Abstract
Background
The appropriate treatment of non-small cell lung cancer (NSCLC) with single brain metastasis (SBM) is still controversial. A systematic review was designed to evaluate the effectiveness of neurosurgery and stereotactic radiosurgery (SRS) in patients with SBM from NSCLC.
Material/Methods
PUBMED, EMBASE, the Cochrane Library, Web of Knowledge, Current Controlled Trials, Clinical Trials, and 2 conference websites were searched to select NSCLC patients with only SBM who received brain surgery or SRS. SPSS 18.0 software was used to analyze the mean median survival time (MST) and Stata 11.0 software was used to calculate the overall survival (OS).
Results
A total of 18 trials including 713 patients were systematically reviewed. The MST of the patients was 12.7 months in surgery group and 14.85 months in SRS group, respectively. The 1, 2, and 5 years OS of the patients were 59%, 33%, and 19% in surgery group, and 62%, 33%, and 14% in SRS group, respectively. Furthermore, in the surgery group, the 1 and 3 years OS were 68% and 15% in patients with controlled primary tumors, and 50% and 13% in the other patients with uncontrolled primary tumors, respectively. Interestingly, the 5-year OS was up to 21% in patients with controlled primary tumors.
Conclusions
There was no significant difference in MST or OS between patients treated with neurosurgery and SRS. Patients with resectable lung tumors and SBM may benefit from the resection of both primary lesions and metastasis.
MeSH Keywords: Brain Neoplasms; Carcinoma, Non-Small-Cell Lung; Craniotomy; Radiosurgery; Review
Background
Lung cancer is one of the leading causes of death worldwide [1,2], and non-small cell lung cancer (NSCLC) accounts for 80% to 85% of this kind of malignancy [3]. Brain metastases are common in patients with lung cancer and reach a rate of approximately 30% to 50% [4,5], about half of which are single brain metastasis (SBM) [6]. Currently, more and more brain metastases are found in NSCLC patients [7,8], and patients with brain metastasis are usually considered to be terminal stage and to have poor prognosis. The median survival time (MST) of NSCLC patients with brain metastases is 1~2 months in patients without any treatment and 4 to 6 months in patients that received radiotherapy, respectively [9,10]. Unlike the patients with multiple brain metastases, patients with SBM exhibit a potential to improve the duration (exceeding 1–2 years) and quality of life [11].
The current methods for treatment of NSCLC patients with SBM are neurosurgery, stereotactic radiosurgery (SRS), and whole-brain radiotherapy (WBRT) [12]. However, the standard treatment schedule for these patients is still unclear. Many researches had investigated the appropriate treatment for NSCLC patients with SBM, with the conclusion that local tumor control rates were similar between surgery and SRS in selected patients [13]. Patients with SBM who received surgical resection or SRS combined with WBRT had better overall survival (OS) and quality of life compared to those treated with WBRT alone [14–16]. Some researcher believe that postoperative WBRT may reduce the recurrence of cancer in the brain, but surgical resection or SRS alone may provide an equivalent survival advantage and less deterioration of neurocognitive functions compared with surgery or SRS plus WBRT [17,18]. In addition, neurosurgery provided longer survival time than SRS or other form of conservative management [19,20]. Which is better, surgery or SRS? To answer the question, systematic review and analysis of the survival data related to different treatments of these patients is necessary.
Currently, there is still no prospective randomized clinical trial comparing surgical resection with SRS for patients with SBM from NSCLC. In this study, we performed a systematic review to evaluate the MST and OS of patients with SBM from NSCLC who received surgical resection and SRS.
Material and Methods
Search strategy
We searched PubMed, EMBASE, the Cochrane Library, Web of Knowledge, Current Controlled Trials, Clinical Trials, 2 conference websites (the American Society for Radiation Oncology (ASTRO), and the American Society of Clinical Oncology (ASCO) for relevant articles published up to April 1, 2014. We scrutinized the reference lists from the selected papers for any other additional studies and limited studies to those written in English. Mesh and text word searches were undertaken in PUBMED, EMBASE, and Cochrane Library databases, including the following: ‘lung neoplasms’, ’brain neoplasms’, ’lung tumor’, ’brain metastases’, ‘surgery’, and ‘radiosurgery’; text word searches were conducted in the other databases using the search terms ‘lung cancer’, ‘brain metastases’, ‘surgery’, and ‘radiotherapy’.
Inclusion criteria
1. Types of studies: randomized controlled trials, clinical control trials, cohort study, and retrospective case series concerning neurosurgery or SRS for SBM from NSCLC. 2. Types of participants: among the selected patients, NSCLC were histologically diagnosed, and brain metastases were confirmed by histological examination, magnetic resonance imaging (MRI), or enhanced computed tomography (CT) scan. 3. Types of interventions: surgery with or without WBRT or SRS (including gamma knife radiosurgery) with or without WBRT. 4. Outcome measures: median survival time (MST) and overall survival (OS).
Exclusion criteria
1. Patients with multiple brain metastases, small-cell lung cancer, solitary brain metastasis from other primary malignancies. 2. NSCLC patients with multiple organ metastases were excluded. 3. Studies of the palliative therapy such as WBRT alone or chemotherapy alone were excluded. 4. Articles with no eligible data were excluded.
Study selection
Eligibility assessment was first performed by screening titles and abstracts, and further assessment was performed by reviewing the full text. All of the eligible articles were selected by 2 reviewers (Qin and Wang) according to the inclusion and exclusion criteria. Disagreement on article inclusion was solved by discussion.
Data collection
All studies were evaluated by 2 reviewers (Qin and Wang) to assess whether the studies matched the inclusion criteria. There were no updated studies. The following information was abstracted from all eligible studies: title, authors, publication time, number of cases, male-to-female ratio, average age, performance status, pathological types, and tumor node metastasis (TNM) stage); treatment of metastases, treatment of primary lung cancer, and outcomes (MST and overall survival rate).
Quality assessment
In this review, all included studies are non-randomized controlled trials, and most of them are non-comparative studies. Few validated instruments are available to determine the methodological quality of observational or non-comparative studies. Finally, the Methodological Index for Non-Randomized Studies (MINORS) [21] was used to assess the quality of eligible studies. MINORS is a valid instrument designed to assess the methodological quality of non-randomized studies, whether comparative or non-comparative which contained 12 items, the first 8 being specifically for non-comparative studies, whereas all 12 items are relevant to comparative studies. Each item is scored 0 (not reported), 1(reported but inadequately), and 2 (reported adequately). The ideal global score would be 16 for the non-comparative studies and 24 for the comparative studies. Two reviewers (Qin and Wang) independently evaluated and cross-checked quality of the studies.
Statistical analysis
SPSS 18.0 statistical software was used to analyze the MST of each group. Stata 11.0 software was used to calculate single-sample rate meta-analyses to determine the survival rate of each group. Chi-square and I-square tests were used to test heterogeneity amongst the studies. The significance threshold of chi-square was set at a=0.1, it was deemed that heterogeneity existed when P<0.1. I2 was used to quantify the heterogeneity across trials and to assess the impact of heterogeneity on the meta-analysis; the value was more than 50% considerable heterogeneity. The OS was estimated using a fixed-effects model if there was no heterogeneity; otherwise the random-effects model was used.
Results
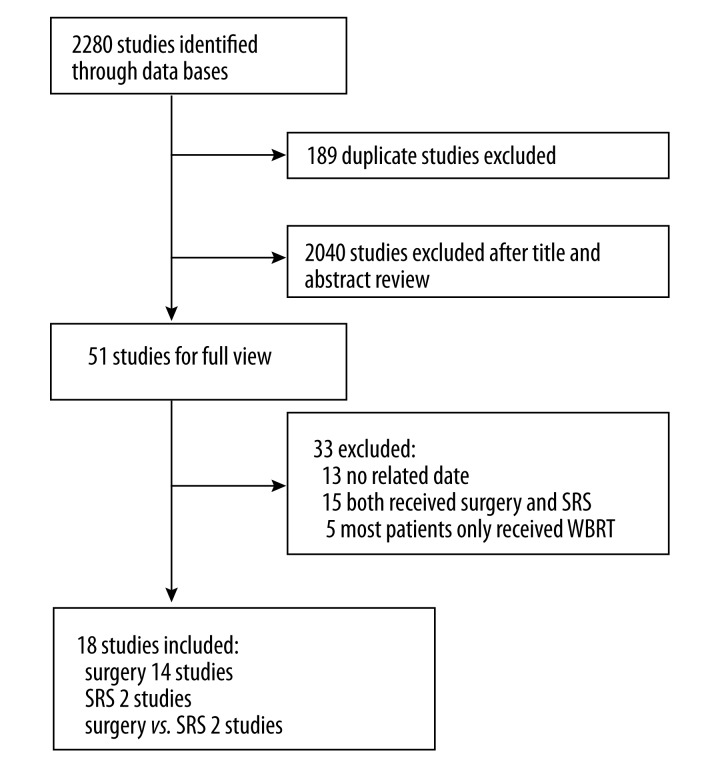
A total of 18 articles are included in our final study after reviewing a total of 2280 selected articles. All studies were observational. There were 2 clinical control studies (Hu, C. S. 2006 Li, H. 2009), 16 retrospective case series, and no randomized controlled trials. The screening process and results of the literature are presented in Figure 1.
Figure 1.
A flow chart of selecting trials included in the meta-analysis.
General characteristics of the eligible studies
There were 713 patients among the 18 studies between 1976 and 2011 (Table 1), including 7 patients with 2 brain metastases and 1 patient with 3 brain metastases. According to the different treatments for brain metastases, 16 eligible studies were included in the surgery group and 4 studies in the SRS group. Data from the 2 clinical control trials are distributed to the surgery and SRS group (e.g., Hu, C. S. et al. (part), Li, H. et al. (part) in Table 1.). For subgroup analysis, the surgery group was divided into a “controlled” primary subgroup (all the participants in this trial with complete resection of the primary lung tumor) and an “uncontrolled” primary subgroup (not all the patients received resection of the primary lung tumor (i.e., some with palliative radiotherapy, chemotherapy, or no treatment), which contained 9 studies and 7 studies, respectively.
Table 1.
General characteristics of the eligible studies.
| Studies | Years | Groups | Cases | Average ages | MST | OS | MINORS score | |
|---|---|---|---|---|---|---|---|---|
| Tummarello, D. et al. | 1985 | Surgery** | 15 | 49 y | 6 M | – | 11 | |
|
| ||||||||
| Saitoh, Y. et al. | 1999 | Surgery* | 24 | 57.8 y | 6.8 M | 3-year | 12.5% | 12 |
| 5-year | 8.3% | |||||||
|
| ||||||||
| Mussi, A. et al. | 1985 | Surgery* | 20 | 50.5 y | – | 5-year | 33.6% | 10 |
|
| ||||||||
| Macchiarini, P. et al. | 1991 | Surgery* | 37 | 56 y | 27 M | 5-year | 37.0% | 10 |
|
| ||||||||
| Louie, A. V. et al. | 2009 | Surgery** | 35 | 58.5 y | 7.8 M | – | 14 | |
|
| ||||||||
| Granone, P. et al. | 2001 | Surgery* | 30 | 58.7 y | 27.8 M | 1-year | 80.0% | 11 |
| 2-year | 41.0% | |||||||
| 3-year | 17.0% | |||||||
|
| ||||||||
| Catinella, F. P. et al. | 1989 | Surgery* | 12 | – | – | 1-year | 90.0% | 8 |
| 4-year | 56.0% | |||||||
|
| ||||||||
| Bai, H. et al. | 2011 | Surgery** | 18 | 54 y | 12.8 M | 1-year | 83.3% | 11 |
| 2-year | 48.8% | |||||||
| 3-year | 13.9% | |||||||
|
| ||||||||
| Wang, Z. M. et al. | 2002 | Surgery** | 32 | 55.8 y | 12 M | 1-year | 40.6% | 12 |
|
| ||||||||
| Mussi, A. et al. | 1996 | Surgery* | 45 | 56.9 y | 19 M | 5-year | 16.0% | 13 |
|
| ||||||||
| Magilligan, D. J., Jr. | 1987 | Surgery* | 41 | – | 13 M | 1-year | 55.0% | 10 |
| 2-year | 31.0% | |||||||
| 4-year | 21.0% | |||||||
| 5-year | 21.0% | |||||||
|
| ||||||||
| Magilligan, D. J., Jr. | 1976 | Surgery* | 22 | 54.8 y | 14 M | 1-year | 45.0% | 9 |
|
| ||||||||
| Bonnette, P. | 2001 | Surgery** | 103 | 54 y | 12.4 M | 1-year | 56.0% | 14 |
| 2-year | 28.0% | |||||||
| 3-year | 13.0% | |||||||
| 4-year | 11.0% | |||||||
|
| ||||||||
| Ampil, F. et al. | 2007 | Surgery** | 28 | – | 5.5 M | 1-year | 21.0% | 16 |
|
| ||||||||
| Hu, C. S. et al. (part) | 2006 | Surgery** | 53 | 60 y | 12 M | – | 23 | |
|
| ||||||||
| Li, H. et al. (part) | 2009 | Surgery* | 35 | – | 13 M | – | 22 | |
|
| ||||||||
| Flannery, T. W. et al. | 2008 | SRS | 42 | 58 y | 18 M | 1-year | 71.3% | 12 |
| 2-year | 34.1% | |||||||
| 5-year | 21.0% | |||||||
|
| ||||||||
| Flannery, T. W. et al. | 2003 | SRS | 72 | 58 y | 15.7 M | 1-year | 61.4% | 12 |
| 2-year | 32.6% | |||||||
| 5-year | 10.4% | |||||||
|
| ||||||||
| Hu, C. S. et al. (part) | 2006 | SRS | 31 | 63 y | 7.4 M | – | 23 | |
|
| ||||||||
| Li, H. et al. (part) | 2009 | SRS | 18 | – | 14 M | 1-year | 44.8% | 22 |
Surgery* – subgroup of surgery which had controlled primary tumor; surgery** – subgroup of surgery which had uncontrolled primary tumor.
The MST range of the patients in the surgery group was 5.5–27.8 months and in the SRS group it was 7.4–18 months. The 1-year, 2-year, and 5-year survival rates ranged from 21–90%, 28–48.8%, and 8.3–37% in the patients treated with surgery, and 44.8–71.3%, 32.6–34.1%, and 10.4–21% in the patients treated with SRS. In the 18 eligible studies, 12 studies reported survival outcomes from the time of craniotomy or lung surgery, 3 studies reported survival from the time of diagnosis of brain metastasis or lung cancer, and 3 studies did not clearly define how survival outcomes were measured.
The basic characteristics of each group
The basic characteristics of the 2 groups are presented in Table 2. There were 550 cases in the surgery group, and 163 cases in the SRS group. The average age was 55.5 years and 59.7 years, respectively. All of the participants exhibited good performance status (PS ≥70). The male/female ratios were 3: 1 in the surgery group and 2: 1 in the SRS group. The pathological types in the surgery group were adenocarcinoma (53.5%), squamous cell carcinoma (25.1%), large-cell carcinoma (5%), and others (16.4%). The T status of the primary tumor in the surgery group was classified as T1–2, and only 2 participants were stage T4. With respect to the N stages in the surgery group, there were 151 patients in N0 stage, 35 patients in N1 stage, 60 patients in N2 stage, and 1 patient in N3 stage. In the SRS group, only N stages were acquired. Furthermore, in the surgery group, approximately 39.6% of patients received WBRT, 71.6% of patients underwent resection of the lung lesion, and 20.5% of patients underwent thoracic radiotherapy. In the SRS group, the number of patients who received WBRT, lung lesion resection, and thoracic radiotherapy were 47.9%, 10.4%, and 8.6%, respectively.
Table 2.
The basic characteristics of each group.
| Surgery group | SRS group | ||
|---|---|---|---|
| Total number | 550 | 163 | |
| Average ages | 55.5 y | 59.7 y | |
| Male: female | 331: 103 | 96: 49 | |
| S: M* | 101: 76 | 81: 33 | |
| Pathological | ADC* | 196 | – |
| SCC* | 103 | – | |
| LC* | 17 | – | |
| T stage | T1–2 | 201 | – |
| T3 | 55 | – | |
| T4 | 2 | – | |
| N stage | N0 | 151 | 20 |
| N1 | 35 | 6 | |
| N2 | 60 | – | |
| N3 | 1 | – | |
| WBRT | 218 | 78 | |
| Thoracic surgery | 394 | 17 | |
| Thoracic radiotherapy | 113 | 14 |
S: M – synchronous versus metachronous brain metastasis; ADC – adenocarcinoma; SCC – squamous cell carcinoma; LC – large cell carcinoma.
Quality of studies
MINORS was used to assess all the included studies. The scores range from 8 to 16 for the non-comparative studies and 22 to 23 for the comparative studies (Table 1), which demonstrated a moderate methodological heterogeneity of the studies included.
Mean survival time (MST) and survival rate
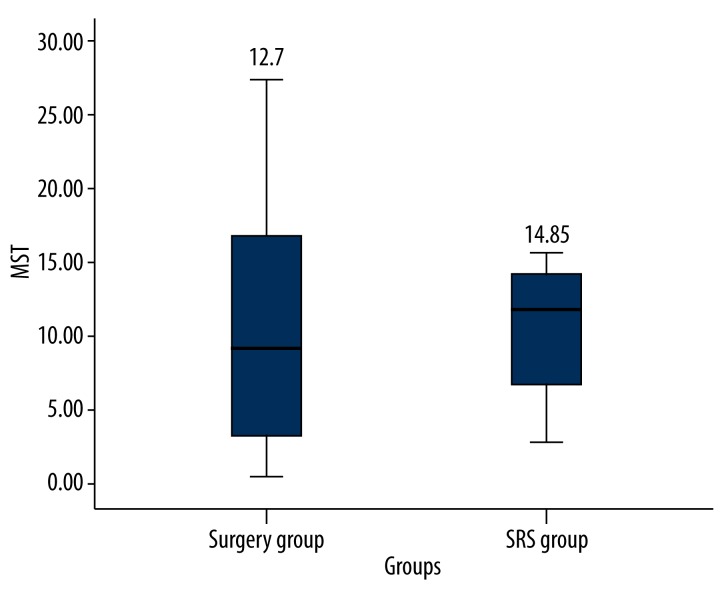
The MST of surgery and SRS were 12.7 months and 14.85 months, respectively (Figure 2). In the surgery group, 2 studies did not report the MST, and statistical analysis was only conducted on 14 studies.
Figure 2.
The MST of the patients was 12.7 months in surgery group and 14.85 months in SRS group.
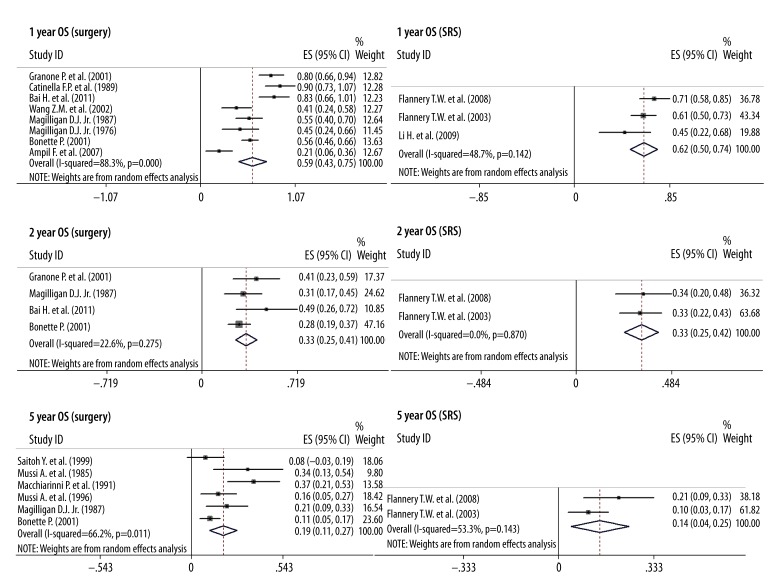
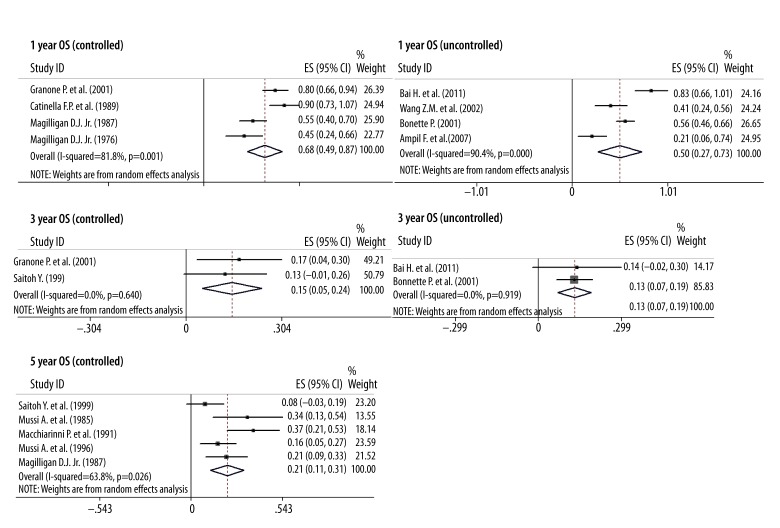
The 1-year, 2-year, and 5-year OS were 59%, 33%, and 19% in the surgery group, and 62%, 33%, and 14% in the SRS group, respectively (Figure 3). Based on the above data, we concluded that there was no significant difference between the surgery and SRS group, and that neurosurgery tends to confer longer survival. In addition, the 1-year, 3-year, and 5-year OS of complete resection of the lung lesions combined with craniotomy in the surgery group were 68%, 15%, and 21%, and the 1-year and 3-year OS of patients with uncontrolled primary lung cancer in the surgery group were 50% and 13%, respectively (Figure 4). Therefore, the resection of the primary tumor and SBM is worthwhile for patients.
Figure 3.
The 1-, 2-, and 5-year OS of the patients were 59%, 33%, and 19% in surgery group, and 62%, 33%, and 14% in SRS group, respectively.
Figure 4.
In the surgery group, the 1- and 3-year OS were 68% and 15% in patients with controlled primary tumors, and 50% and 13% in the other patients with uncontrolled primary tumors. The 5-year OS was up to 21% in patients with controlled primary tumors.
Furthermore, in surgery group, most studies published before the year 2000, and all studies in the SRS group, were published after 2000. Over the last 2 decades, the survival of NSCLC has been improving enormously owing to the progress of systemic therapy. Therefore, when we excluded the studies published before 2000, and reanalyzed the MST and OS in the surgery group, the results showed that the MST was 12.2 months, and the 1- and 2-year OS was 56% and 36%.
Discussion
In our systematic review and meta-analysis, both surgical resection and SRS are believed to be an effective treatment for patients with SBM from NSCLC, and there is no significant difference between effectiveness of surgery and SRS. In the subgroup analysis, combined excision of the primary tumor and the metastasis can achieve a 5-year survival rate up to 21%, which indicates that controlling the primary tumor is associated with better survival in patients with SBM.
WBRT was first reported for treatment of brain metastases in the mid-1950s, and it has long remained a fundamental treatment for patients with brain metastases to improve survival time. In the 1990s, 2 prospective randomized trials [14,15] illustrated that in patients with SBM, surgical resection followed by WBRT improved overall survival and quality of life compared with WBRT alone, which established the role of surgical techniques in the management of brain metastases. Surgical resection was mainly recommended for patients with SBM in an accessible location, especially for those with good functional status and absent or controlled systemic disease.
SRS delivers focused high-dose radiation to a small area and avoids significant damage to the surrounding tissue, which is particularly useful in patients who are not candidates for surgery or with lesions in non-resectable locations [22]. One study (RTOG 9508) showed a survival benefit of SRS plus WBRT over WBRT alone (6.5 months versus 4.9 months, respectively; P=0.0393) in patients with SBM, which greatly contributed to the increased use of SRS for treatment of brain metastases during the last decade [16]. SRS has become popular as a treatment option for brain metastases, especially for the SBM, due to being less invasive than surgical resection and exposing healthy tissue to less radiation than WBRT [23]. In addition, for patients with 1–2 brain metastases, SRS can achieve long-term survival as well as neurosurgery, and both SRS alone and SRS with WBRT offer equivalent levels of survival compared to patients treated with surgery followed by WBRT [25].
Which management is preferable for patients with SBM – neurosurgery or SRS? No convincing results have been found and controversy still exists. A prospective study by Muacevic et al. [24] showed that the treatment results did not differ in terms of survival (P=0.8), neurological death rates (P=0.3), and freedom from local recurrence (P=0.06) between the patients treated with SRS and those treated with surgical resection followed by WBRT. Similarly, several retrospective studies [19,24,25] also indicated that there was no survival difference between the 2 treatment groups. However, Bindal et al. [26] reported that the MST in the surgery group was significantly longer than in the SRS group (16.4 months versus 7.5 months), and that there was a higher incidence of death from neurological causes in the SRS group than in the surgery group (50% and 19%, respectively). In our analysis, there was no statistical different between brain surgery and SRS in patients with SBM from NSCLC. However, 71.6% and 20.5% received thoracic surgery and radiation therapy, respectively, in the surgery group and only 10.4% and 8.6% in the SRS group, so SRS as a new therapy showed a promising role in the treatment of SBM. In addition, several studies evaluated the treatment efficacy of neurosurgery combined with SRS, and demonstrated a significant improvement in survival and local tumor control in those patients.
In several retrospective studies [27,28], univariate analysis showed that survival times could be improved when both the primary lung tumor and brain metastases were resected, thus removal of the primary site and SBM appears to be an effective treatment for long-term survival. The American National Cancer Institute reported that the 5-year survival rates of patients with stage IIIA, IIIB, and IV disease were 14%, 5%, and 1%, respectively, and other studies have also reported similar 5-year survival rates for stages IIIB and IV (1–5%) [29,30]. In this study, sub-analysis of the surgery group showed that patients who received resection of both primary and metastasis lesion had a high 5-year survival rate (up to 21%), which is significantly higher than that of stage IIIA. Therefore, we believe that patients with resectable NSCLC with SBM should be reclassified as a new stage.
There are several limitations in our systematic review. First, no randomized controlled trials were included, and most of the eligible studies were non-comparative studies with low certification strength because of the rarity of this particular NSCLC presentation. Second, there are more cases in the surgery group because surgery is more acceptable clinically, and the number of patients in each group is not well matched. Third, we failed to get the survival outcomes of the stage of intra-thoracic, because no separate outcomes were reported. Fourth, publication bias is also inevitable. Although these limitations exist, we have enough confidence to believe that there are no significant differences in MST and survival rate between the surgery group and SRS group. Furthermore, patients who received resection of both primary and metastasis had a high 5-year survival rate that challenged the present stage IV classification of resectable NSCLC with SBM. Prospective and multi-center studies with larger sample sizes are needed to further confirm the results of our study.
Conclusions
NSCLC with brain metastases often has poor prognosis, but patients with NSCLC with SBM exhibit long-term survival. Our results show that patients with SBM from NSCLC can equally benefit from stereotactic radiosurgery and surgery. In addition, the excision of primary lung lesions and SBM is recommended for patients with resectable NSCLC combined with SBM.
Acknowledgements
The authors thank all the participants in this study.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Li Z, Zhang Z, et al. Meta-analysis of GSTM1 null genotype and lung cancer risk in Asians. Med Sci Monit. 2015;21:1239–45. doi: 10.12659/MSM.890490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunese L, Greco B, Setola FR, et al. Non-small cell lung cancer evaluated with quantitative contrast-enhanced CT and PET-CT: Net enhancement and standardized uptake values are related to tumour size and histology. Med Sci Monit. 2013;19:95–101. doi: 10.12659/MSM.883759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–80. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43(4):795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neuro-Oncol. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen AM, Jahan TM, Jablons DM, et al. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668–75. doi: 10.1002/cncr.22565. [DOI] [PubMed] [Google Scholar]
- 8.Varlotto JM, Flickinger JC, Niranjan A, et al. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57(2):452–64. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 9.Mandell L, Hilaris B, Sullivan M, et al. The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer. 1986;58(3):641–49. [PubMed] [Google Scholar]
- 10.Cai Y, Wang JY, Liu H. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non-small cell lung cancer patients with chemotherapy failure. Asian Pac J Cancer Prev. 2013;14(10):5699–703. doi: 10.7314/apjcp.2013.14.10.5699. [DOI] [PubMed] [Google Scholar]
- 11.Connolly EP, Mathew M, Tam M, et al. Involved field radiation therapy after surgical resection of solitary brain metastases – mature results. Neuro Oncol. 2013;15(5):589–94. doi: 10.1093/neuonc/nos328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng YD, Zhang L, Liao H, et al. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non-small-cell lung cancer: a retrospective study. Asian Pac J Cancer Prev. 2012;13(3):909–14. doi: 10.7314/apjcp.2012.13.3.909. [DOI] [PubMed] [Google Scholar]
- 13.Muacevic A, Kreth FW, Horstmann GA, et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91(1):35–43. doi: 10.3171/jns.1999.91.1.0035. [DOI] [PubMed] [Google Scholar]
- 14.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 15.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33(6):583–90. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 16.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 17.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–89. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 18.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. J Clin Oncol. 2011;29(2):134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol. 2003;55(5):1169–76. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 20.Koutras AK, Marangos M, Kourelis T, et al. Surgical management of cerebral metastases from non-small cell lung cancer. Tumori. 2003;89(3):292–97. doi: 10.1177/030089160308900312. [DOI] [PubMed] [Google Scholar]
- 21.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–16. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Khalsa SS, Chinn M, Krucoff M, et al. The role of stereotactic radiosurgery for multiple brain metastases in stable systemic disease: a review of the literature. Acta Neurochir (Wien) 2013;155(7):1321–27. doi: 10.1007/s00701-013-1701-5. discussion 1327–28. [DOI] [PubMed] [Google Scholar]
- 23.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neuro-Oncol. 2008;87(3):299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 25.Schoggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142(6):621–26. doi: 10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- 26.Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84(5):748–54. doi: 10.3171/jns.1996.84.5.0748. [DOI] [PubMed] [Google Scholar]
- 27.Wronski M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: A follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83(4):605–16. doi: 10.3171/jns.1995.83.4.0605. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg. 2004;26(3):488–93. doi: 10.1016/j.ejcts.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Nelson RA, Bogardus A, et al. Five-year lung cancer survival: which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer. 2010;116(6):1518–25. doi: 10.1002/cncr.24871. [DOI] [PubMed] [Google Scholar]
- 30.Escuín JSdC, Vicente CD, Peñafiel JC, et al. Overall Long-Term Survival in Lung Cancer Analyzed in 610 Unselected Patients. Arch Bronconeumol. 2004;40(6):268–74. [PubMed] [Google Scholar]