Abstract
Rationale: Abdominal adiposity may be an important risk factor for uncontrolled asthma in adults, controlling for general obesity. Whether the relationship, if present, is explained by other factors (e.g., asthma onset age, sex, and/or coexisting conditions) is unclear.
Objectives: To examine whether clinically applicable anthropometric measures of abdominal adiposity—waist circumference and waist-to-height ratio (WHtR)—are related to poorer asthma control in adults with uncontrolled asthma controlling for body mass index (BMI), and whether the relationship (if present) is explained by gastroesophageal reflux disorder (GERD), sleep quality, or obstructive sleep apnea (OSA) or differs by age of asthma onset or sex.
Methods: Patients aged 18 to 70 years with uncontrolled asthma (n = 90) participated in a 6-month randomized clinical trial.
Measurements and Main Results: Baseline measures included sociodemographics, standardized anthropometrics, Asthma Control Test (ACT), GERD Symptom Assessment Scale, Pittsburgh Sleep Quality Index, and Berlin Questionnaire for Sleep Apnea. Participants (mean [SD] age, 52 [12] yr) were racially and ethnically diverse, 67% women, and 69% overweight or obese, and 71% reported their age of asthma onset was 12 years or older. Participants had uncontrolled asthma (mean [SD] ACT score, 14.9 [3.7]) and low GERD symptoms score (0.6 [0.4]); 67% reported poor sleep quality, and 42% had a high OSA risk. General linear regression results showed that worse ACT scores were significantly associated with every SD increase in waist circumference (β= −1.03; 95% confidence interval [CI], −1.96 to −0.16; P = 0.02) and waist-to-height ratio (β= −1.16; 95% CI, −2.00 to −0.33; P = 0.008), controlling for sociodemographics. Waist-to-height ratio remained correlated with ACT (β= −2.30; 95% CI, −4.16 to −0.45; P = 0.02) after further adjusting for BMI. The BMI-controlled relationship between WHtR and ACT did not differ by age of asthma onset or sex (P > 0.05 for interactions) and persisted after additional adjustment for GERD, sleep quality, or OSA scores. Poor sleep quality was associated with worse ACT scores (β= −0.87; 95% CI, −1.71 to −0.03; P = 0.045) controlling for waist-to-height ratio, BMI, and sociodemographics.
Conclusions: Abdominal adiposity by waist-to-height ratio and poor sleep quality correlated with poorer asthma control in adults with uncontrolled asthma, after controlling for BMI and sociodemographics. These results warrant replication in larger studies of diverse populations.
Clinical trial registered with www.clinicaltrials.gov (NCT 01725945).
Keywords: body mass index, abdominal obesity, asthma, gastroesophageal reflux, sleep
Obesity and asthma prevalence have increased concurrently (1–3). Obesity is a recognized risk factor for incident asthma (4). Obese people with asthma also tend to have blunted responsiveness to asthma controller therapies and poorer asthma control compared with their normal-weight counterparts (4, 5). Body mass index (BMI) is a clinically validated, widely used global anthropometric index of adiposity, which, however, has been criticized for its inability to indicate fat distribution (6). Indeed, some adult studies have shown that abdominal adiposity indices, such as waist circumference (WC) or waist-to-height ratio (WHtR), are more strongly associated with asthma prevalence (7–9) and incidence (10) and lung function impairment (11, 12) than BMI.
Although the obesity–asthma relation is established, the extent to which it is explained by other factors (e.g., age of asthma onset, demographics, and comorbidities) is not well understood. Emerging data suggest the possibility of at least two distinct asthma phenotypes in obese adults depending on the age of asthma onset and the presence of atopy (8, 13). Obesity is a recognized risk factor for later-onset (≥12 yr of age), typically nonatopic asthma, the phenotype predominantly found in women and that is more difficult to control than the early-onset, typically atopic asthma that coexists with obesity (8, 13). Among the myriad hypothesized mechanisms, coexisting conditions, such as gastroesophageal reflux disorder (GERD), suboptimal sleep quality, and obstructive sleep apnea (OSA), may contribute to worse asthma control in adults (14).
Symptom control is an asthma treatment goal that, unfortunately, is elusive for many patients with asthma (15). Patients with uncontrolled asthma incur high health-care use and costs, significant functional impairment and psychological distress, and poor quality of life (16–18). Few studies have examined the association of abdominal and general adiposity with asthma control, specifically in adults with uncontrolled asthma, and the possible explanatory factors for such an association, if present. Using data from a well-characterized sample of adults with uncontrolled asthma, we examined whether clinically applicable anthropometrics—higher BMI, WC, and WHtR—were related to poorer asthma control and whether WC and WHtR remained associated with asthma control after adjusting for BMI. Conditional on a positive association between any of the adiposity indices and asthma control, we also explored whether the relationship varied by reported age of asthma onset or sex and whether it might be explained by GERD symptoms, sleep quality, or OSA risk. Some of the results of this study have been previously reported in the form of an abstract (19).
Methods
This study used baseline data from the ongoing DASH (Dietary Approaches to Stop Hypertension) for Asthma trial, a randomized controlled pilot study designed to evaluate the feasibility and potential effects of the DASH eating pattern among adults with uncontrolled asthma. The trial protocol was published previously (20). The Kaiser Foundation Research Institute’s Institutional Review Board in the Northern California region approved the study, and all participants provided written informed consent.
Study Population
Participants aged 18 to 70 years with uncontrolled persistent asthma were recruited from Kaiser Permanente medical centers in San Francisco and Hayward, California. Patients who likely had uncontrolled persistent asthma (based on their emergency visits, hospitalizations, and/or pharmacy records in the past year) were identified by querying the Kaiser Permanente electronic asthma registry. Approval was obtained from primary care providers to further screen patients in their practice. Patients received recruitment letters briefly explaining the study and inviting them to complete the initial screening (online or by phone), which included the five-item Asthma Control Test (ACT) (21) and other eligibility questions. Uncontrolled asthma was defined by an ACT total score less than or equal to 19 or an item score less than 3 for any of the first four questions: symptoms (three to six times per week or more), nighttime awakening (once a week or more), interference with normal activity (at least some of the time), and rescue medication use for symptom relief (two to three times per week or more). Patients screened eligible were invited to attend a 1-hour orientation during which their height and weight were measured and written informed consent obtained. Consenting participants with a BMI between 18.5 and 39.9 underwent further baseline evaluation before randomization.
Data Collection and Measures
Baseline measures reported in this manuscript included sociodemographics, standardized anthropometric measurements, GERD Symptom Assessment Scale (GSAS), Pittsburgh Sleep Quality Index (PSQI), Berlin Questionnaire for Sleep Apnea (BQ), and ACT.
Sociodemographics and age of asthma onset.
Sociodemographics included age, sex, race, ethnicity, education, income, and smoking status. Participants were asked to recall when they were first diagnosed with asthma (< 5, 5–11, or ≥ 12 yr of age).
Anthropometric measurements.
Height and weight, in light indoor clothes without shoes, were measured using a wall-mounted stadiometer and a digital or balance beam scale, respectively, following standardized protocols (22). Waist circumference was measured on bare skin using a nonstretchable tape, according to a standardized protocol, in a horizontal plane around the abdomen at the level of the right iliac crest (23). All measurements were taken in duplicate. A third measurement was taken if the first two measurements differed by greater than 1.0 cm (1/4”) for height, by greater than 0.1 pounds (0.05 kg) for weight, and by greater than 1.0 cm (1/4”) for waist circumference. If a third measurement was taken, the two closest measurements were averaged. If the third measurement fell equally between the first two measurements, all three were averaged.
GSAS.
The GSAS measures the presence, frequency, and distress of 15 GERD-associated symptoms during the previous week (24). Distress is rated on a 4-point scale ranging from 0 (not at all) to 3 (very much). If a symptom is reportedly not present, the distress score is assigned 0. The GSAS score is the average of symptom scores if at least 12 are nonmissing; otherwise, the GSAS score is assigned missing.
PSQI.
The PSQI assesses sleep quality during the past month based on sleep latency, duration, and efficiency; sleep disturbances; use of sleep medication; and daytime dysfunction (25). Each PSQI item is scored from 0 to 3, and higher sum scores indicate worse sleep quality, with poor sleep quality defined as PSQI greater than 5.
BQ for sleep apnea.
The BQ is a validated instrument to assess the frequency of snoring (category 1) and daytime somnolence (category 2) and the presence of obesity (i.e., BMI > 30 kg/m2) or hypertension (category 3) (26). Categories 1 and 2 are positive if the responses indicate frequent symptoms (>3–4 times/wk) and category 3 is positive if either obesity or hypertension is present. Patients with two or more positive categories (i.e., BQ score ≥ 2) are considered to be at high risk for OSA.
ACT.
The ACT is a validated, self-administered composite measure of asthma control. It assesses the frequency of asthma-related normal activity interference, symptoms, nighttime awakening, and rescue medication use for symptom relief, and the patient’s overall perception of asthma control, during the past 4 weeks (21). The sum of the five questions (each scored from 1 to 5) indicates overall asthma control (range, 5–25; higher scores indicate better control). This study enrolled participants meeting the above-mentioned criteria of uncontrolled asthma.
Data Analysis
Frequencies and percentages were calculated for categorical variables, and means and SDs for continuous variables. Unadjusted linear regression analysis examined the associations of BMI, WC, WHtR, GERD, sleep quality, and OSA scores (standardized as [raw value − mean]/SD) with ACT scores. Multivariable linear regression analysis examined the association of each standardized adiposity index with ACT while controlling for sociodemographics (age, sex, race/ethnicity, education, income, and smoking status) (i.e., Model 1). In addition to these covariates, Model 2 controlled for BMI to assess the association of standardized WC and WHtR separately with ACT. The potential modification effects of reported age of asthma onset (early onset, <12 yr; later onset, ≥12 yr) and sex on the association between WC or WHtR and ACT, if shown significant in Model 2, were evaluated by adding each potential modifier and its interaction with the standardized abdominal adiposity index to Model 2.
Standardized OSA, GERD, and sleep quality scores were added separately to a significant Model 2 to examine whether any of them affected the strength of the relationship of WC or WHtR with ACT. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC). Statistical significance was set at P < 0.05 (two-tailed).
No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Participant Characteristics
The 90 participants had an average age of 52 years(SD, 12 yr), were mostly women (67%), and were racially and ethnically diverse (31% Asian/Pacific Islander, 14% Hispanic/Latino, and 11% non-Hispanic black) (Table 1). Overall, 31% were normal weight (BMI, 18.5 to <25), 41% overweight (BMI, 25 to <30), and 28% obese (BMI ≥ 30). Also, 57% had large WC (i.e., ≥102 cm for men; ≥88 cm for women) (27), and 91% had WHtR ≥ 0.5, typical cutoffs for defining abdominal obesity (28). All participants had uncontrolled asthma (mean [SD] ACT score, 14.9 [3.7]; a score < 16 indicates very poorly controlled asthma), with 71% reporting age of asthma onset 12 years or older. They had low GERD symptoms score (mean [SD], 0.6 [0.4]), 67% reported poor sleep quality, and 42% had a high OSA risk.
Table 1.
Study participant characteristics
| Characteristics | Mean ± SD or %* |
|---|---|
| Age, yr, % | 52 ± 12 |
| 18 to <45 | 29 |
| 45 to <60 | 42 |
| 60+ | 29 |
| Sex, % | |
| Male | 33 |
| Female | 67 |
| Race/ethnicity, % | |
| Non-Hispanic white | 43 |
| Non-Hispanic black | 11 |
| Asian/Pacific Islander | 31 |
| Hispanic | 14 |
| Education, % | |
| <High school | 2 |
| High school/GED | 7 |
| Some college | 38 |
| College or above | 53 |
| Family annual income, % (n = 87) | |
| <$35,000 | 15 |
| $35,000 to <$55,000 | 15 |
| $55,000 to <$75,000 | 18 |
| $75,000 to <$100,000 | 18 |
| $100,000 to <$125,000 | 13 |
| $125,000+ | 21 |
| Smoking status, % | |
| Current/ex-smoker | 26 |
| Never smoker | 74 |
| Age of asthma onset, % | |
| Early (<12 yr) | 29 |
| Later (≥12 yr) | 71 |
| BMI, kg/m2 | 27.9 ± 4.8 |
| WC, cm | 96.6 ± 12.7 |
| WHtR | 0.59 ± 0.07 |
| GERD score | 0.6 ± 0.4 |
| Sleep quality score | 7.8 ± 3.9 |
| Poor sleep quality, % | 67 |
| OSA score (n = 88) | 1.3 ± 0.9 |
| High OSA risk, % (n = 88) | 42 |
| ACT | 14.9 ± 3.7 |
Definition of abbreviations: ACT = Asthma Control Test (range, 5–25; higher scores indicate better control; <16, very poorly controlled asthma); BMI = body mass index; GED = General Education Development; GERD = gastroesophageal reflux disorder (range, 0–3; higher scores indicate more distress); OSA = obstructive sleep apnea (range, 0–3; higher scores indicate higher risk; ≥2, high OSA risk); sleep quality (range, 0–21; higher scores indicate poorer quality; >5, poor sleep quality); WC = waist circumference; WHtR = waist-to-height ratio.
n = 90 unless indicated otherwise.
Adiposity Indices and Asthma Control
Unadjusted associations of standardized BMI (β = −0.90; 95% CI, −1.65 to −0.14; P = 0.02), WC (β = −0.95; 95% CI, −1.71 to −0.20; P = 0.01), and WHtR (β = −1.15; 95% CI, −1.89 to −0.42; P = 0.003) with ACT were significant (Figure 1). One SD increase in BMI, WC, and WHtR was associated with a 0.90-, 0.95-, and 1.15-point decrease, respectively, in ACT score (lower scores indicate poorer control). Adjusting for sociodemographics attenuated the association of BMI (β = −0.78; 95% CI, −1.64 to 0.08; P = 0.08) but did not change the significant inverse associations of abdominal adiposity by WC (β = −1.03; 95% CI, −1.96 to −0.16; P = 0.02) and WHtR (β = −1.16; 95% CI, −2.00 to −0.33; P = 0.008), with ACT (Model 1, Figure 1). The association between WHtR and ACT (β = −2.30; 95% CI, −4.16 to −0.45; P = 0.02) persisted even after further adjusting for BMI (Model 2, Figure 1). The BMI-controlled relationship between WHtR and ACT did not differ by age of asthma onset (interaction with WHtR: P = 0.14) or sex (P = 0.61).
Figure 1.
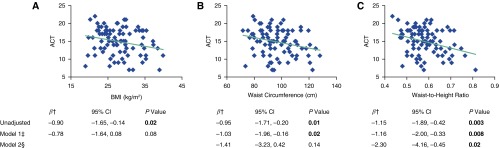
Associations between adiposity indices and Asthma Control Test (ACT) scores (range, 5–25; higher scores indicate better control). Graphs show unadjusted associations between raw values of adiposity indices and ACT scores. †Standardized as (raw value − mean)/SD and examined as a continuous variable in the regression model. ‡Model 1 controlled for age, sex, race/ethnicity, education, income, and smoking status. §Model 2 controlled for all Model 1 covariates and body mass index (BMI). CI = confidence interval. P values in bold were statistically significant (<0.05).
The unadjusted association of ACT scores with standardized sleep quality scores was significant (β = −1.07; 95% CI, −1.81 to −0.33; P = 0.006) but was not with GERD and OSA (Figure 2). One SD increase in sleep quality score (higher scores indicate poorer quality) was associated with a 1.07-point decrease in ACT score. The BMI-controlled relationship between WHtR and ACT persisted after additional adjustment for GERD, sleep quality, or OSA scores (Figure 2). These same models also showed that poor sleep quality was significantly correlated with worse ACT scores (β = −0.87; 95% CI, −1.71 to −0.03; P = 0.045), controlling for WHtR, BMI, and sociodemographics, but that GERD and OSA were not (Figure 2). A sensitivity analysis showed that the unadjusted association between standardized sleep quality scores and ACT scores without the item on nighttime awakenings due to asthma symptoms was β = −0.74 (95% CI, −1.29 to −0.19; P = 0.01) and the adjusted association was β = −0.60 (95% CI, −1.23 to 0.02; P = 0.06).
Figure 2.
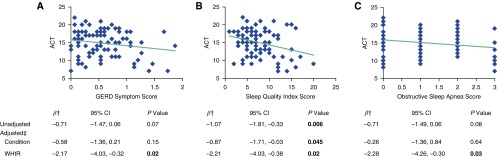
Associations of gastroesophageal reflux disorder (GERD) symptoms (range, 0–3; higher scores indicate more distress), sleep quality, and obstructive sleep apnea risk scores with Asthma Control Test (ACT) scores (range, 5–25; higher scores indicate better control). OSA = obstructive sleep apnea (range, 0–3; higher scores indicate higher risk); SQ = sleep quality (range, 0–21; higher scores indicate poorer quality); WHtR = waist-to-height ratio. Graphs show unadjusted associations of raw values of coexisting conditions with ACT scores. †Standardized as (raw value − mean)/SD and examined as a continuous variable in the regression model. ‡Each adjusted model included WHtR and the coexisting condition (OSA, GERD, or sleep quality), controlling for age, sex, race/ethnicity, education, income, smoking status, and BMI. P values in bold were statistically significant (<0.05).
Discussion
This study showed that in a clinical sample of adults with uncontrolled asthma, increasing abdominal adiposity, as indicated by WC and WHtR, was associated with poorer asthma control by ACT. Moreover, the WHtR association remained significant even after adjusting for BMI. This is consistent with prior cross-sectional and prospective cohort studies suggesting that abdominal obesity not only adversely affects asthma prevalence and incidence and lung function but possibly does so even after accounting for the effect of general obesity (7–12). Hypothesized mechanisms include the effects of abdominal adipose tissue on respiratory mechanics and airway inflammation (29–31). Abdominal adiposity can reduce chest wall compliance, respiratory muscle strength and function, lung volumes, and peripheral airway diameter, which, in turn, may result in airway hyperresponsiveness and asthma (29–31). In addition, abdominal adiposity can lead to an imbalance in the production of adipokines, chemokines, and cytokines, which may cause airway inflammation and consequently promote the expression of asthma (29, 30). Abdominal visceral adipose tissue is more proinflammatory than abdominal and gluteal subcutaneous adipose tissue (32–36). WHtR takes height into consideration. A metaanalysis of studies across different populations concluded that WHtR is superior to WC and BMI in the discrimination of obesity-related cardiometabolic risk (35), because short subjects have higher levels of risk factors than tall subjects in the same WC group, especially in the group with a WC below the commonly used cut-off point (i.e., 102 cm in men; 88 cm in women) (37). Koch and colleagues proposed that adverse environment in early life may program short stature and predisposition to abdominal obesity and cardiometabolic risk factors in adult life (38).
It is worth noting that conflicting findings exist regarding the relation of general and abdominal obesity with asthma outcomes (39–42). Bustos and colleagues (40) found BMI, but not WC, was a significant predictor of asthma symptoms, such as exercise-induced wheezing and breathlessness, whereas other studies (39, 41, 42) showed BMI and WC were comparably associated with asthma prevalence and symptoms. The results of these studies are not directly comparable because of heterogeneous sample characteristics (e.g., different demographics and varied clinical manifestations of asthma and obesity), study designs (e.g., cross-sectional vs. prospective cohort, population samples vs. small convenience samples), and clinical endpoints (e.g., asthma prevalence vs. asthma incidence vs. self-reported asthma symptoms and/or lung function).
Our study showed that the BMI-adjusted relationship between WHtR and ACT was unaffected by further adjustment for GERD, poor sleep quality, or OSA. One cross-sectional study showed that adjustment for OSA and demographics attenuated the bivariate association between obesity (defined as a BMI ≥ 30 kg/m2) and asthma control in adults with controlled or uncontrolled asthma (14). Another cross-sectional study examined the association between OSA and GERD with asthma control in obese patients with asthma and found that OSA, but not GERD, was significantly associated with poorer asthma control after adjusting for lung function, race, and sex (43). These results may not be directly comparable to ours because of different adiposity indices or asthma control levels. Notwithstanding, our study showed that poor sleep quality was correlated with worse asthma control, controlling for WHtR, BMI, and sociodemographics. Luyster and colleagues showed a similar relationship by comparing patients with severe and nonsevere asthma and their healthy counterparts (44). Asthma can lead to poor sleep quality because of nocturnal symptoms. Interestingly, poor sleep quality was also reported by 61% of participants who had no or infrequent (once or twice a month) nighttime asthma symptoms in our study and by 88 to 100% of those with no nighttime symptoms in the Luyster study (44). Our sensitivity analysis also suggested an association of poor sleep quality with worse ACT scores excluding the item on nighttime awakenings due to asthma symptoms. Patients with asthma may experience poor sleep quality for reasons other than asthma symptom–related sleep disturbances (44). Sleep quality also may deteriorate with age (45) and use of certain common asthma medications (e.g., inhaled corticosteroids and short-acting β-agonists) (46–48). Further research is needed to elucidate the mechanism linking sleep quality and disorders (e.g., OSA) to asthma control, especially among individuals who report no or infrequent nighttime asthma symptoms.
Research on factors influencing the level of asthma control in adults is needed to guide clinical interventions targeted at the high-risk, high-cost population with uncontrolled asthma. Leveraging unique baseline data from an ongoing trial, this study focused on indices of general and abdominal adiposity and explored several variables that might modify or explain their relationship with asthma control in a racially and ethnically diverse sample of adult patients with uncontrolled asthma. However, our study had a number of potential limitations. First, it was a cross-sectional study of a small sample with relatively high education and income level, which precludes causal inferences and generalizability of the findings to other populations. Second, GERD, sleep quality, and OSA were assessed by self-report for a short period of time (e.g., 1 wk for GERD and 1 mo for sleep quality), even though the instruments used have been previously validated in diverse populations. Third, age of asthma onset was self-reported and, consequently, subject to recall bias. Despite these limitations, the observed associations of increasing abdominal adiposity as measured by WHtR and poor sleep quality with worse asthma control, adjusted for BMI and sociodemographics, in adults with uncontrolled asthma warrant replication. Larger studies of diverse populations with longitudinal patient data, for example, by leveraging existing prospective cohorts or electronic health records are needed. If replicated, the therapeutic and mechanistic implications will require further interventional studies.
In conclusion, our results suggest that in adults with uncontrolled asthma, abdominal adiposity (as measured by WHtR) and poor sleep quality may contribute to poorer asthma control, controlling for BMI. These findings have potential clinical and public health relevance because of the increasing prevalence of abdominal obesity and sleep disturbances in the United States (49, 50). Waist circumference should be a standard measure like weight and height in clinical research and practice. If confirmed in future research, clinical interventions to address the mechanisms linking abdominal obesity or sleep quality with poorer asthma control may complement standard asthma treatment to improve the quality of care and health outcomes among adults with uncontrolled asthma.
Acknowledgments
Acknowledgment
The authors thank all participants for their continuing support of the project.
Footnotes
Supported by grant R34 HL108753 from the National Heart, Lung, and Blood Institute and internal funding from the Palo Alto Medical Foundation Research Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Contributions: Conception and design: N.L. and J.M.; oversight of data acquisition: J.M. and P.S.; data analysis: N.L. and L.X.; manuscript writing and/or critical revisions for important intellectual content: N.L., L.X., C.A.C., S.R.W., A.S.B., P.S., K.C.N., and J.M.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001--2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 3.Martinez FD. Trends in asthma prevalence, admission rates, and asthma deaths. Respir Care. 2008;53:561–565. [Discussion pp. 565–567.]. [PubMed] [Google Scholar]
- 4.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 5.Farah CS, Salome CM. Asthma and obesity: a known association but unknown mechanism. Respirology. 2012;17:412–421. doi: 10.1111/j.1440-1843.2011.02080.x. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 7.Von Behren J, Lipsett M, Horn-Ross PL, Delfino RJ, Gilliland F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, waist size and prevalence of current asthma in the California Teachers Study cohort. Thorax. 2009;64:889–893. doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Xiao L. Association of general and central obesity and atopic and nonatopic asthma in US adults. J Asthma. 2013;50:395–402. doi: 10.3109/02770903.2013.770014. [DOI] [PubMed] [Google Scholar]
- 9.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE North West Adelaide Health Study Team. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Brumpton B, Langhammer A, Romundstad P, Chen Y, Mai XM. General and abdominal obesity and incident asthma in adults: the HUNT study. Eur Respir J. 2013;41:323–329. doi: 10.1183/09031936.00012112. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Rennie D, Cormier YF, Dosman J. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr. 2007;85:35–39. doi: 10.1093/ajcn/85.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 13.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515.e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teodorescu M, Polomis DA, Hall SV, Teodorescu MC, Gangnon RE, Peterson AG, Xie A, Sorkness CA, Jarjour NN. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138:543–550. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partridge MR. Examining the unmet need in adults with severe asthma. Eur Respir Rev. 2007;16:67–72. [Google Scholar]
- 16.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE, Group TS TENOR Study Group. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007;62:126–133. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 17.Doz M, Chouaid C, Com-Ruelle L, Calvo E, Brosa M, Robert J, Decuypère L, Pribil C, Huerta A, Detournay B. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med. 2013;13:15. doi: 10.1186/1471-2466-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marco F, Verga M, Santus P, Giovannelli F, Busatto P, Neri M, Girbino G, Bonini S, Centanni S. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22–28. doi: 10.1016/j.rmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Lv N, Xiao L, Camargo CA, Wilson SR, Strub P, Buist S, Nadeau KC, Lavori P, Ma J. Abdominal and general obesity, sleep disturbance, and level of control in adults with uncontrolled asthma [abstract] Am J Respir Crit Care Med. 2014;189:A5380. [Google Scholar]
- 20.Ma J, Strub P, Lavori PW, Buist AS, Camargo CA, Jr, Nadeau KC, Wilson SR, Xiao L. DASH for asthma: a pilot study of the DASH diet in not-well-controlled adult asthma. Contemp Clin Trials. 2013;35:55–67. doi: 10.1016/j.cct.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Golan M. Parents as agents of change in childhood obesity–from research to practice. Int J Pediatr Obes. 2006;1:66–76. doi: 10.1080/17477160600644272. [DOI] [PubMed] [Google Scholar]
- 23.NHLBIPractical guide to the identification, evaluation and treatment of overweight and obesity in adults. Bethesda, MD: Public Health Service, US Department of Health and Human Services; 2000. NIH Publication No. 00–4084 [Google Scholar]
- 24.Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) Dig Dis Sci. 2002;47:1530–1537. doi: 10.1023/a:1015815102175. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 27.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 29.McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63:649–654. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 30.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Després JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol. 2012;28:642–652. doi: 10.1016/j.cjca.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 34.DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR. Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr Hypertens Rep. 2010;12:93–98. doi: 10.1007/s11906-010-0095-5. [DOI] [PubMed] [Google Scholar]
- 35.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 36.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 37.Schneider HJ, Klotsche J, Silber S, Stalla GK, Wittchen HU. Measuring abdominal obesity: effects of height on distribution of cardiometabolic risk factors risk using waist circumference and waist-to-height ratio. Diabetes Care. 2011;34:e7. doi: 10.2337/dc10-1794. [DOI] [PubMed] [Google Scholar]
- 38.Koch E, Romero T, Romero CX, Aguilera H, Paredes M, Vargas M, Ahumada C. Early life and adult socioeconomic influences on mortality risk: preliminary report of a ‘pauper rich’ paradox in a Chilean adult cohort. Ann Epidemiol. 2010;20:487–492. doi: 10.1016/j.annepidem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Del-Rio-Navarro BE, Fanghänel G, Berber A, Sánchez-Reyes L, Estrada-Reyes E, Sienra-Monge JJ. The relationship between asthma symptoms and anthropometric markers of overweight in a Hispanic population. J Investig Allergol Clin Immunol. 2003;13:118–123. [PubMed] [Google Scholar]
- 40.Bustos P, Amigo H, Oyarzún M, Rona RJ. Is there a causal relation between obesity and asthma? Evidence from Chile. Int J Obes (Lond) 2005;29:804–809. doi: 10.1038/sj.ijo.0802958. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Rennie D, Cormier Y, Dosman J. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: the Humboldt study. Chest. 2005;128:3048–3054. doi: 10.1378/chest.128.4.3048. [DOI] [PubMed] [Google Scholar]
- 42.Vangeepuram N, Teitelbaum SL, Galvez MP, Brenner B, Doucette J, Wolff MS.Measures of obesity associated with asthma diagnosis in ethnic minority children J Obes 2011. 2011:517417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon AE, Clerisme-Beaty EM, Sugar EA, Cohen RI, Lang JE, Brown ED, Richter JE, Irvin CG, Mastronarde JG. Effects of obstructive sleep apnea and gastroesophageal reflux disease on asthma control in obesity. J Asthma. 2011;48:707–713. doi: 10.3109/02770903.2011.601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, Chung KF, Erzurum S, Israel E, Strollo PJ, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16:1129–1137. doi: 10.1007/s11325-011-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitiello MV. Effective treatment of sleep disturbances in older adults. Clin Cornerstone. 2000;2:16–27. doi: 10.1016/s1098-3597(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 46.Braido F, Baiardini I, Ghiglione V, Fassio O, Bordo A, Cauglia S, Canonica GW. Sleep disturbances and asthma control: a real life study. Asian Pac J Allergy Immunol. 2009;27:27–33. [PubMed] [Google Scholar]
- 47.Sears MR. The evolution of beta2-agonists. Respir Med. 2001;95:S2–6. doi: 10.1053/rmed.2001.1138. [DOI] [PubMed] [Google Scholar]
- 48.Teodorescu M, Xie A, Sorkness CA, Robbins J, Reeder S, Gong Y, Fedie JE, Sexton A, Miller B, Huard T, Hind J, Bioty N, Peterson E, Kunselman SJ, Chinchilli VM, Soler X, Ramsdell J, Loredo J, Israel E, Eckert DJ, Malhotra A. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10:183–193. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 50.Institute of Medicine (US) Committee on Sleep Medicine and Research Colten HR, Altevogt BM, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 200655–136. [PubMed] [Google Scholar]


