; J.B.P.: provided interpretation of data; D.M.N.: participated in study design, completed data acquisition, provided interpretation of data.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hedden M, Ersoz CJ, Donnelly WH, Safar P. Laryngotracheal damage after prolonged use of orotracheal tubes in adults. JAMA. 1969;207:703–708. [PubMed] [Google Scholar]
- 2.Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy: a prospective study of 150 critically ill adult patients. Am J Med. 1981;70:65–76. doi: 10.1016/0002-9343(81)90413-7. [DOI] [PubMed] [Google Scholar]
- 3.Colice GL, Stukel TA, Dain B. Laryngeal complications of prolonged intubation. Chest. 1989;96:877–884. doi: 10.1378/chest.96.4.877. [DOI] [PubMed] [Google Scholar]
- 4.de Larminat V, Montravers P, Dureuil B, Desmonts JM. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit Care Med. 1995;23:486–490. doi: 10.1097/00003246-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Leder SB, Cohn SM, Moller BA. Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia. 1998;13:208–212. doi: 10.1007/PL00009573. [DOI] [PubMed] [Google Scholar]
- 6.Barquist E, Brown M, Cohn S, Lundy D, Jackowski J. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized, prospective trial. Crit Care Med. 2001;29:1710–1713. doi: 10.1097/00003246-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 7.El Solh A, Okada M, Bhat A, Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29:1451–1455. doi: 10.1007/s00134-003-1870-4. [DOI] [PubMed] [Google Scholar]
- 8.Ajemian MS, Nirmul GB, Anderson MT, Zirlen DM, Kwasnik EM. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg. 2001;136:434–437. doi: 10.1001/archsurg.136.4.434. [DOI] [PubMed] [Google Scholar]
- 9.Macht M, Wimbish T, Clark BJ, Benson AB, Burnham EL, Williams A, Moss M. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care. 2011;15:R231. doi: 10.1186/cc10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petheram B, Enderby P. Demographic and epidemiological analysis of patients referred to speech and language therapy at eleven centres 1987-95. Int J Lang Commun Disord. 2001;36:515–525. doi: 10.1080/13682820110075015. [DOI] [PubMed] [Google Scholar]
- 11.Enderby P, Petheram B. Has aphasia therapy been swallowed up? Clin Rehabil. 2002;16:604–608. doi: 10.1191/0269215502cr505oa. [DOI] [PubMed] [Google Scholar]
- 12.Leder SB, Suiter DM. An epidemiologic study on aging and dysphagia in the acute care hospitalized population: 2000-2007. Gerontology. 2009;55:714–718. doi: 10.1159/000235824. [DOI] [PubMed] [Google Scholar]
- 13.Hansel DE, Heinemann D. Improving nursing practice with staff education: the challenges of dysphagia. Gastroenterol Nurs. 1996;19:201–206. doi: 10.1097/00001610-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Massey R, Jedlicka D. The Massey Bedside Swallowing Screen. J Neurosci Nurs. 2002;34:252–253, 257–260. doi: 10.1097/01376517-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Morris H. Dysphagia in the elderly—a management challenge for nurses. Br J Nurs. 2006;15:558–562. doi: 10.12968/bjon.2006.15.10.21132. [DOI] [PubMed] [Google Scholar]
- 16.Martino R, Silver F, Teasell R, Bayley M, Nicholson G, Streiner DL, Diamant NE. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40:555–561. doi: 10.1161/STROKEAHA.107.510370. [DOI] [PubMed] [Google Scholar]
- 17.Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D’Este C, Drury P, Griffiths R, Cheung NW, Quinn C, et al. QASC Trialists Group. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. 2011;378:1699–1706. doi: 10.1016/S0140-6736(11)61485-2. [DOI] [PubMed] [Google Scholar]
- 18.Macht M, Wimbish T, Clark BJ, Benson AB, Burnham EL, Williams A, Moss M. Diagnosis and treatment of post-extubation dysphagia: results from a national survey. J Crit Care. 2012;27:578–586. doi: 10.1016/j.jcrc.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137:665–673. doi: 10.1378/chest.09-1823. [DOI] [PubMed] [Google Scholar]
- 20.Macht M, Wimbish T, Bodine C, Moss M. ICU-acquired swallowing disorders. Crit Care Med. 2013;41:2396–2405. doi: 10.1097/CCM.0b013e31829caf33. [DOI] [PubMed] [Google Scholar]
- 21.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 22.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 23.Needham DM, Dowdy DW, Mendez-Tellez PA, Herridge MS, Pronovost PJ. Studying outcomes of intensive care unit survivors: measuring exposures and outcomes. Intensive Care Med. 2005;31:1153–1160. doi: 10.1007/s00134-005-2656-7. [DOI] [PubMed] [Google Scholar]
- 24.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 25.Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin, TX: PRO-ED; 1998. [Google Scholar]
- 26.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 30.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000. [Google Scholar]
- 33.Mann G. MASA: The Mann Assessment of Swallowing Ability. New York: Delmar Learning; 2002. [Google Scholar]
- 34.Buchholz D, Neumann S. The Burke Dysphagia Screening Test: validation of its use in patients with stroke. Dysphagia. 1996;11:217–218. doi: 10.1007/BF00366390. [DOI] [PubMed] [Google Scholar]
- 35.DePippo KL, Holas MA, Reding MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49:1259–1261. doi: 10.1001/archneur.1992.00530360057018. [DOI] [PubMed] [Google Scholar]
- 36.Edmiaston J, Connor LT, Steger-May K, Ford AL. A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23:712–716. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logemann JA, Veis S, Colangelo L. A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14:44–51. doi: 10.1007/PL00009583. [DOI] [PubMed] [Google Scholar]
- 38.Mandysova P, Skvrňáková J, Ehler E, Cerný M. Development of the Brief Bedside Dysphagia Screening Test in the Czech Republic. Nurs Health Sci. 2011;13:388–395. doi: 10.1111/j.1442-2018.2011.00630.x. [DOI] [PubMed] [Google Scholar]
- 39.Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23:244–250. doi: 10.1007/s00455-007-9127-y. [DOI] [PubMed] [Google Scholar]
- 40.Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, Brainin M. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38:2948–2952. doi: 10.1161/STROKEAHA.107.483933. [DOI] [PubMed] [Google Scholar]
- 41.Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117:919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 42.Chen PH, Golub JS, Hapner ER, Johns MM., III Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia. 2009;24:1–6. doi: 10.1007/s00455-008-9156-1. [DOI] [PubMed] [Google Scholar]
- 43.Wallace KL, Middleton S, Cook IJ. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678–687. doi: 10.1016/s0016-5085(00)70137-5. [DOI] [PubMed] [Google Scholar]
- 44.Woisard V, Andrieux MP, Puech M. [Validation of a self-assessment questionnaire for swallowing disorders (Deglutition Handicap Index)] [Article in French] Rev Laryngol Otol Rhinol (Bord) 2006;127:315–325. [PubMed] [Google Scholar]
- 45.Ghazali N, Kanatas A, Scott B, Lowe D, Zuydam A, Rogers SN. Use of the Patient Concerns Inventory to identify speech and swallowing concerns following treatment for oral and oropharyngeal cancer. J Laryngol Otol. 2012;126:800–808. doi: 10.1017/S0022215112001107. [DOI] [PubMed] [Google Scholar]
- 46.Swigert NB, Steele C, Riquelme L.Dysphagia screening for patients with stroke: challenges in implementing a Joint Commission guidelineThe ASHA Leader2007 March 6[accessed 2014 Nov 20] Available fromhttp://www.asha.org/Publications/leader/2007/070306/070306c.htm [Google Scholar]
- 47.Donnelly WH. Histopathology of endotracheal intubation: an autopsy study of 99 cases. Arch Pathol. 1969;88:511–520. [PubMed] [Google Scholar]
- 48.Benjamin B. Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol Suppl. 1993;160:1–15. doi: 10.1177/00034894931020s401. [DOI] [PubMed] [Google Scholar]
- 49.François B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T, Brenet O, Preux PM, Vignon P Association des Réanimateurs du Centre-Ouest (ARCO) 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369:1083–1089. doi: 10.1016/S0140-6736(07)60526-1. [DOI] [PubMed] [Google Scholar]