Abstract
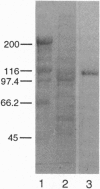
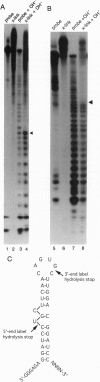
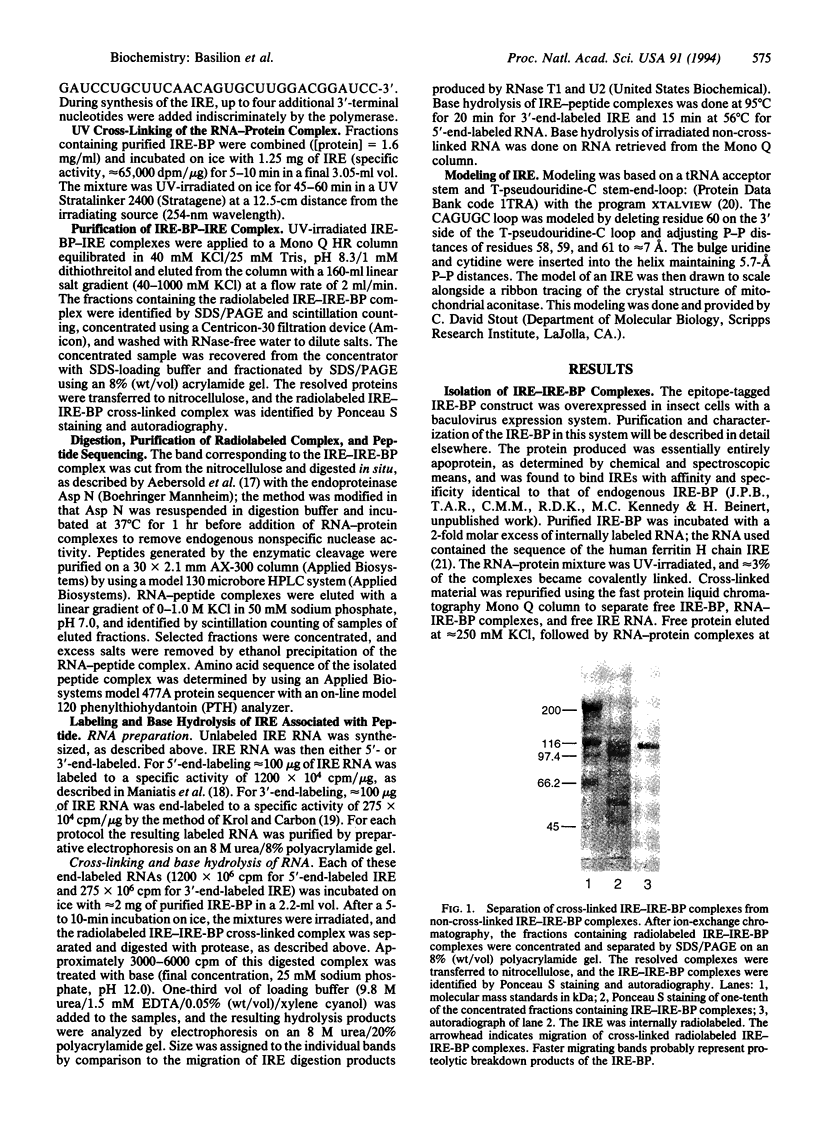
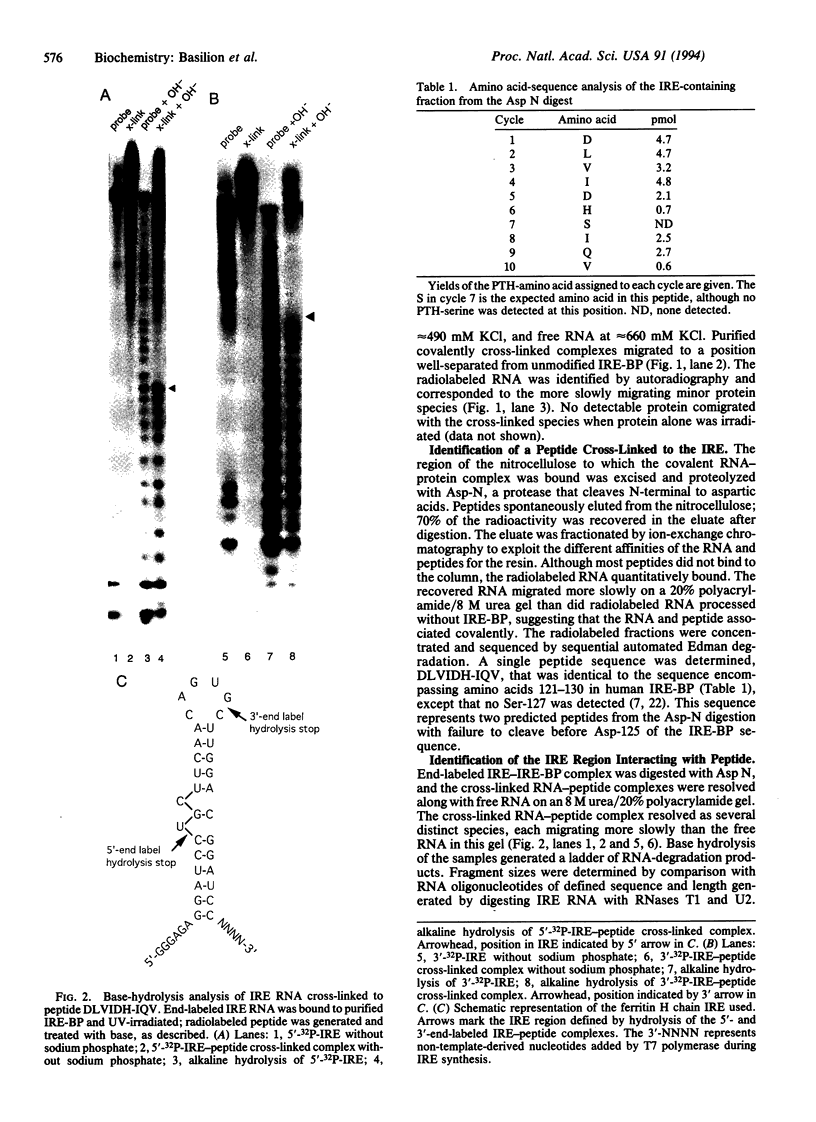
The iron-responsive element-binding protein (IRE-BP) binds to specific stem-loop RNA structures known as iron-responsive elements (IREs) present in a variety of cellular mRNAs (e.g., those encoding ferritin, erythroid 5-aminolevulinate synthase, and transferrin receptor). Expression of these genes is regulated by interaction with the IRE-BP. The IRE-BP is identical in sequence to cytosolic aconitase, and the function of the protein is determined by the presence or absence of an Fe-S cluster. The protein either functions as an active aconitase when the Fe-S cluster is present or as an RNA-binding protein when the protein lacks this cluster. Aconitase activity and IRE-binding activity are mutually exclusive, and interconversion between the two activities is determined by intracellular Fe concentrations. Mapping of the RNA-binding site of the IRE-BP by UV cross-linking studies defines a major contact site between IRE and protein in the active-site region. Modeling based on probable structural similarities between the previously crystallized mitochondrial aconitase and the IRE-BP predicts that these residues would be accessible to the IRE only were there a major change in the predicted conformation of the protein when cells are iron-depleted.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. D., Wick K. L., Matthews K. S. Identification of amino acids in lac repressor protein cross-linked to operator DNA specifically substituted with bromodeoxyuridine. J Biol Chem. 1991 Apr 5;266(10):6113–6119. [PubMed] [Google Scholar]
- Beinert H., Kennedy M. C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989 Dec 8;186(1-2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Emery-Goodman A., Hirling H., Scarpellino L., Henderson B., Kühn L. C. Iron regulatory factor expressed from recombinant baculovirus: conversion between the RNA-binding apoprotein and Fe-S cluster containing aconitase. Nucleic Acids Res. 1993 Mar 25;21(6):1457–1461. doi: 10.1093/nar/21.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsell D. S., Lauble H., Stout C. D., Olson A. J. Automated docking in crystallography: analysis of the substrates of aconitase. Proteins. 1993 Sep;17(1):1–10. doi: 10.1002/prot.340170104. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Ultraviolet light-induced crosslinking of mRNA to proteins. Nucleic Acids Res. 1979 Feb;6(2):715–732. doi: 10.1093/nar/6.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzy T. G., Freeman J. P., Johnson A. E., Merrick W. C. A model for the aminoacyl-tRNA binding site of eukaryotic elongation factor 1 alpha. J Biol Chem. 1992 Jan 25;267(3):1623–1632. [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Krol A., Carbon P. A guide for probing native small nuclear RNA and ribonucleoprotein structures. Methods Enzymol. 1989;180:212–227. doi: 10.1016/0076-6879(89)80103-x. [DOI] [PubMed] [Google Scholar]
- Lauble H., Kennedy M. C., Beinert H., Stout C. D. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry. 1992 Mar 17;31(10):2735–2748. doi: 10.1021/bi00125a014. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Laudano A., Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990 Apr 11;18(7):1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. C., Haile D., Rouault T. A., Klausner R. D. Modification of a free Fe-S cluster cysteine residue in the active iron-responsive element-binding protein prevents RNA binding. J Biol Chem. 1993 Aug 25;268(24):17655–17658. [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. The structure of aconitase. Proteins. 1989;5(4):289–312. doi: 10.1002/prot.340050406. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Haile D. J., Downey W. E., Philpott C. C., Tang C., Samaniego F., Chin J., Paul I., Orloff D., Harford J. B. An iron-sulfur cluster plays a novel regulatory role in the iron-responsive element binding protein. Biometals. 1992 Autumn;5(3):131–140. doi: 10.1007/BF01061319. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R., Chen L., Buettner J. A., Hudson D., Frankel A. D. RNA recognition by an isolated alpha helix. Cell. 1993 Jun 4;73(5):1031–1040. doi: 10.1016/0092-8674(93)90280-4. [DOI] [PubMed] [Google Scholar]