Abstract
Rationale: The relationship between chronic obstructive pulmonary disease (COPD) and cognitive impairment in leading to disability has not been characterized.
Objectives: We aimed to investigate the prevalence and cumulative incidence of disability among adults with and without COPD and the association of COPD and cognitive impairment with disability.
Methods: We analyzed 2006–2008 waves of the Health and Retirement Study, a nationally representative longitudinal health survey. COPD was self-reported. Prevalent disability was defined as baseline dependency in one or more activities of daily living (ADLs) and incident disability as one or more additional ADL dependencies. We used a validated performance-based measure of cognition to identify dementia and mild cognitive impairment. Covariates included seven chronic diseases, four geriatric syndromes, and sociodemographics. We used logistic regression to test associations between COPD, cognitive status, and prevalent/incident disability.
Measurements and Main Results: Of 17,535 participants at least 53 years of age in wave 2006 (representing 77.7 million Americans), 9.5% reported COPD and 13.5% mild cognitive impairment; 17.5% of those with COPD had mild cognitive impairment. Prevalent disability for COPD was 12.8% (5.2% for no-COPD, P < 0.001). An additional 9.2% with COPD developed incident disability at 2 years (4.0% for no-COPD, P < 0.001). In adjusted models, COPD was associated with baseline (odds ratio, 2.0) and incident disability (odds ratio, 2.1; adjusted for baseline disability). Cognitive impairment had an additive effect to COPD. The COPD–disability association, prevalent/incident, was of similar or greater magnitude than that of other chronic diseases (e.g., stroke, diabetes). The associations were maintained in sensitivity analyses using alternative definitions of disability (dependency in two or more ADLs, dependency in instrumental ADLs), and in analysis excluding respondents with dementia.
Conclusions: Both COPD and mild cognitive impairment increase the risk of disability. The risk conferred by COPD is significant and similar or higher than other chronic diseases.
Keywords: chronic obstructive pulmonary disease, cognitive impairment, multimorbidity, disability, geriatrics
In studies on comorbidity and multimorbidity, disability is important both as a descriptor of a patient’s current health status and as an outcome resulting from the disease(s). According to survey data from the Census Bureau, 23.9% of Americans age 45–64 years and 51.8% of those 65 years and older reported disability (broadly defined) (1). In older adults, disability is often defined in terms of activities of daily living (ADLs) and instrumental ADLs (IADLs), with ADL and IADL dependences predictive of future care needs and nursing home admission (2, 3). ADL/IADL evaluation is based on the terminology and concepts derived from the International Classification of Functioning, Disability, and Health. ADLs represent an array of everyday tasks required for self-care, and IADLs are slightly more complex tasks required for domestic management and household tasks (4, 5). Limitations in ADLs were reported by 4.2% of Americans age 45–64 years and by 12.5% of those 65 years and older, and limitations in IADLs were reported by 6.0 and 19.1%, respectively (1).
Diabetes, heart disease, arthritis, and stroke are frequently cited as the chronic diseases strongly associated with the disabling process (1). In contrast, information about the role of chronic obstructive pulmonary disease (COPD) in disability is limited, even as the growing impact of the disease in other measures of burden of disease in America is well known; for example, COPD currently ranks third as a cause of death (6).
The impact of COPD is typically evaluated using physiological descriptors (lung function, walking distance); discrete clinical outcomes (exacerbations, death); or changes in health-related quality of life. The effect of COPD on functional outcomes, such as ADLs and IADLs, has been the subject of only a limited number of cross-sectional studies (7, 8) and longitudinal analyses (9, 10), which, overall, support the role of COPD and its comorbidities in the development of disability, using data from nonrepresentative cohorts, and different instruments and definitions of disability. Investigations of the association between COPD and disability using representative data from the whole American population are also limited (11). Cognitive impairment, an important comorbid geriatric condition (12), may play an additional role in contributing to disability in COPD (10, 13). However, the extent of the impact of both COPD and cognitive function on the disabling process at the population level remains to be examined.
In the current article, using data from a population-based longitudinal health interview study, we evaluated the relationship between COPD and cognitive impairment in contributing to disability in middle-aged and older adults. Our analyses are based on theoretical models of disability in COPD (14) modified by Locke and colleagues (10). The model describes the underlying disease (COPD), influenced by sociodemographic risk factors (age, sex, race, marital status), progressing through impairments (e.g., coexistent chronic diseases) and functional limitations (e.g., cognitive impairment, other geriatric conditions), and resulting in the development of disability. Our goals were to investigate (1) the prevalence and cumulative incidence of disability among American adults 53 years and older, with and without COPD, and (2) the association of COPD and cognitive impairment with disability.
Objective
Our objective was to compare the prevalence and cumulative incidence of disability in American adults 53 years and older with COPD with that of those without COPD, and to examine the association of COPD and mild cognitive impairment with prevalent and incident disability. We hypothesized that, first, adults with COPD, compared with those without, had a higher frequency of coexistent cognitive impairment and, second, both COPD and cognitive impairment were associated with higher risks of prevalent and incident disability.
Methods
Design
This study is a secondary analysis of data from a population-based, longitudinal health survey, the Health and Retirement Study (HRS).
Study Population
The HRS is a nationally representative, biennial longitudinal health interview survey performed by the Institute for Social Research at the University of Michigan (Ann Arbor, MI) and sponsored by the National Institute on Aging (National Institutes of Health, Bethesda, MD), with the main goal of studying adults as they transition into retirement. The HRS information is gathered through interviews performed every 2 years with about 22,000 Americans 50 years and older, and covers topics such as income, wealth, health, health services use, work, retirement, and family dynamics (http://hrsonline.isr.umich.edu/sitedocs/brochure/HRS-brochure.pdf). HRS data have been used previously for the evaluation of COPD, COPD outcomes, and sepsis (12, 15–17). The HRS was approved by the Health Sciences Institutional Review Board at the University of Michigan. Our analysis used data from participants 53 age years and older in the 2006 wave of the HRS and monitored through the 2008 wave. The 2006 wave included 17,535 participants in this age range, representing 77.7 million Americans in that year. The interview questions were answered by the participant or, in the event of medical or cognitive problems, by a proxy. Data used in the current project are publicly available and do not contain unique identifiers, and thus are exempt from institutional review board approval (http://www.hrpp.umich.edu/om/Part4.html).
Main Measures
Exposure variables included COPD and cognitive function. Participants self-reported COPD, based on their responses to the question “Has a doctor ever told you that you have chronic lung disease such as chronic bronchitis or emphysema (not including asthma)”? For self-respondents, cognitive function was determined in each wave using a validated performance-based instrument (scaled from 0 to 35), and for proxy respondents, with a derived instrument (scaled from 0 to 11). The HRS cognitive instrument (http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf) has been subject to intense construct, face, and criteria validation and compared with other instruments (18–21). Scores from both measures were classified as normal, cognitive impairment not dementia (hereafter referred as mild cognitive impairment), and dementia, based on values obtained from the Aging, Demographics, and Memory Study (21). Both COPD and cognitive function were determined at baseline (2006 wave) for all participants.
Outcomes: Baseline and incident disability.
The HRS includes items asking participants about five ADLs (bathing, dressing, eating, transferring, using the toilet) and five IADLs (using the phone, preparing meals, grocery shopping, managing money, managing medications). In a first step, participants are questioned about difficulty with each task and, in a different question, about requiring assistance from anybody else to perform each task. We defined dependency for each ADL and IADL as both having difficulty with the task (because of health or memory problems) and receiving assistance to perform it (22). We defined baseline disability in ADLs as the presence of one or more ADL dependencies in 2006, and incident disability as the development of one or more additional dependencies at follow-up (2008 wave). The ADL/IADL measures have been extensively validated and have appropriate reliability, as evaluated across multiple waves of the HRS(http://hrsonline.isr.umich.edu/sitedocs/userg/dr-008.pdf).
Covariates: Sociodemographic factors, chronic diseases, geriatric conditions.
We included covariates relevant to our model of disability, including those considered risk factors (sociodemographic factors), impairments (chronic diseases), and functional limitations (geriatric conditions). Age, sex, race (white, African American, Hispanic, other), marital status (married or unmarried), and years of education were used as sociodemographic descriptors. Hypertension, heart disease, diabetes, cancer, stroke, musculoskeletal conditions, and psychiatric diseases were considered present if they were severe or active enough as to be currently under treatment. Four geriatric conditions (falls, urinary incontinence, vision impairment, hearing impairment) were included if they were present in their most severe/active forms (e.g., two or more falls in the previous 2 yr; urinary incontinence, ≥15 d/mo).
Statistical Analysis
Respondents were analyzed according to their COPD status in 2006. Prevalence of disability was analyzed using 2006 data. Incident disability was defined and analyzed in terms of the 2-year cumulative incidence of disability using baseline (2006) and 2-year follow-up (2008) data. We calculated this stipulating as the population at risk all respondents from the 2006 wave who were alive at the time of the 2008 interview; we excluded respondents who died subsequent to the 2006 wave. We treated respondents lost to follow-up as deceased. (Note that we did define and analyze incidence in terms of cumulative incidence, because the survey is applied every 2 yr, and the exact time when a respondent developed a new dependency could not be known.) We used multivariate logistic models to evaluate the association of COPD status and mild cognitive impairment, two independent variables, with prevalent and incident disability (dependent variables). Separate logistic models were built introducing groups of variables: COPD, mild cognitive impairment, sociodemographic variables (risk factors), chronic diseases and geriatric syndromes (impairments and functional limitations in the model). Logistic models of incident disability were also adjusted for baseline disability. All models were evaluated by regression diagnostics, including tests for multicollinearity and interaction between COPD and mild cognitive impairment. To better understand the effect of COPD across groups of cognitive impairment, additional analyses were performed, excluding those with dementia. Likewise, to test the robustness of the findings, we performed extensive sensitivity analyses, using alternative definitions of disability (≥2 ADL dependencies, ≥1 IADL dependency, ≥2 IADL dependencies), and changing the at-risk population (considering respondents lost to follow-up as alive and having no additional disability, and considering deceased respondents as having no additional disability).
All analyses were weighted and adjusted to the complex survey design of the HRS, probability of selection and nonresponse, to generate national population estimates. All analyses were performed with Stata version 12 (Stata Corp., College Station, TX).
Results
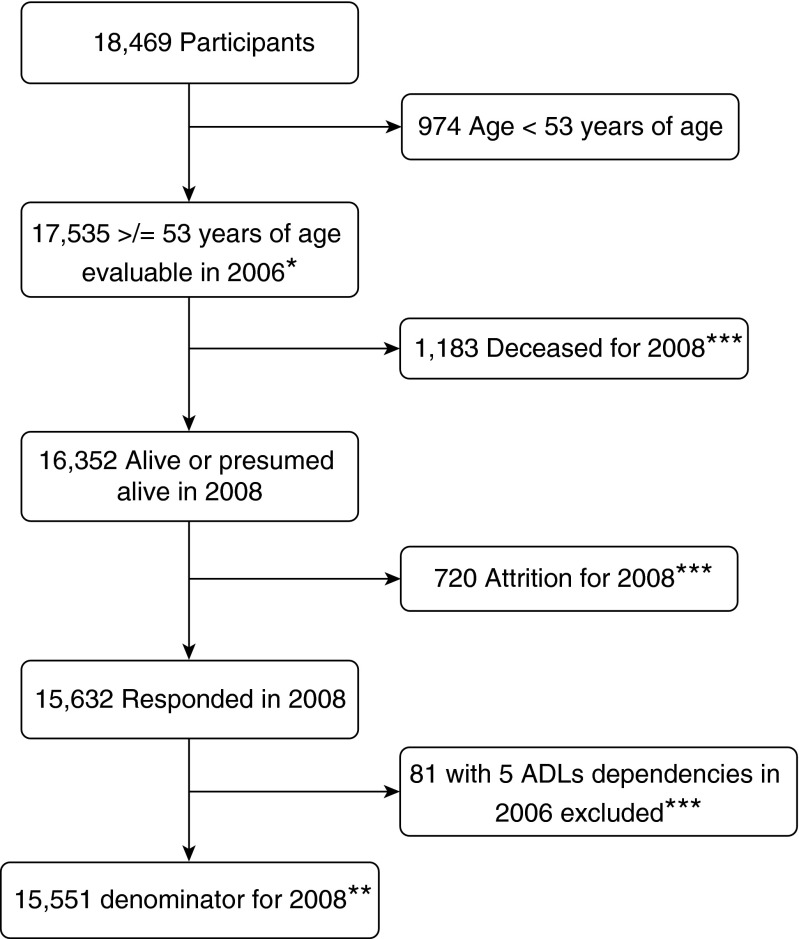
Figure 1 illustrates the flow of respondents in the 2006 wave of the HRS. Overall, COPD was reported by 10.3% (representing 7.4 million Americans). Those who reported COPD were more likely to be older, female, and unmarried and have less education and more comorbid diseases. Mild cognitive impairment was found in 13.5% of respondents (representing 10.5 million Americans). Mild cognitive impairment was more common among respondents with COPD than in those not having the disease (17.5 and 13.1%, respectively; P = 0.001). It is estimated that 1.3 million Americans have both COPD and mild cognitive impairment. There was no difference in the prevalence of dementia (approximately 4.5% for the whole population). The prevalence of disability at baseline (≥1 ADL dependency) was 12.8% for respondents with COPD (representing 940,000 Americans), compared with 5.2% for those without COPD (P < 0.001). We include more detailed information on the characteristics of the population according to COPD status (see Table E1 in the online supplement).
Figure 1.
Flow of participants in the Health and Retirement Study, 2006 wave. *Denominator for 2006 (prevalence). **Denominator for 2008 (incidence). ***Included in denominator for sensitivity analyses. ADLs = activities of daily living.
Table 1 shows the characteristics of four subgroups, according to the presence of COPD and the presence of at least one ADL dependency. Incident disability (that is, 2-yr cumulative incidence of at least one additional ADL dependency) was higher in respondents with COPD, irrespective of whether they reported disability at baseline. During the 2-year follow-up (2006–2008) 1,183 participants died (6.75% of initial respondents). Mortality was higher among those with baseline (2006 wave) disability, regardless of COPD status: 30.5% for those with disability but not COPD versus 29.4% for those with COPD and disability (P = 0.73).
Table 1.
Demographics, chronic diseases, geriatric syndromes, and incident disability according to chronic obstructive pulmonary disease and baseline dependencies among participants in 2006–2008 waves of the Health and Retirement Study*
| |
COPD and Disability |
COPD and No
Disability |
No-COPD and
Disability |
No-COPD and No
Disability |
|---|---|---|---|---|
| (n = 272, representing 0.94 million) | (n = 1,540, representing 6.46 million) | (n = 1,273, representing 3.67 million) | (n = 14,450, representing 66.66 million) | |
| Sociodemographics | ||||
| Age, yr: mean (SD) | 74.2 (10.8) | 70.3 (9.0) | 77.4 (11.8) | 68.7 (9.9) |
| Female sex, % | 69.6 | 57.6 | 63.1 | 53.1 |
| African American race, % | 10.6 | 6.7 | 16.5 | 9.0 |
| Unmarried, % | 45.9 | 44.8 | 41.3 | 40.0 |
| Education beyond high school, % | 27.9 | 33.8 | 32.0 | 50.7 |
| Currently smokes, % | 17.2 | 29.7 | 10.6 | 13.8 |
| Cognitive status, % | ||||
| Normal | 59.0 | 79.8 | 46.2 | 84.4 |
| Mild cognitive impairment | 25.4 | 16.5 | 25.4 | 12.4 |
| Dementia | 15.6 | 3.9 | 27.8 | 3.1 |
| Chronic diseases, % | ||||
| Hypertension | 74.6 | 53.5 | 64.2 | 45.4 |
| Diabetes | 39.3 | 16.4 | 28.0 | 13.5 |
| Heart disease† | 39.3 | 23.6 | 25.6 | 11.9 |
| Stroke | 15.6 | 5.4 | 17.8 | 2.4 |
| Cancer | 5.5 | 4.7 | 4.7 | 3.6 |
| Musculoskeletal disease | 49.2 | 33.7 | 42.4 | 20.8 |
| Psychiatric disease | 39.3 | 19.7 | 20.3 | 8.9 |
| Geriatric syndromes, % | ||||
| Vision problems | 55.7 | 31.3 | 46.2 | 17.1 |
| Hearing problems | 31.1 | 24.4 | 30.0 | 15.4 |
| Urinary incontinence | 30.3 | 11.7 | 19.7 | 5.2 |
| Falls | 27.9 | 12.0 | 27.8 | 6.2 |
| Baseline IADL dependency, % | ||||
| One or more IADL dependencies | 73.8 | 14.0 | 70.1 | 5.9 |
| Incident disability, %‡ | ||||
| One or more new ADL dependencies | 23.7 | 7.5 | 19.0 | 3.3 |
| One or more new IADL dependencies | 24.7 | 13.1 | 21.7 | 5.3 |
Definition of abbreviations: ADL = activity of daily living; COPD = chronic obstructive pulmonary disease; IADL = instrumental activity of daily living.
Note: n = 17,535.
Weighted percentages were derived using Health and Retirement Study respondent population weights.
Heart disease includes history of myocardial infarction, coronary artery disease, angina, and congestive heart failure.
After excluding those who were deceased or did not participate in the 2008 wave (n = 15,551 for 2008).
Next, we examined the association of COPD and cognitive impairment with the prevalence of disability at baseline (Table 2) and with the 2-year cumulative incidence of disability (Table 3). The unadjusted odds ratio for the association of COPD with disability, 2.6, was significant (model 1) and was maintained in models adjusted for cognitive function (model 2), cognitive function plus sociodemographic characteristics (model 3), and the fully adjusted model including cognitive function, sociodemographic characteristics, chronic diseases, and geriatric conditions (model 4). The fully adjusted models show that both COPD and mild cognitive impairment are associated with both prevalent disability (Table 2) and the 2-year cumulative incidence of disability (Table 3). For prevalent disability and for the 2-year cumulative incidence of disability, the associations with COPD and mild cognitive impairment were of similar magnitude to those for other chronic diseases, including cardiovascular disease, diabetes, and stroke. Of note, COPD–cognitive function interaction terms were not significant in fully adjusted models in the analyses of either the prevalent disability or the 2-year cumulative incidence of disability.
Table 2.
Multivariate models of prevalent disability among participants in the 2006 wave of the Health and Retirement Study
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
|---|---|---|---|---|
| COPD status | ||||
| COPD | 2.6 (2.2, 3.2) | 3.55 (2.7, 4.6) | 3.29 (2.5, 4.3) | 1.97 (1.5, 2.6) |
| Cognitive status | ||||
| Mild cognitive impairment | 3.7 (3.0, 4.5) | 2.8 (2.2, 3.5) | 2.11 (1.7, 2.6) | |
| Dementia | 15.9 (13.0, 19.6) | 10.3 (8.0, 13.2) | 7.1 (5.4, 9.3) | |
| Sociodemographics | ||||
| Age, by year increment | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.02) | ||
| Female sex | 1.5 (1.3, 1.8) | 1.4 (1.2, 1.7) | ||
| African American race | 1.4 (1.2, 1.8) | 1.3 (1.0, 1.7) | ||
| Unmarried | 1.3 (1.1, 1.5) | 1.4 (1.2, 1.7) | ||
| Education | ||||
| High school | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.2) | ||
| Beyond high school | 0.7 (0.6, 0.9) | 0.9 (0.7, 1.1) | ||
| Chronic diseases | ||||
| Hypertension | 1.20 (1.0, 1.4) | |||
| Diabetes | 1.8 (1.4, 2.1) | |||
| Heart disease | 1.3 (1.1, 1.6) | |||
| Stroke | 2.9 (2.3, 3.8) | |||
| Cancer | 0.9 (0.7, 1.4) | |||
| Musculoskeletal disease | 1.8 (1.5, 2.1) | |||
| Psychiatric disease | 1.7 (1.4, 2.2) | |||
| Geriatric conditions | ||||
| Vision impairment | 2.1 (1.8, 2.5) | |||
| Hearing impairment | 1.2 (0.9, 1.4) | |||
| Urinary incontinence | 2.2 (1.8, 2.8) | |||
| Falls | 2.1 (1.8, 2.6) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Note: n = 17,535. All measures of association represent odds ratio with 95% confidence interval in parentheses.
Unadjusted.
Adjusted for baseline cognition.
Adjusted for baseline cognition and five sociodemographic descriptors.
Adjusted for baseline cognition, five sociodemographic descriptors, seven chronic diseases, and four geriatric syndromes.
Table 3.
Multivariate models of incident disability among participants in the 2006 wave of the Health and Retirement Study*
| Model 1† | Model 2‡ | Model 3§ | Model 4|| | |
|---|---|---|---|---|
| COPD status | ||||
| COPD | 2.5 (2.0, 3.1) | 3.5 (2.6, 4.7) | 2.7 (1.9, 3.6) | 2.1 (1.6, 2.9) |
| Cognitive status | ||||
| Mild cognitive impairment | 4.2 (3.4, 5.2) | 2.3 (1.8, 2.9) | 2.1 (1.6, 2.7) | |
| Dementia | 15.5 (12., 19.6) | 5.9 (4.4, 8.0) | 5.3 (3.9, 7.1) | |
| Sociodemographics | ||||
| Age, by year increment | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.1) | ||
| Female sex | 1.2 (0.9, 1.4) | 1.2 (0.9, 1.5) | ||
| African American race | 1.0 (0.8, 1.3) | 0.9 (0.7, 1.2) | ||
| Unmarried | 0.9 (0.8, 1.2) | 1.0 (0.8, 1.2) | ||
| Education | ||||
| High school | 0.9 (0.8, 1.2) | 1.1 (0.9, 1.3) | ||
| Beyond high school | 0.7 (0.6, 0.9) | 0.8 (0.6, 1.0) | ||
| Chronic diseases | ||||
| Hypertension | 1.0 (0.8, 1.2) | |||
| Diabetes | 1.6 (1.3, 2.0) | |||
| Heart disease | 1.1 (0.9, 1.4) | |||
| Stroke | 1.6 (1.2, 2.3) | |||
| Cancer | 1.1 (0.8, 1.7) | |||
| Musculoskeletal disease | 1.4 (1.9, 1.8) | |||
| Psychiatric disease | 1.4 (1.1, 1.8) | |||
| Geriatric syndromes | ||||
| Vision impairment | 1.4 (1.2, 1.8) | |||
| Hearing impairment | 1.3 (1.0, 1.6) | |||
| Urinary incontinence | 1.0 (0.8, 1.3) | |||
| Falls | 1.6 (1.3, 2.0) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Note: n = 17,535. All entries represent odds ratio with 95% confidence interval in parentheses.
All models also adjusted for prevalent disability.
Unadjusted.
Adjusted for baseline cognition.
Adjusted for baseline cognition and five sociodemographic descriptors.
Adjusted for baseline cognition, five sociodemographic descriptors, seven chronic diseases, and four geriatric syndromes.
To better understand the combined effect of COPD and mild cognitive impairment on prevalent disability and on the 2-year cumulative incidence of disability we performed separate analyses excluding either respondents with dementia or with mild cognitive impairment. Table 4 shows the comparison between respondents with both COPD and mild cognitive impairment, respondents without COPD and with normal cognition, respondents without COPD but with mild cognitive impairment, and respondents with COPD and with normal cognition (excluding respondents with dementia), using as outcome prevalent disability. The effect of coexistent COPD and mild cognitive impairment was significant, with the risk of disability conferred by having both COPD and mild cognitive impairment (OR, 2.7) being greater than having COPD alone (OR, 1.9) or mild cognitive impairment alone (OR, 2.0). Similarly, the effect on incident disability (Table 5) was higher when COPD and mild cognitive impairment occur together (OR, 2.2), than when participants had either COPD (OR, 2.0) or mild cognitive impairment (1.9). We constructed similar models excluding respondents with mild cognitive impairment and keeping those with dementia, and found similar associations (Table E2).
Table 4.
Multivariate models of prevalent disability among participants in the 2006 wave of the Health and Retirement Study without dementia
| Model 1* | Model 2† | Model 3‡ | |
|---|---|---|---|
| COPD by cognition status | |||
| No COPD, normal cognition | Ref. | Ref. | Ref. |
| No COPD, mild cognitive impairment | 3.7 (3.0, 4.5) | 2.6 (2.0, 3.3) | 2.0 (1.6, 2.5) |
| COPD, normal cognition | 3.5 (2.7, 4.6) | 3.2 (2.4, 4.2) | 1.9 (1.4, 2.5) |
| COPD, mild cognitive impairment | 7.4 (5.3, 10.5) | 5.3 (3.7, 7.6) | 2.7 (1.8, 4.2) |
| Sociodemographics | |||
| Age, by year increment | 1.02 (1.01, 1.03) | 1.00 (0.99, 1.01) | |
| Female sex | 1.5 (1.2, 1.8) | 1.40 (1.1, 1.7) | |
| African American race | 1.6 (1.2, 2.0) | 1.4 (1.0, 1.8) | |
| Unmarried | 1.2 (1.0, 1.5) | 1.4 (1.2, 1.7) | |
| Education | |||
| High school | 0.7 (0.5, 0.9) | 0.8 (0.7, 1.1) | |
| Beyond high school | 0.6 (0.4, 0.7) | 0.7 (0.6, 0.9) | |
| Chronic diseases | |||
| Hypertension | 1.4 (1.1, 1.7) | ||
| Diabetes | 1.8 (1.4, 2.2) | ||
| Heart disease | 1.5 (1.2, 1.8) | ||
| Stroke | 3.1 (2.3, 4.2) | ||
| Cancer | 1.1 (0.7, 1.6) | ||
| Musculoskeletal disease | 2.0 (1.6, 2.4) | ||
| Psychiatric disease | 1.8 (1.4, 2.3) | ||
| Geriatric syndromes | |||
| Vision impairment | 2.0 (1.7, 2.5) | ||
| Hearing impairment | 1.2 (0.9, 1.5) | ||
| Urinary incontinence | 2.0 (1.6, 2.6) | ||
| Falls | 2.3 (1.8, 2.8) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Note: n = 16,164. All entries represent odds ratio with 95% confidence interval in parentheses.
Unadjusted.
Adjusted for five sociodemographic descriptors.
Adjusted for five sociodemographic descriptors, seven chronic diseases, and four geriatric syndromes.
Table 5.
Multivariate models of incident disability among participants in the 2006 wave of the Health and Retirement Study without dementia*
| Model 1† | Model 2‡ | Model 3§ | |
|---|---|---|---|
| COPD by cognition status | |||
| No COPD, normal cognition | Ref. | Ref. | Ref. |
| No COPD, mild cognitive impairment | 4.2 (3.4, 5.20) | 2.1 (1.6, 2.7) | 1.9 (1.5, 2.5) |
| COPD, normal cognition | 3.5 (2.6, 4.7) | 2.5 (1.8, 3.5) | 2.0 (1.5, 2.9) |
| COPD, mild cognitive impairment | 5.9 (3.9, 9.0) | 2.8 (1.8, 4.5) | 2.2 (1.4, 3.5) |
| Sociodemographics | |||
| Age, by year increment | 1.05 (1.04, 1.07) | 1.05 (1.03, 1.06) | |
| Female sex | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.4) | |
| African American race | 1.1 (0.8, 1.4) | 1.0 (0.8, 1.4) | |
| Unmarried | 0.9 (0.7, 1.1) | 0.9 (0.8, 1.2) | |
| Education | |||
| High school | 0.8 (0.6, 0.9) | 0.9 (0.7, 1.1) | |
| Beyond high school | 0.5 (0.4, 0.7) | 0.6 (0.5, 0.8) | |
| Chronic diseases | |||
| Hypertension | 1.1 (0.9, 1.4) | ||
| Diabetes | 1.7 (1.4, 2.2) | ||
| Heart disease | 1.2 (0.9, 1.5) | ||
| Stroke | 2.0 (1.4, 2.9) | ||
| Cancer | 1.1 (0.7, 1.7) | ||
| Musculoskeletal disease | 1.6 (1.3, 2.0) | ||
| Psychiatric disease | 1.5 (1.1, 2.0) | ||
| Geriatric syndromes | |||
| Vision impairment | 1.6 (1.3, 2.1) | ||
| Hearing impairment | 1.4 (1.1, 1.7) | ||
| Urinary incontinence | 1.2 (0.9, 1.6) | ||
| Falls | 1.7 (1.4, 2.2) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Note: n = 16,604. All entries represent odds ratio with 95% confidence interval in parentheses.
All models also adjusted for baseline disability.
Unadjusted.
Adjusted for five sociodemographic descriptors.
Adjusted for five sociodemographic descriptors, seven chronic diseases, and four geriatric syndromes.
Finally, we tested the robustness of the associations in sensitivity analyses using different definitions of disability and varying the definitions of the population at risk, to account for those who died or were lost to follow-up. When disability was defined as two or more ADL dependencies (Tables E3 and E4), COPD was still associated with prevalent disability (OR, 2.5) and the cumulative incidence of disability (OR, 2.2); likewise, mild cognitive impairment remained associated with prevalent disability (OR, 2.9) and the cumulative incidence of disability (OR, 2.9). Defining disability as two or more IADL (instead of ADL) dependencies (Tables E5 and E6) did not change the associations for either COPD with prevalent disability (OR, 1.5) or the cumulative incidence of disability (OR, 2.2) or for mild cognitive impairment with prevalent disability (OR, 2.6) or the cumulative incidence of disability (OR, 2.7). In sensitivity analysis of the cumulative incidence of disability, we considered respondents lost to follow-up as alive and having no additional disability, and we considered deceased respondents as having no additional disability, therefore including both groups in the denominator for cumulative incidence calculations; in these analyses, the associations were maintained both for COPD (OR, 2.2) and for mild cognitive impairment (OR, 1.9), as shown in Table E7.
Discussion
Our investigation of a nationally representative sample of middle-aged and older Americans finds evidence of a significant association between COPD and cognitive impairment with disability. At the population level, COPD is a risk factor for both prevalent and incident disability, an association as strong as or stronger than that for other chronic diseases classically associated with disability (heart disease, diabetes, stroke). We also confirmed the association between mild cognitive impairment and disability in adults with COPD, finding that mild cognitive impairment is common in patients with COPD and has an additive effect in the development of disability.
In the current study, adults with COPD had twice the prevalent disability and had twice the cumulative incidence of disability, compared with those without COPD. Our figures are similar to reports from Rodriguez-Rodriguez and colleagues (7), who found that, in a population-based sample of subjects with COPD in Spain, disability, defined by ADL dependency, occurred in 16.4% (15% in our study) and, defined by IADL dependency, occurred in 27.7% (21.5% in our study).
We also found that nonpulmonary factors, mild cognitive impairment in particular but also other geriatric conditions, contributed to the development of disability in COPD. Our findings are in line with those of Eisner and colleagues and Singer and colleagues, who reported that extrapulmonary factors (body composition, lower extremity strength) are associated with an increased risk of disability (9, 23). There are differences between our definition of disability and those of Eisner and Singer, related to differences in the study populations and the study outcomes of interest. Eisner and Singer studied younger and working adults, whereas the ADL-based definition in the current analysis is appropriate for older adults or when there is a societal perspective (e.g., ADL dependency leading to a need for long-term care) (2, 3, 24). Also, we tested alternative definitions of disability (higher number of ADL dependencies and IADL dependencies) and found the association to be maintained.
When disability is an outcome in older adults, a key confounder is mild cognitive impairment, which is common in patients with COPD (12). The HRS uses a performance-based measure of cognition, which has been validated at the national level (25) and which enables classification of different levels of impairment, (based on the Aging, Demographics, and Memory Study [ADAMS]) (21). We confirmed that mild cognitive impairment and dementia are frequent among older adults with COPD and that cognitive impairment is another factor leading to disability, with additive risk to COPD. Antonelli-Incalzi and colleagues showed, in a small cross-sectional study, that low cognitive function clustered with limitations to perform ADLs (13); we report similar associations. The association between mild cognitive impairment and COPD in the development of disability deserves the attention of clinicians and policymakers; we estimate that in 2006 there were nearly 1.3 million Americans with both COPD and mild cognitive impairment and 392,000 with COPD and dementia. These findings have clinical implications: COPD is a disease requiring skills in self-management. The presence of cognitive impairment can limit participation in disease management programs, a fertile but still underdeveloped area of clinical and implementation research in COPD in the United States (26, 27). Although most practitioners are familiar with and can recognize dementia, it is not clear that mild cognitive impairment is as readily identified.
One finding that deserves particular attention is that the association between COPD and disability is as strong as that for other chronic diseases, including stroke, diabetes, and heart disease. This finding is on par with the recognition of COPD as a major factor impacting health status at the population level, with associations also stronger than those reported for cardiovascular disease and diabetes (28). The biological plausibility of the COPD–disability association may be related to the idea that there are subgroups of subjects with COPD expressing more severe systemic consequences (29), probably mediated by systemic inflammatory pathways. However, although our findings are aligned with the current view of COPD as a disease with extrapulmonary effects, the elucidation of any mechanism involved is beyond the reach of this study.
Although there is growing interest in the investigation of multidimensional effects of COPD, there has been little research addressing the impact of COPD on disability (30). Disability, or even its descriptors such as ADLs or IADLs, is an infrequent outcome in COPD studies (31), and representative national data have rarely been used to test the impact of COPD on any form of disability (11). Overall, our results confirm that COPD is a disabling disease and that disability should be considered as a measure of the impact of the disease.
Some of the strengths of our analysis include the characteristics of the design of the HRS, not always available in other health interview surveys. The HRS includes relevant covariates whose effects are predicted by different models of disability (sociodemographics, presence of chronic diseases and geriatric conditions), and its focus is middle-aged and older adults, with questions addressing geriatric issues. The algorithms to adjudicate ADL and IADL dependencies have been widely used and validated (22). The sampling framework and representativeness of the HRS allow study findings to be generalized to the whole American population. In addition, the robustness of the associations, maintained through different sensitivity analyses, and the participation of subjects with a spectrum of disabling conditions (mild cognitive impairment, chronic diseases, geriatric conditions), frequently excluded from observational studies, provides additional support to our findings.
Potential limitations of the analysis include the lack of spirometry to classify the degree of obstruction, and the description of diseases based on self-report; however, the definition of COPD used here has been previously validated (32, 33). Another potential limitation is the selection of the model of disability used to outline the analysis, as there are many available theoretical models of disability, calling for the exploration of different covariates (34, 35). However, although the pathways leading to disability could be analyzed differently with each model, the one we selected is easy to interpret by the clinician and replicates and extends prior efforts within the COPD research community (10, 13). The inclusion of coexistent chronic diseases and geriatric conditions in our analytic model could be considered by some as a risk for overfitting the regression models, but at the same time this approach prevents the overestimation of the effect of COPD.
Another area of concern is the lack of a “gold standard” for disability, as proved by the different definitions found in the literature; however, we used one that has been validated as associated with relevant individual and policy outcomes (2, 3) and also performed sensitivity analyses using different definitions. In all the scenarios the effect of COPD was maintained and was as strong, if not stronger, than the effect of other chronic diseases. Finally, the HRS does not include the exact time when disability started, restricting our analysis to cumulative incidence, not incident rates.
Conclusions
We demonstrate, using nationally representative data from middle-aged and older Americans, that COPD is associated with prevalent and incident disability, with an effect of similar magnitude to other chronic diseases. Cognitive impairment, especially mild cognitive impairment, is also common in adults with COPD, and the coexistence of COPD and cognitive impairment increases the risk of disability. These data suggest that efforts to identify cognitive impairment and early deficits in ADLs may be as important as other areas of COPD care. To better identify these important risk factors and outcomes, changes at different levels of the health care process could be necessary, including education of pulmonary providers in the evaluation of geriatric aspects of the disease, such as cognition and disability. Our findings have implications for the design of preventive measures of disability and the design of self-management programs for patients with COPD. However, our findings also highlight the need for additional investigative efforts on the mechanisms and trajectories of disability in COPD.
Footnotes
Supported by grant NIH NHLBI T32HL007749-20 (C.H.M.), Department of Veterans Affairs Health Services Research and Development IIR 09-366 (C.R.R.), grant NHLBI K23 HL093351 (M.K.H.), and National Institute on Aging grant 5K08AG031837 and the Claude D. Pepper Older Americans Independence Center at the University of Michigan (C.T.C.). The National Institute on Aging provided funding for the Health and Retirement Study (U01 AG09740).
Author Contributions: C.H.M. and C.T.C. contributed to the conception, design, and analysis and interpretation of the data, and to the drafting of the article. C.R.R. and M.K.H. contributed to the conception and to the analysis and interpretation of the data. All authors revised the article critically for important intellectual content and gave final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1513/AnnalsATS.201405-187OC on October 6, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 2.Hopp FP. Patterns and predictors of formal and informal care among elderly persons living in board and care homes. Gerontologist. 1999;39:167–176. doi: 10.1093/geront/39.2.167. [DOI] [PubMed] [Google Scholar]
- 3.Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S.: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce B, Fries JF, Ambrosini D, Lingala B, Gandek B, Rose M, Ware JE., Jr Better assessment of physical function: item improvement is neglected but essential. Arthritis Res Ther. 2009;11:R191. doi: 10.1186/ar2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuben DB, Rosen S.Principles of geriatric assessment Halter J, Ouslander J, Tinetti M, Studenski S, High K, Asthana S.editors. Hazzard’s principles of geriatric medicine and gerontology, 6th ed New York: McGraw-Hill; 2009. Chapter 11 [Google Scholar]
- 6.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 7.Rodriguez-Rodriguez P, Jimenez-Garcia R, Hernandez-Barrera V, Carrasco-Garrido P, Puente-Maestu L, de Miguel-Diez J. Prevalence of physical disability in patients with chronic obstructive pulmonary disease and associated risk factors. COPD. 2013;10:611–617. doi: 10.3109/15412555.2013.781150. [DOI] [PubMed] [Google Scholar]
- 8.Katz PP, Gregorich S, Eisner M, Julian L, Chen H, Yelin E, Blanc PD. Disability in valued life activities among individuals with COPD and other respiratory conditions. J Cardiopulm Rehabil Prev. 2010;30:126–136. doi: 10.1097/HCR.0b013e3181be7e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner MD, Iribarren C, Blanc PD, Yelin EH, Ackerson L, Byl N, Omachi TA, Sidney S, Katz PP. Development of disability in chronic obstructive pulmonary disease: beyond lung function. Thorax. 2011;66:108–114. doi: 10.1136/thx.2010.137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke E, Thielke S, Diehr P, Wilsdon AG, Barr RG, Hansel N, Kapur VK, Krishnan J, Enright P, Heckbert SR, et al. Effects of respiratory and non-respiratory factors on disability among older adults with airway obstruction: the Cardiovascular Health Study. COPD. 2013;10:588–596. doi: 10.3109/15412555.2013.781148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton Snider J, Romley JA, Wong KS, Zhang J, Eber M, Goldman DP. The disability burden of COPD. COPD. 2012;9:513–521. doi: 10.3109/15412555.2012.696159. [DOI] [PubMed] [Google Scholar]
- 12.Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli-Incalzi R, Corsonello A, Trojano L, Acanfora D, Spada A, Izzo O, Rengo F. Correlation between cognitive impairment and dependence in hypoxemic COPD. J Clin Exp Neuropsychol. 2008;30:141–150. doi: 10.1080/13803390701287390. [DOI] [PubMed] [Google Scholar]
- 14.Jette DU, Manago D, Medved E, Nickerson A, Warzycha T, Bourgeois MC. The disablement process in patients with pulmonary disease. Phys Ther. 1997;77:385–394. doi: 10.1093/ptj/77.4.385. [DOI] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda T, Ojima T, Hayasaka S, Hagihara A, Takayanagi R, Nobutomo K. The health impact of remarriage behavior on chronic obstructive pulmonary disease: findings from the US Longitudinal Survey. BMC Public Health. 2009;9:412. doi: 10.1186/1471-2458-9-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. 2008;23:1757–1762. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 19.Blaum CS, Ofstedal MB, Liang J. Low cognitive performance, comorbid disease, and task-specific disability: findings from a nationally representative survey. J Gerontol A Biol Sci Med Sci. 2002;57:M523–M531. doi: 10.1093/gerona/57.8.m523. [DOI] [PubMed] [Google Scholar]
- 20.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57:163–172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 21.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NH, Hurd MD, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 22.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 23.Singer JP, Katz PP, Iribarren C, Omachi TA, Sanchez G, Yelin EH, Cisternas MG, Blanc PD. Both pulmonary and extra-pulmonary factors predict the development of disability in chronic obstructive pulmonary disease. Respiration. 2013;85:375–383. doi: 10.1159/000338110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland D. Measuring the elderly’s need for home care. Health Aff (Millwood) 1989;8:39–51. doi: 10.1377/hlthaff.8.4.39. [DOI] [PubMed] [Google Scholar]
- 25.Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, Wallace RB, Mucha LM, Straus WL, Fendrick AM. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, Thwin SS, Huang GD, Robbins R, Sriram PS, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Benzo R, Vickers K, Ernst D, Tucker S, McEvoy C, Lorig K. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil Prev. 2013;33:113–123. doi: 10.1097/HCR.0b013e318284ec67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janson C, Marks G, Buist S, Gnatiuc L, Gislason T, McBurnie MA, Nielsen R, Studnicka M, Toelle B, Benediktsdottir B, et al. The impact of COPD on health status: findings from the BOLD Study. Eur Respir J. 2013;42:1472–1483. doi: 10.1183/09031936.00153712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012;96:713–727. doi: 10.1016/j.mcna.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillum LA, Gouveia C, Dorsey ER, Pletcher M, Mathers CD, McCulloch CE, Johnston SC. NIH disease funding levels and burden of disease. PLoS One. 2011;6:e16837. doi: 10.1371/journal.pone.0016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janaudis-Ferreira T, Beauchamp MK, Robles PG, Goldstein RS, Brooks D. Measurement of activities of daily living in patients with COPD: a systematic review. Chest. 2014;145:253–271. doi: 10.1378/chest.13-0016. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DE, Powell-Griner E, Town M, Kovar MG. A comparison of national estimates from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System. Am J Public Health. 2003;93:1335–1341. doi: 10.2105/ajph.93.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121S–126S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 34.Masala C, Petretto DR. From disablement to enablement: conceptual models of disability in the 20th century. Disabil Rehabil. 2008;30:1233–1244. doi: 10.1080/09638280701602418. [DOI] [PubMed] [Google Scholar]
- 35.Oliver M. Theories in health care and research: theories of disability in health practice and research. BMJ. 1998;317:1446–1449. doi: 10.1136/bmj.317.7170.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]