Abstract
Rationale: Factors and outcomes associated with end-of-life decision-making among patients during clinical trials in the intensive care unit are unclear.
Objectives: We sought to determine patterns and outcomes of Do Not Resuscitate (DNR) decisions among critically ill patients with acute respiratory distress syndrome (ARDS) enrolled in a clinical trial.
Methods: We performed a secondary analysis of data from the ARDS Network Fluid and Catheter Treatment Trial (FACTT), collected between 2000 and 2005. We calculated mortality outcomes stratified by code status, and compared baseline characteristics of patients who became DNR during the trial with participants who remained full code.
Measurements and Main Results: Among 809 FACTT participants with a code status recorded, 232 (28.7%) elected DNR status. Specifically, 37 (15.9%) chose to withhold cardiopulmonary resuscitation alone, 44 (19.0%) elected to withhold some life support measures in addition to cardiopulmonary resuscitation, and 151 (65.1%) had life support withdrawn. Admission severity of illness as measured by APACHE III score was strongly associated with election of DNR status (odds ratio, 2.2; 95% confidence interval, 1.85–2.62; P < 0.0001). Almost all (97.0%; 225 of 232) patients who selected DNR status died, and 79% (225 of 284) of patients who died during the trial were DNR. Among patients who chose DNR status but did not elect withdrawal of life support, 91% (74 of 81) died.
Conclusions: The vast majority of deaths among clinical trial patients with ARDS were preceded by a DNR order. Unlike other studies of end-of-life decision-making in the intensive care unit, nearly all patients who became DNR died. The impact of variation of practice in end-of-life decision-making during clinical trials warrants further study.
Keywords: acute lung injury, acute respiratory distress syndrome, resuscitation orders, withholding treatment, clinical trial
Although most intensive care unit (ICU) deaths are preceded by a decision to limit life-sustaining treatment through a Do Not Resuscitate (DNR) directive (1), wide hospital-level variation in use of DNR orders among critically ill patients has been observed (2, 3). This variation may potentially impact results of clinical trials among the critically ill if unaccounted for during randomization (4), but hospital end-of-life practices are rarely reported. Similarly, use of, factors associated with, and outcomes of DNR status during clinical trials among critically ill patients have not been described.
There are reasons to believe that the use and outcomes of DNR directives may be different in the setting of a clinical trial. Patients enrolled in clinical trials are carefully screened and those in a moribund state are generally excluded. Clinicians might be more reluctant to institute limitations on aggressive care in patients involved in trials. In outpatient oncology settings, clinical trial participants have placed a high value on prolonging life and receiving aggressive medical care (5, 6). In this article we explore end-of-life decision-making in the context of an acute respiratory distress syndrome (ARDS) clinical trial, a population expected to have high mortality and the need for surrogate decision-makers to consider goals of care at the end of life (7).
This research was previously presented in the form of an abstract at the American Thoracic Society International Conference on May 21, 2013 (Philadelphia, PA).
Methods
Study Sample
We performed a secondary analysis of data from the ARDS Network (ARDSNet) Fluid and Catheter Treatment Trial (FACTT). The National Heart, Lung, and Blood Institute (NHLBI) ARDSNet FACTT trial was a multicenter, randomized trial performed to evaluate the effect of fluid management strategies on outcomes of ARDS (8, 9). A “commit[ment] to full support” was an inclusion criterion for enrollment in the FACTT trial, with an exception for patients committed to all supportive care except for attempts at cardiopulmonary resuscitation (CPR) in the setting of cardiac arrest. Baseline demographics and clinical information were obtained just before randomization. From the time of enrollment, patients were monitored until they died or were discharged home (or other prehospital residence) with unassisted breathing, or for 90 days if neither outcome was reached.
Outcome Measures
Our primary outcome measure was the proportion of FACTT participants who had a code status of DNR by the end of the trial. We defined “DNR status” using the “end-of-life decision-making” variable, which was recorded at the time of the completion of trial participation as (1) no DNR decision made (includes patients receiving aggressive management, possibly including failed CPR); (2) DNR decision made to withhold only CPR; (3) DNR decision made to withhold life support in addition to CPR; (4) DNR decision made to withdraw life support; (5) diagnosis of brain death; or (6) unknown. For our analysis, full code status was assigned to subjects who fell into categories 1 and 5, as a diagnosis of brain death with withdrawal of care was assumed to represent a prior commitment to full support. DNR status was assigned to categories 2–4. Category 6 was considered to be unknown code status, and these patients were excluded from further analyses. Secondary outcomes included factors associated with DNR status and 90-day mortality.
Statistical Methods
We used Wilcoxon rank-sum tests, t tests, chi-squared tests, and Fisher exact tests to determine differences in continuous and categorical variables. We compared prerandomization characteristics of patients who became DNR during the trial with participants who remained full code, using bivariate analyses. Baseline characteristics included place of residence before admission, origin of ICU admission (e.g., ward, emergency, operating room), demographics, body mass index, comorbidities, primary ARDS risk factor, laboratory values, vital signs, and ratio of PaO2 to fraction of inspired oxygen (FiO2). Double-sided α < 0.05 was used as our threshold for statistical significance. SAS version 9.3 software (SAS, Cary, NC) was used for all analyses. All study procedures were approved by the Boston University School of Medicine (Boston, MA) Institutional Review Board and the NHLBI Biologic Specimen and Data Repository Information Coordinating Center.
Results
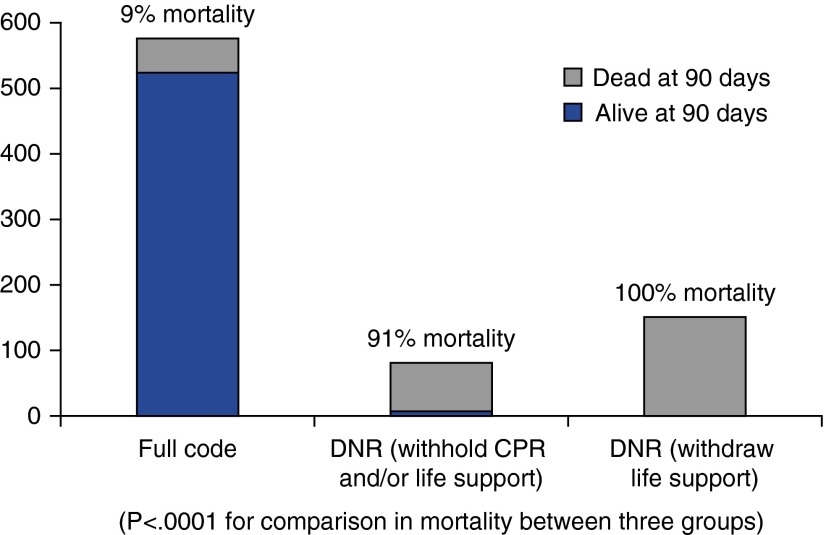
Among the 809 (81%) of 1,000 trial participants with a recorded code status, 232 (28.7%) were made DNR and 577 (71.3%) maintained full code status by the end of trial participation. Specifically, 37 (15.9%) had a directive to withhold CPR alone, 44 (19.0%) had a directive to withhold some life support measures in addition to CPR, and 151 (65.1%) had life support withdrawn. Among patients without documented end-of-life decisions, mortality was 4%. Subject characteristics stratified by code status at the end of trial participation are shown in Table 1. APACHE (Acute Physiology and Chronic Health Evaluation) III score was associated with DNR status in bivariate analysis (odds ratio, 2.20; 95% confidence interval [CI], 1.85–2.62; P < 0.0001). Of 577 patients remaining full code, 52 died (9% mortality; 95% CI, 7–12%), whereas 225 of 232 (97%; 95% CI, 94–99%) DNR patients died. Figure 1 demonstrates the percentage of participants who died, stratified by code status: although strikingly high, mortality among patients with DNR orders who did not withdraw care (91%; 95% CI, 82–96%) did not approximate the universally fatal outcome of patients with withdrawal of life support (100%; 95% CI, 97–100%) (P < 0.001). Of all patients who died in the trial, 225 of 284 (79%; 95% CI, 74–84%) were DNR.
Table 1.
Baseline variables
| Variable | Full Code (n = 577) | DNR (n = 232) | P Value |
|---|---|---|---|
| Age, yr | 47.1 ± 14.6 | 57.0 ± 17.4 | <0.001 |
| Sex, male | 308 (53.4%) | 128 (55.2%) | 0.64 |
| Race | 0.60 | ||
| White | 367 (63.6%) | 139 (59.9%) | |
| Black | 126 (21.8%) | 57 (24.6%) | |
| Other | 84 (14.6%) | 36 (15.5%) | |
| BMI, kg/m2 | 29.0 ± 7.6 | 27.4 ± 7.3 | <0.01 |
| Alcohol use (defined by protocol) | 50 (9.9%) | 24 (10.3%) | 0.56 |
| Origination of ICU admission | <0.001 | ||
| Emergency room | 217 (37.6%) | 70 (30.2%) | |
| Operating room | 63 (10.9%) | 9 (3.9%) | |
| Hospital floor | 129 (22.4%) | 90 (38.8%) | |
| Outside hospital | 128 (22.2%) | 42 (18.1%) | |
| Other | 40 (6.9%) | 21 (9.1%) | |
| Residence before hospitalization | 0.09 | ||
| Home (independently or with help) | 508 (93.4%) | 175 (90.2%) | |
| Skilled nursing facility or rehabilitation center | 15 (2.8%) | 12 (6.2%) | |
| Another acute care hospital or other | 21 (3.9%) | 7 (3.6%) | |
| ARDS risk factor | 0.68 | ||
| Direct lung injury (pneumonia or aspiration) | 349 (60.5%) | 144 (62.1%) | |
| Indirect | 228 (39.5%) | 88 (37.9%) | |
| APACHE III score | 89.1 ± 29.6 | 112.3 ± 29.2 | <0.001 |
| Baseline PaO2/FiO2 | 130.5 ± 59.1 | 119.7 ± 58.8 | 0.01 |
| Vasopressor need at enrollment | 163 (28.2%) | 112 (48.5%) | <0.001 |
| ICU readmission | 23 (4.0%) | 15 (6.5%) | 0.13 |
| Comorbidities | |||
| Cancer (leukemia, lymphoma, or solid tumor with metastases) | 17 (3.1%) | 26 (11.4%) | <0.001 |
| Immunodeficiency* | 61 (11.0%) | 57 (24.9%) | <0.001 |
| Prior stroke | 17 (3.3%) | 14 (7.3%) | 0.02 |
| Hypertension | 142 (27.4%) | 69 (36.1%) | 0.02 |
| Chronic pulmonary disease | 36 (6.9%) | 22 (11.5%) | 0.05 |
| Cardiovascular disease (CHF, prior MI, or peripheral vascular disease) | 54 (10.4%) | 14 (7.3%) | 0.22 |
| Liver disease (hepatic failure or cirrhosis) | 18 (3.3%) | 10 (4.4%) | 0.45 |
| Peptic ulcer disease | 21 (4.0%) | 13 (6.8%) | 0.13 |
| Diabetes mellitus | 98 (17.7%) | 50 (21.8%) | 0.18 |
| Lowest temperature from 24 h preceding randomization, °C | 36.7 ± 1.0 | 36.4 ± 1.0 | <0.001 |
| Highest temperature from 24 h preceding randomization, °C | 38.4 ± 1.0 | 38.2 ± 1.0 | 0.01 |
| Urine output/24 h preceding randomization, cm3 | 2,103.1 ± 1,601.6 | 1,772.8 ± 1,514.2 | 0.00 |
| Systolic blood pressure (just before randomization) | 95.1 ± 19.6 | 88.2 ± 16.6 | <0.001 |
| Heart rate (just before randomization) | 102.3 ± 21.5 | 103.4 ± 21.0 | 0.42 |
| Highest respiratory rate from 24 h preceding randomization | 34.7 ± 10.4 | 36.3 ± 9.8 | 0.04 |
| CVP (before first fluid management instruction), cm H2O | 12.1 ± 4.6 | 11.6 ± 5.0 | 0.13 |
| Creatinine (just before randomization) | 1.2 ± 0.8 | 1.5 ± 0.9 | <0.001 |
| Serum albumin (just before randomization) | 2.3 ± 0.6 | 2.1 ± 0.7 | <0.001 |
| Serum bicarbonate (just before randomization) | 22.5 ± 5.0 | 21.1 ± 5.3 | <0.001 |
| Hemoglobin (just before randomization) | 10.5 ± 1.9 | 10.2 ± 1.8 | 0.02 |
| Serum potassium (just before randomization) | 4.0 ± 0.6 | 4.1 ± 0.7 | 0.03 |
| Serum WBC (just before randomization) | 13.9 ± 11.5 | 13.5 ± 12.5 | 0.17 |
| Serum sodium (just before randomization) | 139.0 ± 5.1 | 138.6 ± 6.3 | 0.27 |
| Serum glucose (just before randomization) | 140.4 ± 77.0 | 142.9 ± 71.8 | 0.85 |
| Lowest platelet count from 24 h preceding randomization | 209.0 ± 129.6 | 184.7 ± 126.2 | 0.01 |
Definition of abbreviations: AIDS = acquired immunodeficiency syndrome; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; BMI = body mass index; CHF = congestive heart failure; CVP = central venous pressure; DNR = Do Not Resuscitate; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; MI = myocardial infarction; WBC = white blood cell count.
Immunodeficiency includes AIDS, recent chemotherapy or radiation, or immunosuppressive medications.
Figure 1.
Mortality by code status. CPR = cardiopulmonary resuscitation; DNR = Do Not Resuscitate.
Discussion
Our findings explore end-of-life decisions, presumably made by surrogate decision-makers in conjunction with ICU staff, among clinical trial patients with ARDS. We found that election of DNR status was associated with death in more than 90% of clinical trial participants with ARDS, even when life support was not withdrawn. As expected, greater baseline severity of illness as measured by APACHE III score was strongly associated with DNR decisions.
Although we explored a novel sample of patients with ARDS enrolled in a clinical trial and reported different gradations of DNR choices, the association between disease severity and DNR status in our study is similar to what has been observed in other ICU populations and, indeed, among patients with ARDS (10). Cook and colleagues showed that disease severity and increasing age were associated with DNR directives (11). Likewise, use of inotropes or vasopressors was strongly associated with the withdrawal of mechanical ventilation in a prior study of mechanically ventilated ICU patients (12). We did not identify an association between race and DNR orders, a finding that differs from prior work that has consistently shown that white patients are more likely to limit aggressiveness of care at the end of life (13–17). The reason for this is not clear, but it is possible that it is related to the nature of DNR decisions made in the context of a clinical trial.
Prior studies evaluating outcomes of DNR directives instituted in the ICU demonstrated hospital mortality rates ranging from 32 to 98% (12, 18–21). Most comparable to our study, Sinuff and colleagues identified a hospital mortality of 83% among mechanically ventilated patients with a DNR directive instituted more than 24 hours after ICU admission; mortality decreased to 65% when subjects from whom life support was withdrawn were excluded (12). By contrast, we observed greater 90-day mortality rates among clinical trial participants with ARDS: 97% among all DNR patients and 91% when excluding subjects from whom life support was withdrawn. The higher mortality observed in our study may relate to the focus on patients with ARDS, who typically have a higher fatality rate than other mechanically ventilated patients, or the measure of 90-day rather than hospital mortality.
However, another possibility for the higher mortality we observed is that factors associated with involvement in a clinical trial may have resulted in a higher “threshold” for initiating DNR directives. For example, patients (and/or their families) who decide to participate in a clinical trial may be inclined to pursue more aggressive care (5, 6). In addition, physicians may be less inclined to withdraw or withhold life support in patients enrolled in a clinical trial. This higher threshold for conversion to DNR status among clinical trial participants may explain the lower rate of DNR directives observed in our study of patients with ARDS (28%), as compared with an observational cohort of patients with ARDS (39%) (22). A reticence to initiate DNR orders in trial participants may result in physicians, patients, and their proxies waiting longer, until the patient is effectively moribund, before converting to DNR status. Indeed, because almost all DNR patients died in our study, and most DNR patients had life support withdrawn, we speculate that DNR decisions may have been “late” DNR decisions made during clinical decline, close to the time of death. Studies have demonstrated that the later in the clinical course a DNR decision is made, the more strongly it is correlated with mortality (23, 24). Our hypothesis is supported by findings of Turnbull and colleagues, which showed that patients with ARDS who failed to show improvement in Sequential Organ Function Failure Assessment (SOFA) scores had higher rates of life support limitation, and that DNR decisions were often made after 2–3 weeks for patients without improvement in SOFA score (10).
Although the high mortality among DNR patients supports the possibility that patients in a clinical trial had a “higher threshold” to initiate DNR status, most patients (79%) in our study who died had been converted to DNR status by the time of death. The 21% of patients who died with full code status is similar to the proportion observed in prior studies. For example, a large prospective multicenter survey study conducted in the mid-1990s found that 31% of patients who died in U.S. ICUs were full code at the time of death (1). Other work has shown that the number of critically ill patients who transition to DNR status before death is rising over time (1, 7, 25). Stapleton and colleagues investigated deaths due to ARDS at a single institution, and found a steady increase in the percentage of ARDS deaths occurring after withdrawal of life support over a period of two decades, culminating in 67% of ARDS deaths occurring after support withdrawal in 1998 (7). As a point of comparison, in the multicenter FACTT, 53% of deaths occurred after withdrawal of support. Our findings suggest that although thresholds to initiate DNR orders may be higher during a trial, physicians and surrogates were willing to limit aggressive measures, including CPR, in dying clinical trial participants.
These variable thresholds for de-escalation of aggressive care and the subjective definition of the “dying” patient raise the possibility of a significant unmeasured impact on both critical care clinical trial outcomes and—just as importantly—the management of individual patients. These issues might be remedied by a more formally protocoled approach to prognostication and consideration of life support withdrawal, along with robust documentation of the circumstances and timing of de-escalation of care during critical care trials. As an example, a multicenter randomized trial that addressed temperature management after cardiac arrest undertook both of these measures (26).
Our study has limitations. We excluded 19% of study subjects from analysis because of missing DNR status. The 90-day mortality among these 191 subjects was lower than that observed in both the full code and DNR groups. In addition, we lack temporal data on code status; the code status variable was recorded as a “snapshot” at the time of trial completion. Thus, we were unable to examine associations between time-varying clinical status and change in code status. Finally, although a “commitment to full support” was mandatory for inclusion in FACTT, subjects were allowed to enroll if they were committed to all forms of support except CPR. Thus, although we treated all DNR decisions as having been made during the trial, it is possible that some patients had limits on CPR when they entered the study. However, given the fact that all trial participants were receiving aggressive care including mechanical ventilation, we suspect that few patients were DNR at the time of study entry.
In conclusion, participants in FACTT who adopted DNR status had high mortality (>90%, regardless of “level” of DNR selected), which may be a function of both severity of illness and possibly a higher “threshold” for DNR status associated with involvement in a clinical trial. Despite this, few trial participants died without a DNR decision before death. Given the strong association between DNR status and death, robust monitoring and documentation of code status in future ICU clinical trials is warranted to better understand trial outcomes and to further study end-of-life care among the critically ill.
Acknowledgments
Acknowledgment
This manuscript was prepared using FACTT Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the FACTT investigators or the NHLBI. The authors acknowledge the work by the FACTT investigators, without which this work would not be possible.
Footnotes
Supported by grants K01 HL116768 and R21 HL112672 from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (A.J.W.). R.S.W. received support from NIH grant K07 CA138772 and the Department of Veterans Affairs.
Author Contributions: H.M.M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. H.M.M. contributed to the study design, analysis and interpretation of the data, and drafting and critical review of the manuscript and has seen and approved the final version. R.S.W. contributed to the study design, interpretation of the data, and drafting and critical review of the manuscript and has seen and approved the final version. A.J.W. contributed to the study conceptualization, study design, analysis and interpretation of the data, and drafting and critical review of the manuscript and has seen and approved the final version.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158:1163–1167. doi: 10.1164/ajrccm.158.4.9801108. [DOI] [PubMed] [Google Scholar]
- 2.Zingmond DS, Wenger NS. Regional and institutional variation in the initiation of early do-not-resuscitate orders. Arch Intern Med. 2005;165:1705–1712. doi: 10.1001/archinte.165.15.1705. [DOI] [PubMed] [Google Scholar]
- 3.Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146:573–582. doi: 10.1378/chest.13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabak YP, Johannes RS, Silber JH, Kurtz SG. Should Do-Not-Resuscitate status be included as a mortality risk adjustor? The impact of DNR variations on performance reporting. Med Care. 2005;43:658–666. doi: 10.1097/01.mlr.0000167106.09265.4e. [DOI] [PubMed] [Google Scholar]
- 5.Nurgat ZA, Craig W, Campbell NC, Bissett JD, Cassidy J, Nicolson MC. Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer. 2005;92:1001–1005. doi: 10.1038/sj.bjc.6602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore S. A need to try everything: patient participation in phase I trials. J Adv Nurs. 2001;33:738–747. doi: 10.1046/j.1365-2648.2001.01715.x. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 8.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42:296–302. doi: 10.1097/CCM.0b013e3182a272db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook D, Rocker G, Marshall J, Sjokvist P, Dodek P, Griffith L, Freitag A, Varon J, Bradley C, Levy M, et al. Level of Care Study Investigators and the Canadian Critical Care Trials Group. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349:1123–1132. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 12.Sinuff T, Cook DJ, Rocker GM, Griffith LE, Walter SD, Fisher MM, Dodek PM, Sjokvist P, McDonald E, Marshall JC, et al. Level of Care Study Investigators and the Canadian Critical Care Trials Group. DNR directives are established early in mechanically ventilated intensive care unit patients. Can J Anaesth. 2004;51:1034–1041. doi: 10.1007/BF03018494. [DOI] [PubMed] [Google Scholar]
- 13.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31(5) Suppl:S373–S378. doi: 10.1097/01.CCM.0000065121.62144.0D. [DOI] [PubMed] [Google Scholar]
- 14.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45:634–641. doi: 10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- 15.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139:1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29:1792–1797. doi: 10.1097/00003246-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24:695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azoulay E, Pochard F, Garrouste-Orgeas M, Moreau D, Montesino L, Adrie C, de Lassence A, Cohen Y, Timsit JF Outcomerea Study Group. Decisions to forgo life-sustaining therapy in ICU patients independently predict hospital death. Intensive Care Med. 2003;29:1895–1901. doi: 10.1007/s00134-003-1989-3. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay E, Metnitz B, Sprung CL, Timsit JF, Lemaire F, Bauer P, Schlemmer B, Moreno R, Metnitz P SAPS 3 Investigators. End-of-life practices in 282 intensive care units: data from the SAPS 3 database. Intensive Care Med. 2009;35:623–630. doi: 10.1007/s00134-008-1310-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen YY, Connors AF, Jr, Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133:1312–1318. doi: 10.1378/chest.07-1500. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JP, Ho KM, Webb SA. Outcome of patients who have therapy withheld or withdrawn in ICU. Anaesth Intensive Care. 2007;35:387–392. doi: 10.1177/0310057X0703500312. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull AE, Lau BM, Ruhl AP, Mendez-Tellez PA, Shanholtz CB, Needham DM. Age and decisions to limit life support for patients with acute lung injury: a prospective cohort study. Crit Care. 2014;18:R107. doi: 10.1186/cc13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenger NS, Pearson ML, Desmond KA, Brook RH, Kahn KL. Outcomes of patients with do-not-resuscitate orders: toward an understanding of what Do-Not-Resuscitate orders mean and how they affect patients. Arch Intern Med. 1995;155:2063–2068. [PubMed] [Google Scholar]
- 24.Shepardson LB, Youngner SJ, Speroff T, Rosenthal GE. Increased risk of death in patients with Do-Not-Resuscitate orders. Med Care. 1999;37:727–737. doi: 10.1097/00005650-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 25.McLean RF, Tarshis J, Mazer CD, Szalai JP. Death in two Canadian intensive care units: institutional difference and changes over time. Crit Care Med. 2000;28:100–103. doi: 10.1097/00003246-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]