Abstract
Background
Giardia is now considered the most common enteric parasite in well cared for dogs and cats in developed countries. The ecology, epidemiology and clinical impact of infections with this parasite in such animals is still not fully understood due to variable results across different studies.
Methods
Faecal samples were collected between 2009 and 2012 from privately owned cats and dogs in Germany presented to local veterinarians for a variety of reasons. Giardia positive samples were identified by microscopy and coproantigen methods. Total faecal DNA was extracted from Giardia positive samples and multilocus genotyping methods (18S rDNA, β-giardin, GDH) were applied. Relationships between host age, sex, and breed, season of presentation and the different species of Giardia detected were assessed.
Results
A total of 60 cat and 130 dog samples were identified as Giardia positive. Potentially zoonotic Giardia was identified in both animal species. Cats had a similarly high rate of infection with the G. duodenalis and G. cati. Cats less than 1 year were more likely to have G. duodenalis than cats older than 1 year. Pure breed cats demonstrated a greater proportion of zoonotic species than mixed breed cats. In samples from dogs, G. canis (C and D genotypes) were identified most commonly. Male dogs were more likely to have G. canis (genotype D) than female dogs. The 18S rDNA PCR protocol was the most successful followed by the β-giardin and GDH (amplifying from 92%, 42% and 13% of samples respectively).
Conclusions
The potentially zoonotic species G. duodenalis and G. enterica were found in cat and dog samples, with G. duodenalis found in greater numbers; however, this may be due to the detection techniques utilised. Cats appeared to show a relationship between G. duodenalis and G. cati with age and breed, which may be explained by different housing habitats for pure and mixed breed cats. The different success rates for the three loci utilised highlights the usefulness of the 18S locus as a screening tool, as well as the importance of using multiple loci for genotyping to fully determine the level of multiple infection of Giardia present.
Keywords: Giardia duodenalis, Giardia spp, Domestic dogs, Cats, Germany, Molecular epidemiology
Background
The only way to achieve a meaningful understanding of the ecology and epidemiology of Giardia infections is to obtain more data on the distribution of species and genotypes in well-defined host populations. A variety of molecular genotyping tools are available which can be used in multilocus studies to produce such data. Interpretation is not always clear cut and is open to differing hypotheses but it is only with the accumulation of such data that a better picture of the ecology of Giardia infections will be obtained. This is particularly the case for Giardia in companion animals, dogs and cats. Giardia is now the most common enteric parasite in well cared for dogs and cats in developed countries [1-3]. This raises questions about the clinical significance of Giardia infections, per se and in cases of polyparasitism, and if this varies between different breeds of hosts [4,5].
Since dogs and cats are susceptible to different species of Giardia which vary in zoonotic potential, it is also important to obtain data on the frequency of infection in urban areas. Many surveys to date have focused purely on the assessment of Giardia prevalence in dogs and cats [2]. Some have attempted to determine the prevalence of specific species and their genotypes in various populations, often with an emphasis on determining their zoonotic potential [6]. While many studies frequently identify host adapted species as the most prevalent species within populations of dogs there are also contrasting studies where a higher prevalence of potentially zoonotic species have been identified [7-9]. Studies on cats describe a higher prevalence of potentially zoonotic species [2,10]. Many of these studies, however, rely on a single genetic locus for characterisation of the infections present in these companion animals. There is already detailed cautionary criticism of such an approach [6,11,12]. In brief, it is evident that the use of small fragments of highly conserved genetic targets (18S rDNA) can result in the misidentification of isolates, while preferential amplification of a species at a single genetic locus can mean that heterogeneous (mixed) templates are not identified. The present study was undertaken to provide additional data on the situation in Germany. Multiple genetic loci (18S rDNA, β-giardin and Glutamate Dehydrogenase (GDH)) were utilised not only with a view to identify the frequency of zoonotic species in dogs and cats but also to determine the frequency and impact of host adapted species on cohorts within this population of well cared for animals.
A revised taxonomy for the genus Giardia has been developed over the last few years [13] based on the original host specificity recognised by early taxonomists and reinforced by more recent genetic characterisation and molecular epidemiological studies. A summary of the proposed taxonomic revision for the genus is shown in Table 1. In the current study the revised species nomenclature is used; however, where discussion involves the subtypes within these species the sub-assemblage terminology is applied as described by Caccio et al. [14].
Table 1.
Species of Giardia
| Species | Assemblage | Host(s) |
|---|---|---|
| G. duodenalis | A | Humans and other primates, dogs, cats, livestock, rodents and other wild animals. |
| G. enterica | B | Humans and other primates, dogs, cats, and some species of wild animals. |
| G. canis | C/D | Dogs and other canids |
| G. bovis | E | Cattle and other hoofed animals |
| G. cati | F | Cats |
| G. simondi | G | Rats |
Adapted from Thompson and Monis [13].
Methods
Sampling strategy
Between October 2009 and January 2012 faecal samples from privately owned dogs and cats were routinely examined for endoparasites by the commercial Veterinary Laboratory Freiburg (Germany). The distribution of submitting veterinary clinics is illustrated in Figure 1(a-b). Animals were presented to veterinarians for a variety of reasons including gastrointestinal disorders, routine examination and vaccination or general health checks. Age, breed and sex data of Giardia positive cats and dogs provided the basis to analyse any relationships between these factors and the presence of species of Giardia. Samples detected as positive for Giardia spp. were collected from dogs between September 2009 and March 2011 with sampling occurring continuously throughout this period. Samples detected as Giardia spp. positive were collected from cats between October 2009 and February 2012. Due to low numbers of cyst positive cat samples the study was extended; therefore, collection occurred more sporadically throughout the sampling period than for the dog samples.
Figure 1.
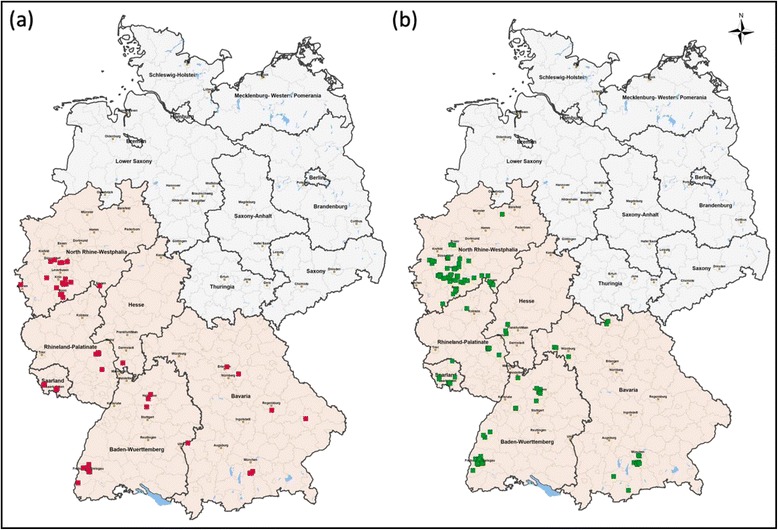
Geographical origin of animals positive for Giardia spp. by SAF technique and copro-antigen test. (a) Giardia positive cats (n = 60) and (b) Giardia positive dogs (n = 130).
Faecal examination
For detection of Giardia spp. samples were analysed by a coproantigen ELISA (ProSpecT® Giardia Microplate Assay, Remel Europe Ltd., distributed by Sekisui Virotech GmbH, Germany) as per manufacturer’s instructions or by sodium acetate formaldehyde SAF technique [15] to concentrate cysts of Giardia. Samples found positive for Giardia spp. by the identification of cysts and also by the coproantigen method were preserved in 70% ethanol for shipping.
DNA extraction
Samples preserved in 70% ethanol were received at Murdoch University (Western Australia) in three consignments. In total, 60 cat samples and 130 dog samples were received for Giardia genotyping. Upon arrival at Murdoch sub-samples were transferred into 1.7 ml microfuge tubes and pelleted, the supernatant was removed and discarded. Total DNA was extracted from the pellets using the Maxwell® 16 Tissue DNA Purification Kit (Promega, Madison, USA) with the Maxwell® 16 SEV Instrument (Promega) according to manufacturer’s instructions. Total DNA extracts were stored at -20°C until processing.
Amplification of 18S rDNA
Polymerase chain reactions (PCR) were carried out using 1 μl of both 1:4 diluted and neat total DNA template, 2.5 μl of 10 X reaction buffer, 2.5 μl of MgCl2 (25 mM), 0.1 μl Tth Plus DNA polymerase (Fisher Biotech Perth, Australia), 1 μl of dNTPs (10 mM) (Promega), 1 μl of each primer (10 μM), 5% dimethyl sulfoxide (DMSO)(Sigma-Aldrich St. Louis, Missouri) and water-ultra pure grade, to a final volume of 25 μl. The first-round PCR conditions were: 96°C for 5 min for 1 cycle, 96°C for 45 s, 50°C for 30 s and 72°C for 45 s for 35 cycles followed by 72°C for 7 min, using RH11, 5′- CATCCGGTCGATCCTGCC −3′ and RH4, 5′- AGTCGAACCCTGATTCTCCGCCAGG −3′ from Hopkins et al. [16]. Template for the secondary PCR consisted of 1 μl of first round PCR reaction. Where a particularly strong or double banded product was produced a 1:4 dilution of the primary template that was used. Second-round PCR conditions were: 96°C for 5 min for 1 cycle, 96°C for 45 s, 55°C for 30 s and 72°C for 45 s for 35 cycles followed by 72°C for 7 min. For dog samples PCR primers GiarF, 5′- GACGCTCTCCCCAAGGAC −3′ and GiarR, 5′- CTGCGTCACGCTGCTCG −3′ [17] were utilised. For cat samples a semi nested approach was used using the primary oligo RH11 as the secondary forward primer and GiarR as the secondary reverse, allowing for a longer secondary fragment at the 5′ end of the sequence, where crucial nucleotide polymorphisms for G. cati are found.
Amplification of β-giardin gene
PCR reactions used 1 μl of both the 1:4 diluted and neat DNA template, 2.5 μl of 10 X reaction buffer, 2.5 μl of MgCl2 (25 mM), 0.1 μl Tth Plus DNA polymerase (Fisher Biotech Perth, Australia), 1 μl of dNTPs (5 mM) (Promega), 1 μl of each primer (10 μM), 5% DMSO (Sigma-Aldrich St. Louis, Missouri) and water ultra-pure grade (Fisher Biotech Perth, Australia), to a final volume of 25 μl. The first round PCR conditions were: 95°C for 5 min for 1 cycle, 95°C for 30 s, 50°C for 30 s and 72°C for 60 s for 40 cycles followed by 72°C for 7 min, using primers G7 5′-AAGCCCGACGACCTCACCCGCAGTGC −3′ and G759 5′-GAGGCCGCCCTGGATCTTCGAGACGAC −3′ [18]. Again 1 μl from the first-round PCR was used as template for the secondary PCR. Secondary PCR conditions were: 96°C for 5 min for 1 cycle, 96°C for 45 s, 55°C for 30 s and 72°C for 45 s for 35 cycles followed by 72°C for 7 min using primers 5′- GAACGAACGAGATCGAGGTCCG −3′ and 5′-CTCGACGAGCTTCGTGTT −3′ [19].
Amplification of Glutamate Dehydrogenase gene (GDH)
PCR reactions used 1 μl of both the 1:4 diluted and neat DNA template, 2.5 μl of 10 X reaction buffer, 2.5 μl of MgCl2 (25 mM), 0.1 μl Tth Plus DNA polymerase (Fisher Biotech Perth, Australia), 1 μl of dNTPs (5 mM) (Promega), 1 μl of each primer (10 μM), 5% DMSO (Sigma-Aldrich St. Louis, Missouri) and water ultra-pure grade (Fisher Biotech Perth, Australia), to a final volume of 25 μl. The primary PCR conditions were: 94°C for 5 min for 1 cycle, 94°C for 30 s, 50°C for 30 s and 72°C for 60 s for 40 cycles followed by 72°C for 7 min. One micro litre from the first round PCR reaction was used in the second-round PCR. Cycling conditions for second round PCR were: 94°C for 5 min for 1 cycle, 94°C for 30 s, 60°C for 30 s and 72°C for 60 s for 40 cycles followed by 72°C for 7 min. The primary PCR used primers GDHeF, 5′- TCAACGTYAAYCGYGGYTTCCGT -3′and GDHiR 5′-GTTRTCCTTGCACATCTCC -3′. The secondary PCR reaction used GDHiF 5′- CAGTACAACTCYGCTCTCGG -3′ and GDHiR [20].
Sequencing
PCR products were purified using Agencourt® AMPure® XP PCR Purification (Beckman Coulter) in a 96 well format as per the manufacturer’s instructions. Sequence reactions were performed using the Big Dye Terminator Version 3.1 cycle sequencing kit (Applied Biosystems) according to manufacturer’s instructions. PCR products were sequenced with second round primers (1 μl [2.25 μM]). The cycling conditions for nucleotide sequencing were 1 cycle of 96°C for 2 min and 25 cycles at 96°C for 10 s, 50°C for 5 s and 60°C for 4 min. Reactions were electrophoresed on an ABI 3730 48 capillary machine. Sequence chromatograms were analysed using Sequencher® version 5.2 sequence analysis software (Gene Codes Corporation, Ann Arbour, MI USA (http://www.genecodes.com).
Species, subtype and genotype identification
To confirm the species (assemblage) sequences were aligned with published sequences as described previously [21]. To determine G. duodenalis subassemblage and subtypes, sequences were aligned and compared with published sequences as defined by Feng and Xiao [6] (Table 2). It should be noted that the fragment of the GDH gene amplified by the protocol utilised here does not exhibit enough sequence divergence to distinguish between G. duodenalis subtypes A1 and A5 within subassemblage AI. In the results this ambiguity has been noted for isolates falling within this grouping at the GDH locus. For G. enterica (assemblage B) confident grouping was possible to species level only. Sequence comparisons were made using reference sequences for the alloenzyme based subassemblage groups BIII and BIV [14,22]. Where different sequences contained heterogenous bases or where different loci produced incongruent results samples were considered to contain templates from mixed species, subassemblages or subtypes.
Table 2.
Subassemblage subtype reference sequences
| Species | Locus | ||
|---|---|---|---|
| G. duodenalis* | β-giardin | GDH | |
| Subassemblage AI | Subtype | ||
| A1 | X14185 | AY178735 | |
| A5 | DQ984131 | M84604 | |
| Subassemblage AII | A2 | AY072723 | AY178737 |
| A3 | AY072724 | EU278608 | |
| A4 | EF507657 | ||
| Subassemblage AIII | A6 | DQ650649 | DQ100288 |
| G. enterica # | |||
| Subassemblage BIII | AY072726 | AF069059 | |
| Subassemblage BIV | AY072725 | AY178738 | |
Statistical analysis
Differences in infection ratios between groups (species, breeds, sexes) were tested by Chi-squared analysis. To test whether the prevalence of mixed infections differed from an independent random distribution pattern, observed frequencies were compared with those expected under a multiple kind lottery (MKL) model [23] using Chi-squared analysis. All statistical tests were performed with JMP® version 4.0.4 (SAS Institute Inc., Cary, NC, 1989–2007), at an alpha level of 0.05.
Results
Sample distribution
A total of 60 cat and 130 dog faecal samples were identified as Giardia spp. positive by both microscopy and coproantigen test and submitted to Murdoch University for genotyping. The geographical origins of these Giardia positive samples are illustrated in Figure 1(a-b). Samples from dogs originated from 53 different clinics with 1 sample as the lowest number submitted by any individual clinic and 8 as the highest. Samples from cats originated from 31 different clinics with 1 as the lowest number submitted by any individual clinic and 7 as the highest. Data for Giardia negative animals was not available for this study; the following results are therefore based on analysis of those samples that were submitted for genotyping only.
A summary of the distribution of Giardia positive samples collected for both animal cohorts by sex, breed, symptoms and age is illustrated (Figure 2(a–d). Symptom assignment (symptomatic or asymptomatic) was based on clinical history where animals were noted as displaying any gastrointestinal dysfunction on presentation to the veterinary clinic.
Figure 2.
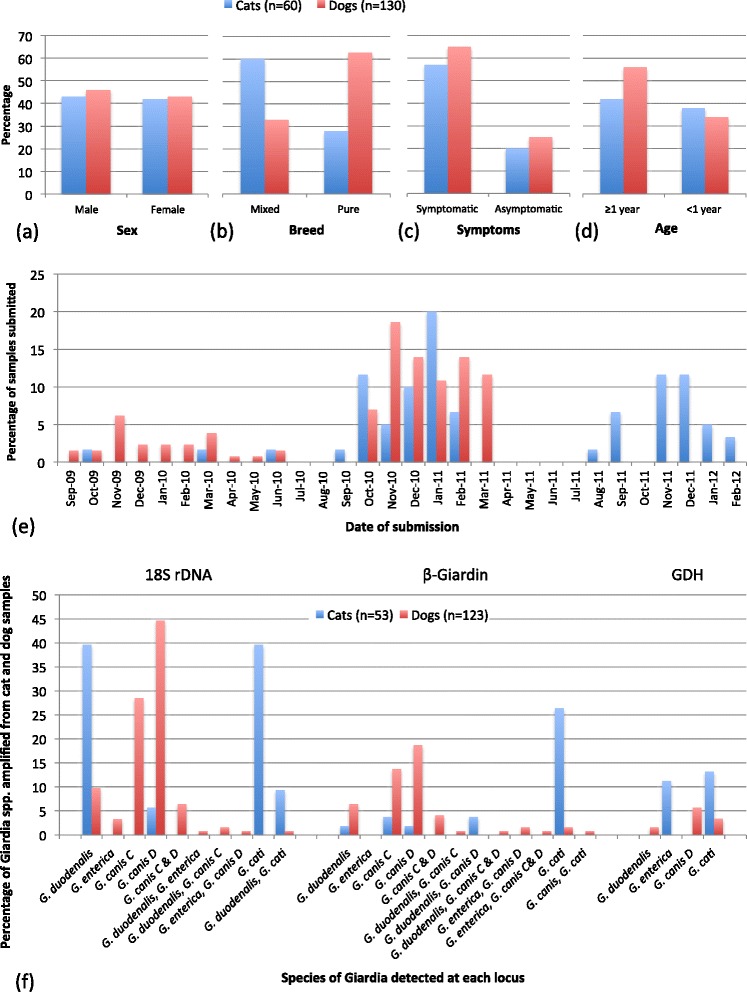
Distribution of samples from cats and dogs positive for Giardia spp. by microscopy and coproantigen. Percentage distribution is shown by: (a) sex, (b) Breed type, (c) Symptoms, (d) Age of animal, (e) Date of submission and (f) Species and mixed species combinations amplified at each locus used.
Samples from dogs, which were collected continuously throughout the study, demonstrated a marked fluctuation in season, with the submission of Giardia positive samples peaking in the autumn and winter months (Figure 2e). The cat samples were collected more sporadically throughout the sampling period; however, there was still a similar pattern identified with submission of Giardia positive samples also peaking in the autumn and winter months (Figure 2e). In addition to the seasonal peaks in Giardia there appeared to be relationships between the presence of Giardia cysts with age and with season in both cats and dogs. In the Giardia positive cats 62% of samples from younger animals were submitted in the autumn months while 65% of samples from older cats were submitted in the winter months (X2(3, N = 48) = 8.43, p = 0.04). In Giardia positive dogs overall no such trend was identified; however, in mixed breed dogs an opposite trend was identified with 61% of samples from younger animals submitted in the winter (X2(1, N = 39) = 3.13, p = 0.07). Significantly more polyparasitic infections (samples detected with concurrent infections of Giardia and one or more other parasites) were detected in dogs than in cats (X2 (1, N = 190) = 5.94, p = 0.02). In addition, Giardia positive dogs showed significant relationships between age and sex with polyparasitic infections, where younger animals (X2 (1, N = 117) = 5.5, p = 0.02) and female animals (X2 (1, N = 124) = 4.45, p = 0.04) bore a significantly greater number of mixed infections. This was the case for polyparasitism in general but particularly so for co-infections of Giardia spp. and Cystoisospora spp. in female dogs (X2 (1, N = 124) = 6.37, p = 0.01). In the cats there were too few samples to statistically test for risk factors of polyparasitism. Given the relationship between polyparasitism and sex in dogs, however, it is interesting to note that in the cats four out of the six samples identified with polyparasitic infections were in female cats. A summary of the polyparasitic infections identified in the cat and dog samples is presented (Table 3). Despite the low number of cat samples demonstrating polyparasitism with Giardia spp. a positive relationship between the presence of G. duodenalis (assemblage A) and co-infection with Cystoisospora spp. (X2(1, N = 45) = 3.36, p = 0.07) was also noted. There were no significant relationships between mixed infections of different Giardia spp. with any other factor.
Table 3.
Polyparasitic infections detected in dog and cat samples positive for Giardia spp
| Polyparasitic infections detected with Giardia sp. | Cats | Dogs |
|---|---|---|
| Hookworm* | 1 | |
| Toxascaris leonina | 2 | |
| Toxocara canis | 1 | 8 |
| Angiostrongylus vasorum | 1 | |
| Crenosoma vulpis | 2 | |
| Cystoisospora spp. | 4 | 6 |
| Hammondia/Neospora sp. | 1 | |
| Sarcocystis spp. | 2 | |
| Toxoplasma gondii | 1 | |
| A. vasorum, C. vulpis, Trichuris vulpis | 1 | |
| T. canis, Taenia spp. | 1 | |
| T. canis, T. vulpis. | 1 | |
| T. vulpis, Cystoisospora spp. | 1 | |
| Hookworm*, T. canis, Sarcocystis spp. | 1 | |
| Hookworm*, T. vulpis, Cystoisospora spp. | 1 | |
| Capillaria spp., Cystoisospora spp. | 1 | |
| Total | 6 | 30 |
*Ancylostoma or Uncinaria.
Molecular genotyping
Cats
Of the 60 cat samples submitted for genotyping, 88.3% (53) were typeable at one or more loci with the 18S rDNA being the most successful and the GDH the least (Figure 2(f)). Overall, using one or more loci, single species of Giardia were identified in 67.9% (36/53) of cat samples, with multiple species identified in the remaining 32.1% (17/53). In cat samples that were amplified at multiple loci the genotypes concurred in 48% (12/25) of cases (including instances where a second locus confirmed the genotype identified at the first while simultaneously detecting another genotype – mixed template) (Table 4). In the remaining 52% (13/25) the genotype amplified at a second locus differed to the species detected at the first locus (Table 4).
Table 4.
Summary of molecular genotyping results for Giardia in dogs and cats
| Host species | Sample size | Number amplified ≥1 loci | Number amplified ≥2 loci | Assemblage concurrence at multiple loci* | Assemblage divergence at multiple loci |
|---|---|---|---|---|---|
| Cats | 60 | 53 (88.3%) | 25 (41.7%) | 48% (n = 25) | 52% (n = 25) |
| Dogs | 130 | 123(94.6%) | 59 (45.4%) | 71% (n = 59) | 29% (n = 59) |
*Includes instances where a genotype identified at the first locus was again amplified at the second locus as part of a mixed sequence (indicating a mixed template). Figures are expressed as a percentage of the number of samples amplified at ≥2 loci.
Of the 53 samples that were successfully amplified, 18S rDNA PCR sequencing yielded genotype information for 94.3% (50/53), β-giardin PCR sequencing for 39.6% (21/53) and GDH PCR sequencing produced limited results with genotype information for only 24.5% (13/53) cat samples. The species and genotypes of Giardia amplified at each locus are summarised in Figure 2f. Sub-genotype information was obtained for 4 cat samples at the β-giardin and 6 at the GDH loci (Tables 5 and 6); however, due to the heterogeneity present at the GDH it was not possible to unequivocally assign a subtype to these isolates (Table 6).
Table 5.
Species and sub-genotypes for 53 cat and 123 dog samples at one, two or three loci
| Host species | Single locus amplifications (n) | Multiple locus amplifications (n) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18S | GDH | β-giardin | 18S | GDH | 18S | β-giardin | 18S | GDH | β-giardin | |||||||
| Cats | A | (13) | BIV | (2) | C | (1) | A,F | F | (1) | A | A1 | (1) | F | BIV | F | (3) |
| D | (3) | F | BIV | (1) | A | C | (1) | F | F | F | (3) | |||||
| F | (9) | F | F | (2) | A | D | (1) | A | F | F | (1) | |||||
| A | F | (4) | ||||||||||||||
| A,F | A2,D | (1) | ||||||||||||||
| A,F | A2,F | (1) | ||||||||||||||
| A,F | F | (2) | ||||||||||||||
| F | A1,D | (1) | ||||||||||||||
| F | F | (2) | ||||||||||||||
| Dogs | A | (8) | F | (1) | C | (1) | D | D | (1) | A | A5 | (1) | A | A1 | A1 | (1) |
| A,B | (1) | D | (1) | A | BIII,D | (1) | A | A1 | A5 | (1) | ||||||
| A,C | (1) | D | (1) | A,F | C | (1) | A,C | F | C | (1) | ||||||
| B | (3) | B | C,D | (1) | D | D | A2 | (1) | ||||||||
| C | (18) | C | A | (1) | D | D | C,D | (1) | ||||||||
| C,D | (5) | C | A1 | (1) | ||||||||||||
| D | (24) | C | BIII,D | (1) | ||||||||||||
| C | C | (13) | ||||||||||||||
| C | D | (1) | ||||||||||||||
| C,D | A1,C | (1) | ||||||||||||||
| C,D | C,D | (2) | ||||||||||||||
| D | A5 | (2) | ||||||||||||||
| D | C | (1) | ||||||||||||||
| D | C,D | (1) | ||||||||||||||
| D | D | (20) | ||||||||||||||
| D | A,C,D | (1) | ||||||||||||||
| D,B | BIII,C,D | (1) | ||||||||||||||
| D | D,F | (1) | ||||||||||||||
| D | F | (2) | ||||||||||||||
A = G. duodenalis, B = G. enterica, C and D = G. canis, F = G. cati. Heterogeneous sequences identified at a single locus are identified by letters separated by commas. The figure in parentheses indicates the number of samples with this result. For example in the first row 13 cats were detected with G. duodenalis only at the 18S, 2 cats were detected with G. enterica (BIV) only at the GDH, 1 cat was detected with G. canis (C genotype) only at the β-giardin, 1 cat was detected with both G. duodenalis and G. cati at the 18S as well as G. cati only at the GDH, 1 cat was detected with G. duodenalis at both the 18S and the GDH and finally 1 cat was detected with G. cati at both the 18S and the β-giardin while in the same sample G. enterica (BIV) was detected only at the GDH.
Table 6.
Sub-genotypes of G. duodenalis and G. enterica detected in cat and dog samples
| Sub-genotypes detected | Cats | Dogs |
|---|---|---|
| G. duodenalis (Assemblage A) | ||
| subtype A1 | 2 (β-giardin) | 3 (β-giardin) |
| subtype A2 | 2 (β-giardin) | 1 (β-giardin) |
| subtype A5 | 4 (β-giardin) | |
| subtype A1/A5 | 2 (2 GDH) | |
| G. enterica (Assemblage B) | ||
| subassemblage BIII | 3 (β-giardin) | |
| subassemblage BIV | 6 (GDH) |
Overall, across all three loci the most commonly detected species in the 53 cat samples was G. cati 56.6% (30/53), followed by G. duodenalis 50.9% (27/53) and then low levels of G. enterica 11.3% (6/53), G. canis (genotype D) 11.3% (6/53) and G. canis (genotype C) 3.7% (2/53) (Table 5).
Mixed Giardia spp. included 10 samples with G. duodenalis and G. cati (one of these also included G. canis (D)), 4 samples included a mix of G. enterica with G. cati and 3 samples included a mix of G. cati with G. canis (C in one and D in two samples) (Table 5). There were no strong correlations between the presence of mixed species of Giardia with any other factor.
Dogs
Of the 130 dog samples submitted for genotyping, 94.6% (123) were typeable at one or more loci. As with the cat samples the 18S rDNA was the most successful and the GDH the least (Figure 2(f)). Overall, using one or more loci, single species or genotypes (including mixed G. canis C or D genotypes or mixed G. duodenalis subtypes) were identified in 75.6% (93/123) dog samples, with multiple species or genotypes identified in the remaining 24.3% (30/123). In dog samples that amplified at multiple loci the species or genotypes detected concurred in 71.2% (42/59) of cases (Table 4). In the remaining 28.8% (17/59) the species or genotypes amplified at a second locus differed to the genotype detected at the first (Table 4).
Of the 123 dog samples that were successfully amplified, 18S rDNA PCR sequencing was able to genotype 95.9% (118/123), the β-giardin PCR yielded genotype information for 48.7% (60/123) and the GDH PCR again produced limited results with genotype information for only 5.7% (7/123). Sub-genotype information was acquired for 11 dog samples at the β-giardin locus and 2 at the GDH locus. Again due to the heterogeneity present at the GDH it was not possible to unequivocally assign a subtype to these isolates (Tables 5 and 6).
Overall, across the three loci the most commonly detected species in the 123 dog samples was G. canis (D) 56.1% (69/123), followed by G. canis (C) 42.2% (52/123), and then lower levels of G. duodenalis 19.5% (24/123), G. enterica 6.5% (8/123) and G. cati 4.9% (6/123).
The presence of mixed Giardia spp. was detected in 15 dog samples, these included 13 samples with one or both of the zoonotic species (mainly G. duodenalis) with G. canis (genotype C or D or C and D) and 2 samples included a mix of G. canis (D or C and D) with G. cati (Table 5). There were no strong correlations between the presence of mixed Giardia spp. with any other factor although there was a trend for samples from female dogs to contain more mixed species (X2 (1, N = 117) = 3.28, p = 0.07). A mix of the two G. canis genotypes was detected in 15 (Table 5) dog samples.
Host adapted assemblages
Age and sex were identified as significant factors for the presence of the host adapted G. cati in samples from mixed breed cats only. Older and male cats in the mixed breed group had a significantly higher prevalence of this species of Giardia (X2 (1, N = 28) = 4.37, p = 0.04) and (X2 (1, N = 30) = 5.79, p = 0.02) respectively, than their younger or female counterparts. Due to the low number of samples it was not possible to carry out multivariate analyses on this data to determine a more complex relationship between the presence of G. cati with sex, age or breed in this group of animals.
Of the two recognised genotypes of G. canis (C and D) samples from male dogs had a significantly greater proportion of the D genotype detected in comparison to female dogs (X2 (1, N = 117) = 4.45, p = 0.04). This significance strengthened when only pure breed dogs were considered (X2 (1, N = 73) = 4.77, p = 0.03). An opposite trend was detected with the C genotype occurring in a greater proportion of samples from female pure breed dogs than from males (X2 (1, N = 72) = 3.56, p = 0.06). There was no similar correlation for the C genotype in the smaller cohort of mixed breed dogs. In addition, overall there appeared to be a negative association with the C genotype and symptoms (X2(1, N = 110) = 4.55, p = 0.03). Given the prevalence of G. canis C and D genotypes detected in the dog samples (40% and 53% respectively), under an MKL model the expected prevalence of a mixture of C and D genotypes in the dog samples would be 21%. The actual prevalence of such a mix was significantly lower at 11.5%, (X2 (1, N = 130) = 6.45, p = 0.01).
Zoonotic species
Due to the low number of G. enterica positive samples identified, the significance of zoonotic species was examined as a whole (both G. duodenalis and G. enterica together) or with G. duodenalis considered alone. A significantly greater proportion of the potentially zoonotic species were detected in the cats than in the dog samples (X2 (1, N = 174) = 21.87, p = <0.01). In both hosts zoonotic species of Giardia were as likely to be identified in samples with mixed genotypes as in those with only single genotypes detected. For the cat samples there was an association with the presence of potentially zoonotic Giardia spp. with age. Cats ≤1 year were significantly more likely than cats >1 year to harbour either of the two zoonotic species (X2 (1, N = 40) = 5.63, p = 0.02).
G. enterica alone was identified in too few samples to determine any significance with any particular factor in either of the animal groups. It is worth noting, however, that in both cats and dogs the greater proportion of samples identified with G. enterica came from animals ≤1 year (5/6 and 5/8 respectively). G. enterica was identified in 3 dogs all amplifying with the β-giardin PCR only. These sequences matched most closely to the BIII subassemblage grouping (Table 6). Conversely the 6 cats identified with G. enterica were identified with the GDH PCR only and all of these matched most closely to the BIV subassemblage grouping (Table 6). Nucleotide changes from subassemblage B sequences from both the β-giardin and GDH PCRs are presented in Table 7. Subtype information at multiple loci was obtained for 2 dog samples. Both grouped in subassemblage AI, one sample with subtypes A1 and A1/A5 and the second with A5 and A1/A5 at the β-giardin and GDH locus respectively.
Table 7.
Nucleotide changes in G. enterica subassemblage B isolates from cat and dog samples
| Isolate | GDH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 309 | 356 | 357 | 429 | 447 | 456 | 482 | 502 | 540 | 561 | 572 | 577 | 606 | 608 | 612 | |
| STBIII reference sequence | C | T | T | T | T | G | T | G | C | C | T | G | C | T | G |
| STBIV reference sequence | T | . | . | C | C | . | . | . | T | T | . | . | . | . | A |
| 1116074 | T | C/T | C | C | C | A | C/T | . | T | C/T | . | . | C/T | . | A/G |
| 1201420 | T | . | C | C | C | A | . | . | T | . | . | . | . | . | A |
| 1116246 | . | . | C | C | C/T | A/G | . | . | C/T | . | C/T | . | . | C/T | A |
| 1114218 | T | . | C | C | C | A | . | A | T | . | . | . | . | . | . |
| 1111290 | T | . | C | C | C | A | . | . | C/T | . | . | . | . | . | . |
| 1111426 | T | . | C | C | C/T | A/G | . | . | T | C/T | . | A/G | T | . | A/G |
| β-giardin | |||||||||||||||
| 165 | 171 | 189 | 234 | 249 | 264 | 288 | 315 | 318 | 396 | 399 | 549 | ||||
| STBIII reference sequence | G | C | A | G | C | G | C | C | C | C | C | C | |||
| STBIV reference sequence | . | T | . | A | . | . | T | T | T | . | T | . | |||
| 1015139 | A | C/T | . | A | . | A/G | . | C/T | T | C/T | . | C/T | |||
| 1102417 | . | . | A/G | A | . | . | . | . | T | . | . | C/T | |||
| 1014340 | . | . | . | A | T | . | . | . | T | . | . | . | |||
In both cats and dogs there was a trend (significant in dogs) for G. duodenalis in particular to be identified more often in female animals, (X2 (1, N = 46) = 3.14, p = 0.08) and (X2 (1, N = 114) = 4.23, p = 0.04), in cats and dogs respectively. For the cat samples there was also an association with breed. There was a trend approaching significance in pure breed cats, which appeared to harbour a greater proportion of zoonotic species (X2 (1, N = 44) = 3.73, p = 0.05) in comparison to mixed breed cats. All G. duodenalis subassemblage sequences matched with 100% identity to their respective reference sequences.
PCR Amplification success of Giardia species in dogs and cats
The cat samples demonstrated a significantly higher PCR amplification failure rate at the 18S rDNA locus than dog samples 11/60 (18%) and 11/130 (8.5%) respectively (X2(1, N = 181) = 8.29, p = <0.01). There was a similar trend although not significant for the β-giardin PCR, with a failure rate of 39/60 (65%) and 69/130 (53%) for cat and dog samples respectively (X2(1, N = 183) = 3.10, p = 0.08). The reverse was true, however, for the GDH PCR where despite having a very high rate of failure in both species, the cat samples were more successfully amplified by this assay with 47/60 (78%) failing compared to 123/130 (95%) in the cats and dogs respectively (X2(1, N = 178) = 9.87, p = <0.01).
Mixed genotypes and/or species were detected in 49 samples in total (including samples with mixed G. canis C and D genotypes) and these were identified at all three loci to varying degrees (Table 5). As discussed previously, 14 of these samples were identified with heterogeneous sequences at a single locus, 10 (22%) at the 18S rDNA locus and 4 (7%) at the β-giardin locus. The remaining 35 samples were identified by the combined amplification at a second or third locus. Twenty five (51%) were identified with a combination of the 18S and the β-giardin PCRs and 2 (4%) by a combination of the 18S and GDH PCRs. The remaining 6 (12%) samples were identified by a combination of all three methods (Table 5).
The effectiveness of each PCR protocol to amplify each Giardia species was variable. A comparison of the number of times each PCR amplified each species as a percentage of the total species identifications (across all three PCR loci) is illustrated (Figure 3). Despite amplifying at least one species or genotype from 92% of all the samples submitted, the 18S rDNA PCR, performed comparatively poorly with G. enterica isolates amplifying from 6 of the 14 samples (43%) detected with this species. The second most successful protocol used, the β-giardin PCR (amplifying from 42% of samples overall), not only performed poorly when amplifying G. enterica (3/14, 14%) but in addition amplified from only 14 of a possible 50 G. duodenalis positive samples. The GDH protocol, despite being the least successful method utilised here, (amplifying only 13% of the total number of samples submitted) was comparable or better than the two other loci when amplifying G. enterica, equalling the 18S PCR in detecting 6 of 14 possible samples (42%) identified with this species. Interestingly, in both dogs and cats a trend showing that samples from males were more likely to amplify than samples from females was evident for the GDH PCR. When both animal species were combined this trend became significant (X2(1, N = 164) = 4.38, p = <0.04).
Figure 3.
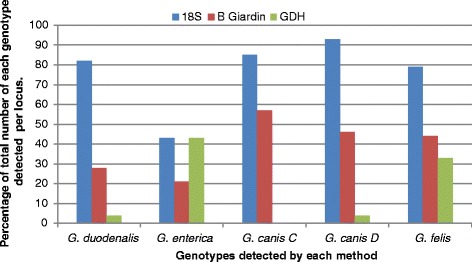
The relative effectiveness of each PCR protocol utilised at amplifying each Giardia sp. Effectiveness of each protocol is estimated by comparing the number of samples in which each protocol amplified a particular species compared to the total number of samples in which that species was amplified across all of the three protocols utilised.
Discussion
Previously Giardia has been identified as the most prevalent parasite found in both cats and dogs (12.6% and 18.6% respectively) in this geographical area [3]. This is one of the highest prevalence rates across several European countries [24]. Whilst in the current study data to determine the overall prevalence of Giardia was not collected, a stable prevalence of endoparasites in dogs and cats from the same area in Germany has been demonstrated for nearly 10 years [3,25].
The bias towards samples from symptomatic animals is probably a reflection of the sampling strategy of this study, in that animals were sampled once presented to a veterinarian rather than as part of a cross sectional survey. In addition, the variation between the two animal species with respect to breed (in cats more Giardia spp. positive samples were collected from mixed breed animals, while in dogs more were collected from pure breed animals) is likely to be a reflection of the distribution of these breed types within each of the two populations of well cared for cats and dogs. This is in contrast to one previous study which noted a reduced prevalence of Giardia spp. in mixed breed dogs [26]. There is substantial evidence supporting the increased susceptibility to Giardia spp. in younger dogs and cats [2,27] and this factor would account for the greater representation of dogs ≤1 year in this study although this was not demonstrated in the cat samples.
The overall seasonal distribution for both Giardia positive cats and dogs in this study closely corresponds to that of an earlier study in Germany [3]. Similar seasonal patterns have been identified in the USA and Argentina [2] with peaks in prevalence in the winter months, although this is not the case in other European based studies [28-30]. The reasons for such a persistent and striking seasonal fluctuation for Giardia spp. in well cared for dogs and cats in Germany, while neighbouring countries with a similar climate fail to show similar associations remain unclear.
The possibility of a relationship between younger cats and sample submission during autumn months is interesting, but this should be regarded with caution. Giardia positive cat samples were difficult to obtain and sampling for this cohort was not consistent throughout the study period. There did not appear to be a clear correlation between sample submissions in autumn or winter with the species of Giardia detected despite positive relationships between younger cats and the potentially zoonotic G. duodenalis and G. enterica, and similarly in older cats with the host adapted species G. cati. Such a relationship should be investigated with sufficient samples to enable multivariate analysis.
Previously, analysis of endoparasite infection in well cared for dogs and cats in Germany identified an increased prevalence of infection in animals less than one year of age [27]. This was also reflected here with a positive relationship observed between young dogs and polyparasitic infections with Cystoisospora spp. and Toxocara canis being the most common. Other studies have identified an increased risk in cats for Giardia spp. and co-infections with Cryptosporidium spp. and other coccidia [31]. While being too few to draw any statistical significance it should be noted that in the current study five out of the six co-infections in cats were with coccidian parasites. It is also interesting to note that coinfections with Giardia and Tritrichomonas foetus in cats [32,33] or Pentatrichomonas hominis in dogs [34] have also been identified, but examination for these parasites did not occur in this study.
In both animal groups presented here the most commonly detected species of Giardia was the host adapted species with which they are commonly associated, G. cati in cats and G. canis (C and D) in dogs. This agrees with many other studies [2,35-37] but is at odds with a previous study from the Munich area [7] and in the USA [8] where the authors found a predominance of zoonotic species of Giardia. The potentially zoonotic G. duodenalis and G. enterica were detected to varying degrees in both species of animal. Subtyping of some of these potentially zoonotic isolates identified several that are commonly associated with humans (Table 6) [6,10].
G. enterica was identified in too few samples to determine significance on its own; however, in both cats and dogs the greater proportion were identified in animals ≤1 year (5/6 and 5/8 respectively).
There was a significant difference between the two species of animals and the proportion of samples detected with potentially zoonotic species of Giardia, 50% and 21% in the cat and dogs samples respectively. The high prevalence of G. duodenalis in the cat samples in this study is consistent with other studies [10,38-40]. Discussion around the allocation of genetic subtypes into particular subassemblages and multilocus genotypes (MLGs) is confusing and at times contradictory or incomplete e.g. [10,14]. Without consistent well defined reference sequences, meaningful comparison and interpretation of zoonotic potential is difficult. For the purposes of this study G. duodenalis subtype reference sequences were used as defined by Feng and Xiao [6], although even here it appears that Portland-1 strain is used as a defining sequence for subtype A1 at the β-giardin locus and subtype A2 at the GDH. The subtypes detected in the present study suggest that the G. duodenalis isolates present fell within subassemblage AI. With the fragments we used, it was not possible to separate these further into MLG types. Likewise the G. enterica isolates present were split between BIII in the dog samples and BIV in the cats. The host distribution of G. enterica (assemblage B) is considered to be predominantly human, and, to a lesser extent, dogs and wildlife [10]; however, there are no obvious genotypes associated with zoonotic transmission.
There has been much discussion surrounding the pattern of transmission for zoonotic and host adapted species of Giardia particularly in dogs [4,41]. The hypothesis has been offered that transmission of host adapted species may be favoured by intensive contact between animals and these may then out-compete other non-host adapted species [42]. In the current study, the fact that cats had a much higher prevalence of non-host adapted species (or zoonotic species) of Giardia than the dogs may lend weight to this argument if this difference is placed in the context of the different animal behaviours. Owned dogs are generally gregarious animals that are often exercised in communal areas set aside particularly for that purpose, thus favouring the transmission of host-adapted G. canis [43]. Owned cats, however, are more territorial outside of home groupings [44] and when allowed to range freely do so in areas that have not been assigned for that purpose, potentially lessening their direct contact with other cats (in comparison with dog to dog contact). This behaviour therefore increases their propensity to acquire and retain the potentially zoonotic species of Giardia. In the present study, younger cats were seen to have a greater proportion of samples positive for the zoonotic species of Giardia in general. This was particularly so for the larger cohort of mixed breed animals. In addition, older animals in this mixed breed group were also more commonly infected with the host adapted G. cati. The opposite was true in the pure breed cats (although based on low numbers of samples) where older cats had a greater proportion of G. duodenalis. The difference in the pattern of infection between these two groups of cats (mixed and pure breed) may be related to housing conditions. Such restrictions may go some way in explaining the difference seen here. In both breed types in these well cared for animals one would expect younger animals to be kept indoors at least until vaccination was complete and the risk of them acquiring the zoonotic species would be increased due to their disproportionate contact with humans in comparison to other cats. As the animals mature they would be allowed free access to the outside and would therefore increase their contact with the environment contaminated with G. cati by other cats. If some of those animals, however, (more particularly pedigree animals) continue to be restricted to an exclusively indoor existence then they would have a significantly lower risk of contracting the host adapted G. cati and would therefore maintain their infections with the zoonotic species. In the current study, pure bred cats (particularly males) demonstrated a higher proportion of zoonotic Giardia spp. than their mixed breed counterparts (71% to 50% respectively). No data exists for the housing arrangements of the animals in this study or indeed for the proportions of pedigree cats that are housed exclusively indoors across European countries in general. There are studies from several countries such as the UK, USA, France, Canada and Australia that estimate varying levels of exclusively indoor housing (10% to 65%) of cats [45-52]. None of these studies give the breed status of the cats kept indoors although there is some evidence to suggest that expensive pedigree cats are more likely to be housed indoors [48]. In Australia, there is evidence supporting a greater level of exclusive indoor housing for pure breed cats. In Japan there has been some suggestion that this factor has an effect on the overall prevalence of Giardia spp. in such cats) [53,54]. It is therefore possible that pure breed cats may have been at greater risk of acquiring G. duodenalis and G. enterica due to greater proportions of them being restricted to an exclusively indoor environment. Further study is needed to investigate this association properly.
There are a number of studies that have examined the different species of Giardia affecting different populations of cats and dogs, e.g. shelter/stray and veterinary presentations, urban and rural, etc., and some have seen associations with Giardia presence and breed (Rottweilers) and sex (higher levels in female household dogs) [4,55]. As far as we are aware this is the only study to date that has attempted to correlate particular genotypes or species of Giardia to factors such as age, sex, breed or season within a single population type, i.e. well cared for cats and dogs. The possibility that female dogs and cats are more likely than their male counterparts to harbour not only potentially zoonotic Giardia spp. (rather than host adapted species) but also concurrent endoparasitic infections along with Giardia needs verification. Future studies should also aim to understand if this bias is due to behavioural (affiliative or gender based) or intrinsic biological reasons, if indeed this bias does exist. In addition, this is the first study to identify possible factors (age, sex and breed) involved in the acquisition of host adapted species or genotypes of Giardia. The relationship suggested in this study, between transmission of host adapted species/genotypes and their host’s sex, age or breed deserves further investigation. For clarity such studies would need to look at these factors within particular groups of animals, since previous studies comparing across differing groups (owned, stray or shelter) of animals have given variable results that are difficult to interpret in this context [41].
The observed difference between the actual and expected occurrence of mixed C and D genotypes in dog samples may well be explained by an association between these genotypes and gender as noted in this study. Further investigation is needed to rule out the role of any competitive exclusion that may be occurring between these two genotypes. Coupled with the observed association of C genotype and fewer symptoms as well as the genetic difference between these two genotypes [12] there may well be cause to revisit the classification of C and D genotypes within G. canis.
The success rates for each of the PCR methods used on the samples in this study are consistent with several other studies using large numbers of clinical samples [4,7,40]. There has been some criticism with regards to the inclusion of sequence data based on the 18S rDNA [12,41]. Indeed, while the limitations of the information gleaned from this locus (short, highly conserved fragments giving species (assemblage) level information only) are acknowledged here, had this locus been excluded from this study over half of the samples would have remained without any genetic characterisation whatsoever and many instances of mixed genotypes would have gone undetected. The results of this study clearly support the inclusion of the 18S rDNA locus in similar genotyping studies, particularly for difficult samples such as those from dogs; however, until a suite of reliable and well characterised loci are identified for Giardia genotyping it is critically important that multiple loci are utilised alongside, and that 18S rDNA data is not used in isolation. In this case the β-giardin was the next most cost effective option. Further work must include the development or enhancement of protocols including other locus options such as the triose-phosphate isomerase (tpi), GDH and possibly the more recently utilised ITS [56-59] to apply to such notoriously difficult sample types.
For the purposes of this study, the assumption has been made that each genotype detected within a sample represents a single infection from a non-recombining population and that where mixed genotypes were detected a coexisting multiple infection was present. Given this assumption, a total of 73 and 167 different isolates were identified in 53 cats and 123 dogs respectively. The use of multiple loci was responsible for detecting almost 70% of the mixed species/genotypes detected in this study, with the most successful combination being the 18S rDNA PCR with the β–giardin PCR. The use of multiple loci was attempted for all Giardia positive samples and while advocated by the authors here this ideal proved practically difficult and expensive to pursue. Such difficulties have been discussed previously [8,58] and the use of freshly extracted DNA has been recommended to improve the overall amplification success of the various loci included. This is not our experience; more recent studies have used freshly extracted DNA from fresh samples as a template for the same PCR protocols used here, resulting in the same variable success rates (Unpublished data). The relative strengths and weaknesses of each of the PCR protocols at amplifying from particular species of Giardia should be examined further and taken into account when deciding which methods to employ to detect potentially zoonotic species in animal samples. Preferential amplification of genotypes by particular protocols has been described [8]. In the present study the 18S rDNA PCR amplified fragments from 6 of the total 14 samples detected as positive for G. enterica. The β-giardin PCR amplified poorly from both zoonotic species, particularly G. enterica and while the GDH performed very poorly in general it did amplify from as many (and different) G. enterica positive samples as with the 18S rDNA protocol. This has been noted previously in studies from our laboratory [60]. There did appear to be a gender bias with the GDH protocol, however, this may be due to gender differences in the species or genotypes that are preferentially amplified. The likelihood is therefore, that for this study the number of potentially zoonotic species as well as the number of mixed genotypes actually present in these samples has been underestimated.
Conclusions
This study has demonstrated the complexity of Giardia ecology in domestic dogs and cats, and has reinforced the influence exposure to different species of Giardia may have on which dominates under certain environmental and/or anthropogenic circumstances. As with other studies, the results reported here reinforce the public health significance of Giardia in companion animals with the occurrence of zoonotic species in dogs and cats, but demonstrate that it is impossible to extrapolate from one geographical area to another on the prevalence of zoonotic versus host adapted species, even in the same country. Polyparasitism, whether this involves mixed infections of Giardia species and/or other parasites, particularly Cystoisospora, is clearly an aspect of enteric parasitism that requires further study, especially in dogs and cats less than one year of age. Is G. duodenalis or G. canis/G. cati more pathogenic in dogs and cats, and what is the impact of co-infections with Cystoisospora in young animals particularly prior to weaning? Finally, our results have demonstrated the importance of taking a multilocus approach in studies on the molecular epidemiology of Giardia infections, and particularly the relevance of including 18S rDNA as one of the loci examined.
Acknowledgements
We would like to thank Bayer Animal Health for funding this research. We would also like to thank Alan Lymbery (Murdoch University) for assistance and advice with statistical analysis and Aileen Elliot for technical assistance.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DB, RS, AT conceived of the study and participated in its design. DB coordinated the collection and screening of samples by microscopy and coproantigen. LP carried out the molecular genetic studies, performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Louise Pallant, Email: L.Pallant@murdoch.edu.au.
Dieter Barutzki, Email: Barutzki@labor-freiburg.de.
Roland Schaper, Email: roland.schaper@bayer.com.
RC Andrew Thompson, Email: A.Thompson@murdoch.edu.au.
References
- 1.Thompson RC, Palmer CS, O’Handley R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet J. 2008;177:18–25. doi: 10.1016/j.tvjl.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballweber LR, Xiao L, Bowman DD, Kahn G, Cama VA. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 2010;26:180–189. doi: 10.1016/j.pt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Barutzki D, Schaper R. Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol Res. 2011;109(Suppl 1):S45–S60. doi: 10.1007/s00436-011-2402-8. [DOI] [PubMed] [Google Scholar]
- 4.Upjohn M, Cobb C, Monger J, Geurden T, Claerebout E, Fox M. Prevalence, molecular typing and risk factor analysis for Giardia duodenalis infections in dogs in a central London rescue shelter. Vet Parasitol. 2010;172:341–346. doi: 10.1016/j.vetpar.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Lymbery AJ, Thompson RC. The molecular epidemiology of parasite infections: tools and applications. Mol Biochem Parasit. 2012;181:102–116. doi: 10.1016/j.molbiopara.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonhard S, Pfister K, Beelitz P, Wielinga C, Thompson RC. The molecular characterisation of Giardia from dogs in southern Germany. Vet Parasitol. 2007;150:33–38. doi: 10.1016/j.vetpar.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Covacin C, Aucoin DP, Elliot A, Thompson RC. Genotypic characterisation of Giardia from domestic dogs in the USA. Vet Parasitol. 2011;177:28–32. doi: 10.1016/j.vetpar.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Sotiriadou I, Pantchev N, Gassmann D, Karanis P. Molecular identification of Giardia and Cryptosporidium from dogs and cats. Parasite. 2013;20:8. doi: 10.1051/parasite/2013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprong H, Caccio SM, van der Giessen JW, network Z, partners Identification of zoonotic genotypes of Giardia duodenalis. PLoS Neglect Trop D. 2009;3:e558. doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wielinga CM, Thompson RC. Comparative evaluation of Giardia duodenalis sequence data. Parasitology. 2007;134:1795–1821. doi: 10.1017/S0031182007003071. [DOI] [PubMed] [Google Scholar]
- 12.Ryan U, Caccio SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43:943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RC, Monis P. Giardia–from genome to proteome. Adv Parasit. 2012;78:57–95. doi: 10.1016/B978-0-12-394303-3.00003-7. [DOI] [PubMed] [Google Scholar]
- 14.Caccio SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int J Parasitol. 2008;38:1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Marti H, Escher E. SAF–Eine alternative Fixierlösung für parasitologische Stuhluntersuchungen. Schweiz Med Wochenschr. 1990;120:1473–1476. [PubMed] [Google Scholar]
- 16.Hopkins RM, Meloni BP, Groth DM, Wetherall JD, Reynoldson JA, Thompson RC. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J Parasitol. 1997;83:44–51. doi: 10.2307/3284315. [DOI] [PubMed] [Google Scholar]
- 17.Read C, Walters J, Robertson ID, Thompson RCA. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–231. doi: 10.1016/S0020-7519(01)00340-X. [DOI] [PubMed] [Google Scholar]
- 18.Caccio SM, De Giacomo M, Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int J Parasitol. 2002;32:1023–1030. doi: 10.1016/S0020-7519(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 19.Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Caccio SM. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardiaduodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Read CM, Monis PT, Thompson RC. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infecn Genet Evol. 2004;4:125–130. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Gillhuber J, Pallant L, Ash A, Thompson RA, Pfister K, Scheuerle MC. Molecular identification of zoonotic and livestock-specific Giardia-species in faecal samples of calves in Southern Germany. Parasite Vector. 2013;6:346. doi: 10.1186/1756-3305-6-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monis PT, Mayrhofer G, Andrews RH, Homan WL, Limper L, Ey PL. Molecular genetic analysis of Giardia intestinalis isolates at the glutamate dehydrogenase locus. Parasitology. 1996;112(Pt 1):1–12. doi: 10.1017/S0031182000065021. [DOI] [PubMed] [Google Scholar]
- 23.Mehlotra RK, Kasehagen LJ, Baisor M, Lorry K, Kazura JW, Bockarie MJ, Zimmerman PA. Malaria infections are randomly distributed in diverse holoendemic areas of Papua New Guinea. Am J Trop Med Hyg. 2002;67:555–562. doi: 10.4269/ajtmh.2002.67.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epe C, Rehkter G, Schnieder T, Lorentzen L, Kreienbrock L. Giardia in symptomatic dogs and cats in Europe—Results of a European study. Vet Parasitol. 2010;173:32–38. doi: 10.1016/j.vetpar.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Barutzki D, Schaper R. Endoparasites in dogs and cats in Germany 1999–2002. Parasitol Res. 2003;90(Suppl 3):S148–S150. doi: 10.1007/s00436-003-0922-6. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed AS, Glickman LT, Camp JW, Jr, Lund E, Moore GE. Prevalence and risk factors for Giardia spp. infection in a large national sample of pet dogs visiting veterinary hospitals in the United States (2003–2009) Vet Parasitol. 2013;195:35–41. doi: 10.1016/j.vetpar.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Barutzki D, Schaper R. Age-dependant prevalence of endoparasites in young dogs and cats up to one year of age. Parasitol Resh. 2013;112(Suppl 1):119–131. doi: 10.1007/s00436-013-3286-6. [DOI] [PubMed] [Google Scholar]
- 28.Dubná S, Langrová I, Nápravník J, Jankovská I, Vadlejch J, Pekár S, Fechtner J. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet Parasitol. 2007;145:120–128. doi: 10.1016/j.vetpar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Tzannes S, Batchelor DJ, Graham PA, Pinchbeck GL, Wastling J, German AJ. Prevalence of Cryptosporidium, Giardia and Isospora species infections in pet cats with clinical signs of gastrointestinal disease. J Feline Med Surg. 2008;10:1–8. doi: 10.1016/j.jfms.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mircean V, Gyorke A, Cozma V. Prevalence and risk factors of Giardia duodenalis in dogs from Romania. Vet Parasitol. 2012;184:325–329. doi: 10.1016/j.vetpar.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Vasilopulos RJ, Mackin AJ, Rickard LG, Pharr GT, Huston CL. Prevalence and factors associated with fecal shedding of Giardia spp. in domestic cats. J Am Anim Hosp Assoc. 2006;42:424–429. doi: 10.5326/0420424. [DOI] [PubMed] [Google Scholar]
- 32.Bissett SA, Stone ML, Malik R, Norris JM, O’Brien C, Mansfield CS, Nicholls JM, Griffin A, Gookin JL. Observed occurrence of Tritrichomonas foetus and other enteric parasites in Australian cattery and shelter cats. J Feline Med Surg. 2009;11:803–807. doi: 10.1016/j.jfms.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingsbury DD, Marks SL, Cave NJ, Grahn RA. Identification of Tritrichomonas foetus and Giardia spp. infection in pedigree show cats in New Zealand. New Zea Vet J. 2010;58:6–10. doi: 10.1080/00480169.2010.65054. [DOI] [PubMed] [Google Scholar]
- 34.Grellet A, BrunoPolack, Feugier A, Boucraut-Baralon C, Grandjean D, Vandewynckel L, Cian A, Meloni D, Viscogliosi E: Prevalence, risk factors of infection and molecular characterization of trichomonads in puppies from French breeding kennels.Vet Parasitol 2013, 197:418–426. [DOI] [PMC free article] [PubMed]
- 35.Itagaki T, Kinoshita S, Aoki M, Itoh N, Saeki H, Sato N, Uetsuki J, Izumiyama S, Yagita K, Endo T. Genotyping of Giardia intestinalis from domestic and wild animals in Japan using glutamete dehydrogenase gene sequencing. Vet Parasitol. 2005;133:283–287. doi: 10.1016/j.vetpar.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 36.Rimhanen-Finne R, Enemark HL, Kolehmainen J, Toropainen P, Hanninen ML. Evaluation of immunofluorescence microscopy and enzyme-linked immunosorbent assay in detection of Cryptosporidium and Giardia infections in asymptomatic dogs. Vet Parasitol. 2007;145:345–348. doi: 10.1016/j.vetpar.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Scorza AV, Ballweber LR, Tangtrongsup S, Panuska C, Lappin MR. Comparisons of mammalian Giardia duodenalis assemblages based on the beta-giardin, glutamate dehydrogenase and triose phosphate isomerase genes. Vet Parasitol. 2012;189:182–188. doi: 10.1016/j.vetpar.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 38.Souza SL, Gennari SM, Richtzenhain LJ, Pena HF, Funada MR, Cortez A, Gregori F, Soares RM. Molecular identification of Giardia duodenalis isolates from humans, dogs, cats and cattle from the state of Sao Paulo, Brazil, by sequence analysis of fragments of glutamate dehydrogenase (gdh) coding gene. Vet Parasitol. 2007;149:258–264. doi: 10.1016/j.vetpar.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Vasilopulos RJ, Rickard LG, Mackin AJ, Pharr GT, Huston CL. Genotypic analysis of Giardia duodenalis in domestic cats. J Vet Intern Med. 2007;21:352–355. doi: 10.1111/j.1939-1676.2007.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 40.McDowall RM, Peregrine AS, Leonard EK, Lacombe C, Lake M, Rebelo AR, Cai HY. Evaluation of the zoonotic potential of Giardia duodenalis in fecal samples from dogs and cats in Ontario. Can Vet J. 2011;52:1329–1333. [PMC free article] [PubMed] [Google Scholar]
- 41.Beck R, Sprong H, Pozio E, Caccio SM. Genotyping Giardia duodenalis isolates from dogs: lessons from a multilocus sequence typing study. Vector-Borne Zoonot. 2012;12:206–213. doi: 10.1089/vbz.2011.0751. [DOI] [PubMed] [Google Scholar]
- 42.Thompson RC, Monis PT. Variation in Giardia: implications for taxonomy and epidemiology. Adv Parasit. 2004;58:69–137. doi: 10.1016/S0065-308X(04)58002-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang A, Ruch-Gallie R, Scorza V, Lin P, Lappin MR. Prevalence of Giardia and Cryptosporidium species in dog park attending dogs compared to non-dog park attending dogs in one region of Colorado. Vet Parasitol. 2012;184:335–340. doi: 10.1016/j.vetpar.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Crowell-Davis SL, Curtis TM, Knowles RJ. Social organization in the cat: a modern understanding. J Feline Med Surg. 2004;6:19–28. doi: 10.1016/j.jfms.2003.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patronek GJ, Beck AM, Glickman LT. Dynamics of dog and cat populations in a community. J Am Vet Med Assoc. 1997;210:637–642. [PubMed] [Google Scholar]
- 46.Gurfield AN, Boulouis HJ, Chomel BB, Kasten RW, Heller R, Bouillin C, Gandoin C, Thibault D, Chang CC, Barrat F, Piemont Y. Epidemiology of Bartonella infection in domestic cats in France. Vet Microbiol. 2001;80:185–198. doi: 10.1016/S0378-1135(01)00304-2. [DOI] [PubMed] [Google Scholar]
- 47.Clancy EA, Moore AS, Bertone ER. Evaluation of cat and owner characteristics and their relationships to outdoor access of owned cats. J Am Vet Med Assoc. 2003;222:1541–1545. doi: 10.2460/javma.2003.222.1541. [DOI] [PubMed] [Google Scholar]
- 48.Neville PF. An ethical viewpoint: the role of veterinarians and behaviourists in ensuring good husbandry for cats. J Feline Med Surg. 2004;6:43–48. doi: 10.1016/j.jfms.2003.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rochlitz I. A review of the housing requirements of domestic cats (Felis silvestris catus) kept in the home. Appl Anim Behav Sci. 2005;93:97–109. doi: 10.1016/j.applanim.2005.01.002. [DOI] [Google Scholar]
- 50.Jongman EC. Adaptation of domestic cats to confinement. J Vet Behav. 2007;2:193–196. doi: 10.1016/j.jveb.2007.09.003. [DOI] [Google Scholar]
- 51.Slater MR, Di Nardo A, Pediconi O, Villa PD, Candeloro L, Alessandrini B, Del Papa S. Cat and dog ownership and management patterns in central Italy. Prev Vet Med. 2008;85:267–294. doi: 10.1016/j.prevetmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Stull JW, Peregrine AS, Sargeant JM, Weese JS: Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada.Bmc Public Health 2013, 13:520 [DOI] [PMC free article] [PubMed]
- 53.Itoh N, Muraoka N, Kawamata J, Aoki M, Itagaki T. Prevalence of Giardia intestinalis infection in household cats of Tohoku district in Japan. J Vet Med Sci. 2006;68:161–163. doi: 10.1292/jvms.68.161. [DOI] [PubMed] [Google Scholar]
- 54.Itoh N, Muraoka N, Saeki H, Aoki M, Itagaki T. Prevalence of Giardia intestinalis infection in dogs of breeding kennels in Japan. J Vet Med Sci. 2005;67:717–718. doi: 10.1292/jvms.67.717. [DOI] [PubMed] [Google Scholar]
- 55.Meireles P, Montiani-Ferreira F, Thomaz-Soccol V. Survey of giardiosis in household and shelter dogs from metropolitan areas of Curitiba, Parana state, Southern Brazil. Vet Parasitol. 2008;152:242–248. doi: 10.1016/j.vetpar.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 56.Caccio SM, Beck R, Almeida A, Bajer A, Pozio E. Identification of Giardia species and Giardia duodenalis assemblages by sequence analysis of the 5.8S rDNA gene and internal transcribed spacers. Parasitology. 2010;137:919–925. doi: 10.1017/S003118200999179X. [DOI] [PubMed] [Google Scholar]
- 57.Beck R, Sprong H, Lucinger S, Pozio E, Caccio SM. A large survey of Croatian wild mammals for Giardia duodenalis reveals a low prevalence and limited zoonotic potential. Vector-Borne Zoonot. 2011;11:1049–1055. doi: 10.1089/vbz.2010.0113. [DOI] [PubMed] [Google Scholar]
- 58.Beck R, Sprong H, Bata I, Lucinger S, Pozio E, Caccio SM. Prevalence and molecular typing of Giardia spp. in captive mammals at the zoo of Zagreb, Croatia. Vet Parasitol. 2011;175:40–46. doi: 10.1016/j.vetpar.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 59.Elwin K, Fairclough HV, Hadfield SJ, Chalmers RM. Giardia duodenalis typing from stools: a comparison of three approaches to extracting DNA, and validation of a probe-based real-time PCR typing assay. J Med Microbiol. 2014;63:38–44. doi: 10.1099/jmm.0.066050-0. [DOI] [PubMed] [Google Scholar]
- 60.Ash A, Lymbery A, Lemon J, Vitali S, Thompson RC. Molecular epidemiology of Giardia duodenalis in an endangered carnivore–the African painted dog. Vet Parasitol. 2010;174:206–212. doi: 10.1016/j.vetpar.2010.08.034. [DOI] [PubMed] [Google Scholar]


