SUMMARY
The aim of this study was to describe our experience with benign parapharyngeal space tumours resected via a transcervical route without mandibulotomy and to investigate associated postoperative sequelae and complications. The study investigated and analysed the retrospective charts of 44 patients who underwent surgery for benign parapharyngeal space tumours over a 10-year period. The diagnosis was reached in all patients with clinical and radiologic findings; preoperative fine-needle aspiration biopsy was not performed in any case. The preferred means of accessing the parapharyngeal space in all patients was a transcervical route. In 5 of these patients, transparotid extension was performed due to the position of the tumour. Tumours were classified radiologically as poststyloid in 27 cases and prestyloid in 17 cases. The final histopathologic diagnosis was vagal paraganglioma in 16 cases, pleomorphic adenoma in 13 cases, schwannoma in 10 cases and comparatively rarer tumours in the remaining 5 cases. In three patients, cranial nerve paralysis was observed during preoperative evaluation. Permanent cranial nerve paralysis occurred in 19 cases (43.2%) in the postoperative period, the majority of which were neurogenic tumours such as vagal paraganglioma (n = 16) and schwannoma (n = 2), and one case of non-neurogenic parapharyngeal tumour. The median duration of follow-up was 61 ± 33 months. There was no local recurrence in any patient during the follow-up period. A transcervical approach should be the first choice for excision of parapharyngeal space tumours, except for recurrent or malignant tumours, considering its advantages of providing direct access to the neoplasm, adequate control of neurovascular structures from the neck and optimal aesthetic outcomes due to preservation of mandibular continuity with minimal morbidity and hospitalisation time.
KEY WORDS: Parapharyngeal, Benign, Tumour, Transcervical, Mandibulotomy
RIASSUNTO
Scopo del presente studio è di descrivere la nostra esperienza riguardo i tumori benigni della regione parafaringea sottoposti a resezione chirurgica per via transcervicale senza mandibulotomia e valutarne le complicanze post-operatorie. Questo studio analizza retrospettivamente una serie di 44 pazienti sottoposti ad intervento chirurgico per tumori benigni della regione parafaringea nell'arco temporale di 10 anni. La diagnosi è stata formulata in tutti i pazienti sulla base dei dati clinici e radiologici; in nessun caso è stato utilizzato lo studio citologico su agoaspirato (FNAB). In tutti i casi l'approccio di scelta alla neoformazione è stato quello transcervicale. In 5 pazienti è stato necessario un allargamento alla regione parotidea per via della localizzazione anatomica della lesione. I tumori sono stati classificati radiologicamente in post-stiloidei in 27 casi e in prestiloidei in 17 casi. La diagnosi istopatologica definitiva è risultata in 16 casi di paraganglioma vagale, in 13 casi di adenoma pleomorfo, in 10 casi di schwannoma e di tumori relativamente rari nei rimanenti 5 casi. In tre pazienti è stata osservata paralisi di nervi cranici nel pre-operatorio. Paralisi permanente di nervi cranici è stata osservata in 19 casi (43.2%) nel post-operatorio, nella maggioranza di casi si trattava di tumori neurogenici quali paragangliomi del vago (n:16) e schwannoma (n:2) e in un caso di tumore non-neurogenico della regione parafaringea. Il periodo medio di follow up è stato di 61 mesi (SD +/- 33.10) e in questo lasso di tempo non sono state osservate recidive locali di malattia in nessun paziente. L'approccio per via transcervicale dovrebbe costituire il trattamento chirurgico di prima scelta nei tumori della regione parafaringea eccezion fatta per le forme ricorrenti o maligne. I vantaggi sono legati all'accesso diretto alla regione parafaringea, adeguata esposizione chirurgica delle strutture neurovascolari del collo, miglior risultato estetico associato al mantenimento della continuità della mandibola e ridotte morbidità ed ospedalizzazione.
Introduction
The parapharyngeal space (PPS) is defined as the deep space that forms an inverted triangular pyramid in the neck where the posterior belly of the digastric muscle and hyoid bone forms the apex of the pyramid, and the temporal bone, its base. The fascia stretching from the styloid process to the tensor veli palatini muscle divides the PPS into prestyloid and poststyloid compartments. The prestyloid compartment contains the deep lobe of the parotid gland, fibroadipose tissues, medial and lateral pterygoid muscles and several lymph nodes. Additionally, the internal maxillary artery and vein, lingual, inferior alveolar and auriculotemporal nerves course through the prestyloid compartment. In contrast, the poststyloid compartment contains more vital structures such as the internal carotid artery, internal jugular vein and cranial nerves (CN) IX, X and XI. The sympathetic nerve chain and numerous lymph nodes are also located in the poststyloid compartment.
Primary tumours of the PPS are very rare, comprising approximately 0.5% of all head and neck tumours 1.They often present asymptomatic growth and can stay undetected for long periods of time or may be detected as an incidental mass during screening for another reason. These tumours frequently manifest via medial displacement of the lateral wall of the oropharynx or via a growth on the upper neck, and nearly 50% of patients present with a neck mass 2. Symptoms are generally related to the position of the tumour and may include foreign body sensation in the pharynx, difficulty in deglutition and hoarseness. Cranial nerve deficits and otologic manifestations such as hearing loss are rarely observed. A wide variety of primary tumours may be seen in this anatomical region; fortunately most are benign (70-80%) 3 4. The most frequent benign tumour is pleomorphic adenoma followed by paraganglioma; the most common malignancies are also of salivary gland origin 3.
There are several approaches for surgery of PPS. The most preferred approaches involve a transcervical route for tumours in the prestyloid compartment and a combined transparotid-transcervical route for tumours in the poststyloid compartment or for those originating from the deep lobe of parotid gland. Transcervical approaches can also be combined with mandibulotomy for removal of malignant tumours, tumours with vascular origin and recurrent tumours 3 4. In surgical approaches combined with mandibulotomy, damage to the inferior alveolar nerve, malocclusion and non-union-malunion defects and loss of dentition may occur. Additionally, in some types of osteotomies, lip-splitting may be required. Due to damage to the floor of the mouth during the surgery, tracheostomy and nasogastric tube feeding may be required. Fisch described an infratemporal fossa approach for extremely large PPS tumours invading the temporal bone and middle cranial fossa 5. An alternative to this approach is the transcervical-transmastoid technique, which obtains proximal and distal control of the jugular bulb and the internal carotid artery by approaching the skull base from the neck and mastoid 4. In the classic transoral approach to PPS described by Ehrlich in 1950, a curved incision is made along the palatopharyngeal arch and the tumour is enucleated with blunt dissection 6. Due to its major drawbacks, Ducic et al. described a new superior parapharyngeal space approach involving transsection of the soft palate 7. Transoral robotic surgical excision of PPS tumours is an evolving technique. Although robotic surgery is performed in the same way as the traditional transoral approach, there is less damage to the surrounding major neurovascular structures than with the transoral approach; furthermore, in cases of pleomorphic adenoma, the likelihood of capsular violation is relatively high and there is insufficient long-term data on recurrence rates 8-10. Other disadvantages of this technique are high cost and unavailability of the robotic device.
As seen above, due to the complex anatomy of PPS, many surgical approach techniques have been utilised, and all are associated with adverse effects. Herein, we discuss the efficacy, results and complications of transcervical approaches for accessing the PPS in the presence of benign primary tumours.
Materials and methods
In this study, the records of 67 patients who underwent surgery for PPS tumours between January 2001 and December 2010 in a tertiary referral centre were retrospectively reviewed. All patients had the same surgical team. Only tumours originating from the PPS were included and metastatic lesions or tumours extending to the PPS from other parts of the head and neck were excluded. The preoperative clinical signs, symptoms, neurological evaluation of cranial nerves, operative technique, radiologic and histopathologic findings and operative complications were collected from clinical records.
In 19 cases (28.4%), mandibulotomy was performed due to high suspicion of malignancy according to radiological findings or revision surgery; therefore, all of these patients were excluded from the study. The remaining 48 patients were called for a follow-up examination to check for locoregional recurrence and cranial nerve deficits. Four patients could not be contacted and were excluded.
Diagnosis was made with the help of clinical and radiological findings. Magnetic resonance imaging (MRI) was the preferred technique, except for patients who were unsuitable for MRI and were consequently examined by contrasted computerised tomography (CT). In cases with a high suspicion of a vascular tumour, MRI angiography was additionally performed. The proximity of tumours to major blood vessels and the parotid gland were determined and their position was classified as prestyloid or poststyloid via imaging techniques. Preoperative evaluation did not involve FNAB or angiography and embolisation in any case.
During follow-up, at months 1, 2 and 6 after surgery, clinical examination was considered sufficient because all tumours had benign histopathologic diagnoses. At month 12 and yearly thereafter, a head and neck MRI was performed to detect possible recurrence of disease.
Results
Of the 44 cases, there were 15 males and 29 females with an age from 27 to 79 years (mean 44.6 years, SD ± 10.77). The most common clinical findings were neck mass (n = 24, 54.5%) and oropharyngeal mass pushing the pharyngeal structures medially (n = 16, 36.4%) (Table I). Other presenting symptoms were tinnitus, hoarseness, cough and dysphagia. In two patients (4.5%), the parapharyngeal mass was discovered incidentally during radiologic studies for other irrelevant pathologies of the head and neck.
Table I.
Clinical presentation of parapharyngeal space tumours.
| Symptom | Number of patients | % |
|---|---|---|
| Neck mass | 24 | 54.5 |
| Oropharyngeal mass | 16 | 36.4 |
| Pulsatile tinnitus | 3 | 6.8 |
| Incidental | 2 | 4.5 |
| Hoarseness | 2 | 4.5 |
| Dysphagia | 2 | 4.5 |
| Cough | 1 | 2.2 |
In 3 cases a contrast CT scan was preferred due to contraindications for MRI. In the remaining 41 cases, a gadolinium contrasted MRI study was done. In the evaluation of tumours with MRI findings compatible with paraganglioma, routine use of MRI angiography was considered unnecessary, but in 5 cases with a suspicion of vascular origin, an MRI angiography was also performed following the primary radiological study. Radiologic findings compatible with benign tumour histology, which were used to assess the eligibility of the transcervical surgical approach, were defined as the following: well-circumscribed, encapsulated tumour without invasion of surrounding tissues.
The final histopathologic examination revealed vagal paraganglioma in 16 cases (36.4%), pleomorphic adenoma in 13 cases (29.5%) and schwannoma in 10 cases (22.7%) (Table II). Only one schwannoma originated from a cranial nerve, which was expectedly identified as a hypoglossal schwannoma; the remaining schwannomas were from unidentified origins. The comparatively rare tumours observed were giant cell inflammatory granulation tissue (n = 2), neurofibroma (n = 1), lipoma (n = 1) and haemangiopericytoma (n = 1). MRI findings were consistent with histopathologic findings in all cases, and tumours defined radiologically as benign were likewise histopathologically benign.
Table II.
Final histopathologic diagnosis.
| Histology | Number of patients | % |
|---|---|---|
| Paraganglioma | 16 | 36.4 |
| Pleomorphic adenoma | 13 | 29.5 |
| Schwannoma | 10 | 22.7 |
| Giant cell inflammatory granulation tissue | 2 | 4.5 |
| Neurofibroma | 1 | 2.3 |
| Lipoma | 1 | 2.3 |
| Haemangiopericytoma | 1 | 2.3 |
When tumours were classified radiologically according to location, 27 (61.4%) were discovered to originate from the poststyloid PPS and the remaining 17 (38.6%) originated from the prestyloid PPS. The mean tumour diameter was 5.51 cm (SD ± 1.13). The largest tumour was a pleomorphic adenoma with the longest axis of 11 cm and the smallest was a vagal paraganglioma with a diameter of 3 cm.
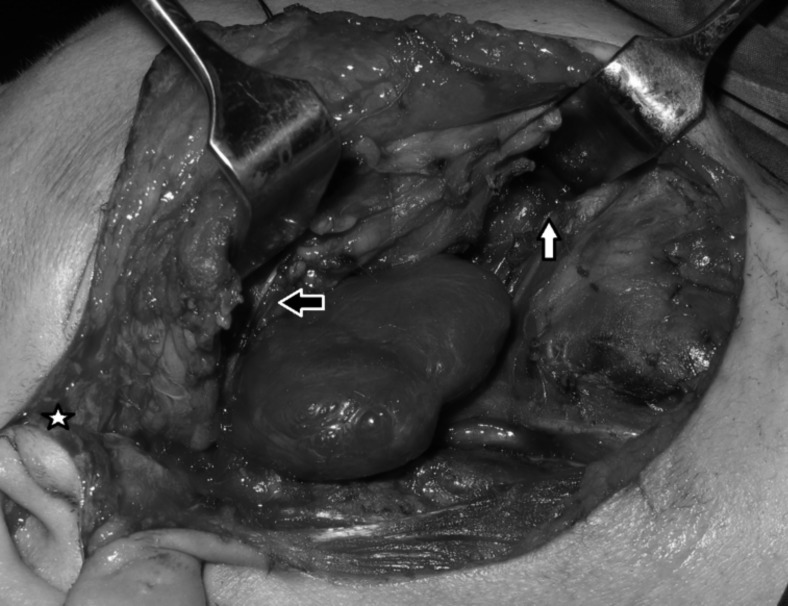
In all 44 patients included in the study, transcervical approaches were preferred. In 5 of these patients, a transparotid extension was also done due to the location of the tumour. Of these 5 cases, 4 were pleomorphic adenomas originating from the deep lobe of the parotid and the remaining case was a haemangiopericytoma originating from the poststyloid PPS (Fig. 1).
Fig. 1.
Haemangiopericytoma of the parapharyngeal space. Note the submandibulary gland is excised, the digastric muscle is transected and its posterior belly is resected (white arrow). A parotidectomy extension is done (white star) and following identification of the facial nerve, its marginal branch is retracted superiorly (black arrow).
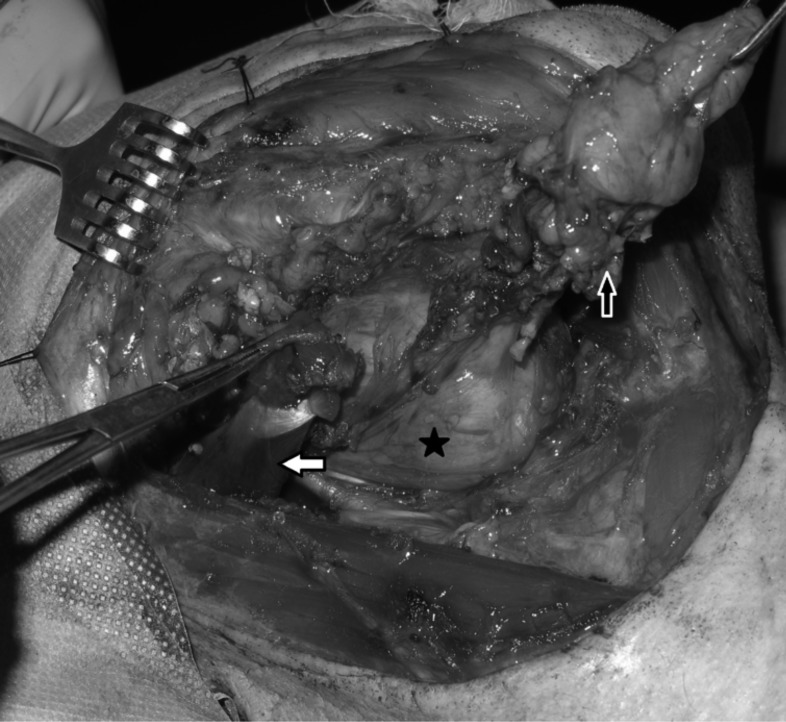
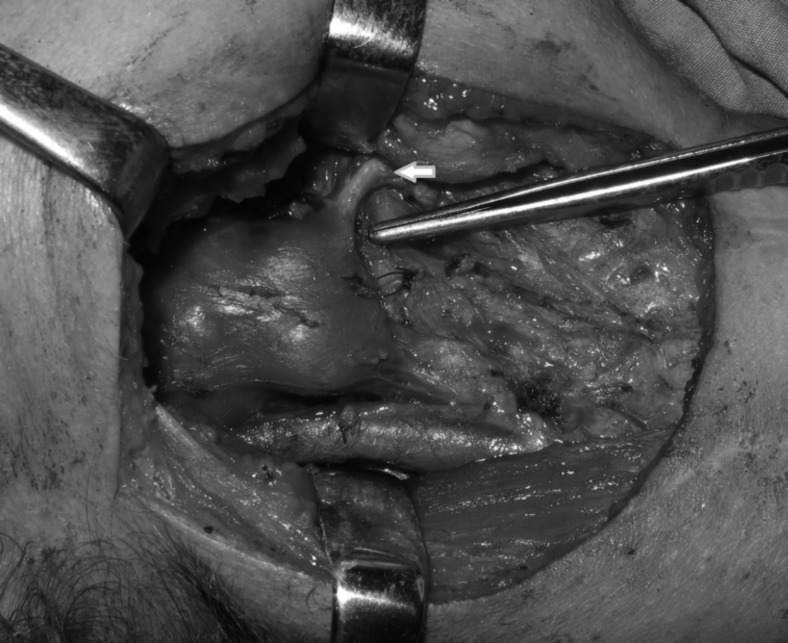
Cranial nerve paralysis was observed in three patients during preoperative evaluation (Table III). The first patient underwent surgery for a PPS pleomorphic adenoma with the longest axis of 11 cm, where the preoperative cranial nerve (CN) IX, X and XII paralysis did not recover during post-operative follow-up of 18 months (Fig. 2). This was attributed to long-standing presence of the tumour in the PPS and consequent atrophy of the nerves under pressure. One patient underwent surgery for a vagal paraganglioma, with preoperative CN X paralysis, and another had surgery for a schwannoma originating from the hypoglossal nerve (Fig. 3), with preoperative CN XII paralysis. CN paralysis did not recover in any of these patients in the postoperative period because the cranial nerves had to be sacrificed for adequate tumour removal. In the remaining 41 patients (93.2%), no preoperative CN deficit was detected.
Table III.
Complications.
| Complications | Tumour histology | Details |
|---|---|---|
| CN Paralysis |
Patients with preoperative paralysis (n = 3, 6.8%) Pleomorphic adenoma (n = 1) Vagal paraganglioma (n = 1) Hypoglossal schwannoma (n = 1) Patients with postoperatively developed paralysis (n = 19, 43.2%) Vagal paraganglioma (n = 15) Schwannoma (n = 2) Hemangiopericytoma (n = 1) Giant cell inflammatory granulation tissue (n = 1) |
Affected CN IX, X, XII (permanent) X (permanent) XII (permanent) X X IX X, XII |
| Vascular Injury (n = 2) | Schwannoma Vagal paraganglioma |
Laceration of the internal carotid artery Right-sided diffuse cerebral infarction caused by arterial embolism |
| Tracheotomy (n = 2) | Pleomorphic adenoma Vagal paraganglioma |
Elective tracheotomy for difficult intubation Right-sided diffuse cerebral infarction, prolonged intubation |
Fig. 2.
Pleomorphic adenoma of the parapharyngeal space (black star). The submandibulary gland and anterior belly of the digastric muscle is pulled up anteriorly (black arrow). The posterior belly of the digastric muscle is retracted posteriorly (white arrow).
Fig. 3.
A case of schwannoma originating from the hypoglossal nerve. The submandibulary gland is pulled anteriorly and the digastric muscle is retracted superiorly. The white arrow shows the hypoglossal nerve.
In the 41 patients without preoperative CN paralysis, no CN paralysis was observed postoperatively in 22 (50%). In addition to the three patients who showed no improvement in preoperative CN paralysis, permanent CN paralysis developed in 19 cases, the details of which are listed in Table III. No patient with vagal paralysis had obstructive respiratory problems postoperatively owing to the one-sided sacrifice of the nerve. However, all patients had problems with feeding due to aspiration, which was resolved by an Isshiki type I thyroplasty procedure performed under local anaesthesia within the first postoperative week. Patients with preoperative CN X paralysis did not require any medialisation procedure because the long-lasting paralysis was compensated spontaneously in the preoperative period. The patient with CN IX paralysis had a moderate velopharyngeal insufficiency postoperatively, which was compensated within the second postoperative week.
One patient who underwent surgery for a vagal paraganglioma experienced a right-sided diffuse cerebral infarction caused by an arterial embolism at the second post-operative day, and a left-sided hemiplegia developed. In another patient with a hypoglossal schwannoma, the internal carotid artery was injured during surgery and was repaired primarily. However, during the postoperative 24 hours, an arterial embolism to the middle cerebral artery developed, which resolved in the second postoperative month without a permanent neurological deficit.
Tracheotomy was performed in two patients (4.5%). The first patient was the one who had a 11 cm pleomorphic adenoma. Due to the risk of difficult intubation, preoperative elective tracheotomy was performed. The second patient was monitored in the intensive care unit for a long period after having diffuse cerebral infarct caused by an arterial embolism.
The longest follow-up period was 150 months and the shortest was 30 months. The median follow-up duration was 61 months (SD ± 33.10), which was considered long enough to evaluate local recurrence and possible late complications. There was no local recurrence in any patient during follow-up; however, 4 patients died due to reasons other than the primary disease (9.1%).
Discussion
The transcervical route, first described in 1955 by Morfit 11, is most preferred surgical approach for resection of PPS tumours 2-4. A transcervical incision is performed at the level of the hyoid bone following orotracheal intubation. The incision can be extended to the submental area to perform lip splitting if a mandibulotomy is necessitated during the operation. For larger tumours originating from the deep lobe of the parotid and for tumours with a retrostyloid location, partial parotidectomy is required. In the parotidectomy extension, following the identification of the main trunk of the facial nerve, its marginal branch is isolated and the lower half of the superficial lobe of the parotid is removed. The stylohyoid muscle, the posterior belly of the digastric muscle and the stylomandibular ligament is transected and the mandible is dislocated anteriorly. The internal and external carotid arteries, internal jugular vein, sympathetic chain and CN IX, X and XI are identified. The submandibular gland is pulled anteriorly or may be resected for exposure, and the mylohyoid muscle may be transected to reach the parapharyngeal space. Thereafter, the tumour is dissected bluntly from the surrounding tissues. The limited exposure of the parapharyngeal space is accepted as the major disadvantage of the transcervical route by some authors.2 However, this issue remains controversial and conflicts with the results of our study.
Reviewing the literature on parapharyngeal tumours approached transcervically, two case series were found. Chang et al. reported on 51 cases with the largest tumour size of 6.8 cm, while Presutti et al. described 18 cases with the largest tumour size of 8 cm 12 13. Our case series involved 44 cases and the largest tumour was a pleomorphic adenoma with a horizontal diameter of 11 cm. It should be emphasised that the vertical diameter should be evaluated rather than the horizontal diameter to determine whether the tumour is suitable for excision through a transcervical route. If the vertical extension of the tumour is suspicious for intracranial extention, a transcervical approach is dispensable. Furthermore, it should be taken into consideration, particularly in neurogenic tumours, that it might be very difficult to dissect the tumour from the surrounding tissues, especially in the vicinity of the cranial base. The schwannoma that originated from the hypoglossal nerve is a good example for this situation among our patients. In this case, the tumour extended to the level of the hypoglossal canal and the internal carotid artery was gradually thinned due to pressure applied by the mass. Consequently, the internal carotid artery was lacerated during blunt dissection of the mass. For this reason, we do not recommend a transcervical approach for tumours with a long vertical dimension and radiologically suspected to invade the cranial foramens.
The indications for mandibulotomy in PPS are malignant neoplasms, recurrent neoplasms, large benign neoplasms and highly vascular neoplasms with the need for improved vascular control 14. In our opinion, the indications for mandibulotomy should be limited only to malignant or recurrent tumours; however, size and hypervascularity of the tumour are not definite indications for mandibulotomy. As mentioned in our series, some hypervascular tumours such as haemangioperistoma and vagal paragangliomas can be safely excised via a transcervical approach. Additionally, the giant pleomorphic adenoma with a diameter of 11 cm was a good example for large PPS tumours underlining that mandibulotomy is unnecessary just owing to the size of the tumour.
In addition to transcervical and transmandibular techniques, transoral routes should be considered for well-selected cases. Nevertheless, we do not employ this technique regardless of tumour size in any parapharyngeal masses. Surgical exposure is extremely poor in this technique; the risk of tumour rupture is very high, and in the event of rupture, it is extremely difficult to clean the spilled tumour cells from the operative field successfully, so recurrence in such a vital body part is unavoidable. Furthermore, control of neurovascular structures is mostly inadequate, resulting in massive intraoperative blood loss and cranial nerve deficits. MRI seems to be superior to CT in diagnosis and assessment of PPS tumours because it demonstrates the size of the tumour and the neighbouring tissues more clearly. The presence of dystrophic calcifications seen on CT and the well-defined, smoothly lobulated tumour contour detected on MRI are the best predictors for pleomorphic adenoma, which is the most common PPS tumour in the literature 15. The second most common PPS tumour in the literature and the most common one in our series is vagal paraganglioma, the diagnosis of which is usually made correctly depending on MRI characteristics 16. On MRI, the most characteristic finding of a paraganglioma is the presence of serpentine or punctate low–signal intensity regions, termed as the "salt and pepper" appearance, which results from its hypervascularity 17. Other radiological findings of a PPS paraganglioma are the easily discernible delineation of tumour borders and the anterior displacement of the internal carotid artery. The second most common tumour type in our case series was the nerve sheet tumour, which also have characteristic radiological signs including well defined tumour margins and homogenous overall appearance 18. These tumours should be considered in the differential diagnosis of PPS paragangliomas and are also characterised with CN paralysis without the "salt and pepper" appearance on MRI 17. Radiological studies can also distinguish infiltration in surrounding soft tissues and regional metastasis, which is considered as an important criterion of malignancy 19-21. It may be difficult to distinguish malignant from benign tumours clinically, and thus the role of radiological assessment is very critical. CN palsies and pain are significant symptoms that may be related to malignancy. Nevertheless, they do not specifically point to a malignant tumour. The radiological signs of a malignancy are finding of irregular tumour margins, spread into surrounding tissues and fat planes on CT or MRI and the evidence of enlarged necrotic lymph nodes in the retropharyngeal and cervical area. In our study, none of the patients radiologically diagnosed as having a benign tumour were reported to have a malignant tumour in postoperative pathological assessment. Therefore, we conclude that preoperative radiological evaluation is sufficient to distinguish malignant from benign tumours.
PPS is an unusual target for FNAB and has some disadvantages such as difficulty in access, tumour spillage and necessity for an experienced cytologist due to the rarity of these tumours. Transcervical FNAB with or without ultrasound guidance, which is used routinely in head and neck clinical applications, may not be adequate in PPS lesions. Instead of the transcervical route, FNAB may be performed via a transoral approach in the outpatient suite or under CT guidance through a transfacial approach. However, its reliability is controversial in the literature because the diagnostic accuracy of FNAB in PPS tumours (other than pleomorphic adenomas) is dubious 22. A diagnosis of the paraganglioma is made mostly with the help of radiology as mentioned above; moreover, FNAB of paragangliomas is not widely accepted due to its extreme vascularity and possible risks of haemorrhage inside the tumour 23. The third common tumour type in our series was schwannoma, in which the presence of hypocellular (Antoni B) areas and the particularly cystic degeneration in larger tumours also makes FNAB ineffective 24. Consequently, FNAB of PPS tumours is technically difficult and time consuming due to its need for a interdisciplinary team including head and neck surgeon, radiologist and cytopathologist. The distinction between benign and malignant or hypo-hypervascular tumours can be made easily with the help of radiology, so we believe that preoperative FNAB will not change preoperative treatment planning. Moreover, we do not recommend incisional biopsy in order to prevent tumour spillage and recurrence.
Due to its complex anatomic content, many types of tumours are expected to occur in the PPS. Shahab published the second largest series in the literature with 114 cases and among those, 96 patients (84%) had benign pathology, of which 34 were pleomorphic adenoma, 33 were paraganglioma (including carotid paragangliomas), 11 were schwannoma and 3 were neurofibroma 3. Cohen published 166 cases, 145 of which were benign PPS tumours: 34 tumours of salivary gland origin, 65 paraganglioma, 16 schwannoma, and 7 neurofibroma 4. All of our 44 cases had benign histopathologic diagnoses, which were vagal paraganglioma in 16 cases (36.4%), pleomorphic adenoma in 13 cases (29.5%) and schwannoma in 10 cases (22.7%). Although tumours with glandular origin are accepted to be the most common neoplasms in the PPS, neurogenic tumours had the greatest prevalence in our series, which is consistent with Cohen's series 4. Our clinic is a tertiary referral centre, which may account for the high proportion of neurogenic tumours due to the difficulty of their surgical excision and the higher rate of preoperative and postoperative complications relative to glandular tumours.
There is much controversy recently as to whether these neurogenic tumours should be resected or monitored in order to observe their biological behaviour. Our principle in the treatment of such tumours is as follows: depending on the patient's preference after informed consent, we prefer surgical treatment for younger patients because of their long life expectancy. It is also clear that the sooner the tumour is excised, the fewer the resultant complications. Moreover, in the event of a complication such as nerve deficit, their tolerance is greater and rehabilitation is easier owing to better physical capacity compared with older patients. In contrast, we prefer to monitor older patients radiologically if tumours are likely to be benign neurogenic neoplasms and patients are asymptomatic. Nonsurgical treatments such as radiotherapy and stereotactic radiosurgery can be used in symptomatic cases.
The most serious complication of PPS surgery is CN paralysis involving CN VII, IX, X, XI and XII 13. Among our patients, excluding three cases that had preoperative paralysis, permanent CN paralysis occurred in 19 cases (43.2%) in the postoperative period. However, regarding the fact that 15 of these cases were vagal paragangliomas and two were schwannomas, the high rate of paralysis is considered reasonable since CN X has to be sacrificed in the surgery of vagal paraganglioma. Neurogenic tumours like paragangliomas, particularly vagal paragangliomas are accepted to have the greatest risk of neurological sequels in comparison with other PPS tumours 4. Informing the patient about the possible neurological complications prior to the operation will improve the patients' compliance with the rehabilitation program because speech and swallowing therapy may be necessary during postoperative rehabilitation of patients with paralysis of CN IX, X or XI. Because most of these tumours are benign and grow slowly, the morbidity that would be caused by CN sacrifice should be taken into consideration while making the decision of surgical treatment, especially in older patients. The principle of 'primum non nocere' should be kept in mind.
There were no recurrences during the follow-up period of 61 months. This result demonstrates the efficacy of the transcervical technique, but might also be related to the fact that the majority of cases were well-capsulated neurogenic tumours.
Conclusions
The ideal surgical approach for the PPS should be one that does not damage important surrounding structures. To prevent possible perioperative vascular and postoperative neurological morbidities, all of the lower cranial nerves, internal carotid artery and internal jugular vein must be identified. The transcervical approach should be the first choice for excision of PPS tumours owing to its advantages of providing direct access to the PPS and control of neurovascular structures from the neck. With improvements in combination with video-assisted and image-guided minimally-invasive surgical techniques, the transcervical approach will be much more useful in the future 25. Because the majority of these tumours are benign and their en-bloc excision with safe margins is sufficient for treatment, it is unnecessary to increase the postoperative morbidity by performing mandibulotomy or other highly invasive procedures.
References
- 1.Stell PM, Mansfield AO, Stoney PJ. Surgical approaches to tumors of the parapharyngeal space. Am J Otolaryngol. 1985;6:92–97. doi: 10.1016/s0196-0709(85)80045-4. [DOI] [PubMed] [Google Scholar]
- 2.Carrau RL, Myers EN, Johnson JT. Management of tumors arising in the parapharyngeal space. Laryngoscope. 1990;100:583–589. doi: 10.1288/00005537-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Shahab R, Heliwell T, Jones AS. How we do it: a series of 114 primary pharyngeal space neoplasms. Clin Otolaryngol. 2005;30:364–367. doi: 10.1111/j.1365-2273.2005.00993.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SM, Burkey BB, Netterville JL. Surgical management of parapharyngeal space masses. Head Neck. 2005;27:669–675. doi: 10.1002/hed.20199. [DOI] [PubMed] [Google Scholar]
- 5.Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978;92:949–967. doi: 10.1017/s0022215100086382. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich H. Mixed tumors of the pterygomaxillary space; operative removal; oral approach. Oral Surg Oral Med Oral Pathol. 1950;3:1366–1371. doi: 10.1016/0030-4220(50)90297-0. [DOI] [PubMed] [Google Scholar]
- 7.Ducic Y, Oxford L, Pontius AT. Transoral approach to the superomedial parapharyngeal space. Otolaryngol Head Neck Surg. 2006;134:466–470. doi: 10.1016/j.otohns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley BW, Jr, Quon H, Leonhardt FD, et al. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72:332–336. doi: 10.1159/000320596. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Kim J, Lee HJ, et al. Transoral robotic surgery for neurogenic tumors of the prestyloid parapharyngeal space. Auris Nasus Larynx. 2012;39:434–437. doi: 10.1016/j.anl.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Chan JY, Tsang RK, Eisele DW, et al. Transoral robotic surgery of the parapharyngeal space: A case series and systematic review. Head Neck. 2013 Nov 29; doi: 10.1002/hed.23557. doi: 10.1002/hed.23557. [DOI] [PubMed] [Google Scholar]
- 11.Morfit HM. Retromandibular parotid tumors; their surgical treatment and mode of origin. AMA Arch Surg. 1955;70:906–913. [PubMed] [Google Scholar]
- 12.Chang SS, Goldenberg D, Koch WM. Transcervical approach to benign parapharyngeal space tumors. Ann Otol Rhinol Laryngol. 2012;121:620–624. doi: 10.1177/000348941212100910. [DOI] [PubMed] [Google Scholar]
- 13.Presutti L, Molteni G, Malvè L, et al. Parapharyngeal space tumors without mandibulotomy: our experience. Eur Arch Otorhinolaryngol. 2012;269:265–273. doi: 10.1007/s00405-011-1594-y. [DOI] [PubMed] [Google Scholar]
- 14.Kolokythas A, Eisele DW, El-Sayed I, et al. Mandibular osteotomies for access to select parapharyngeal space neoplasms. Head Neck. 2009;31:102–110. doi: 10.1002/hed.20934. [DOI] [PubMed] [Google Scholar]
- 15.Som PM, Sacher M, Stollman AL, et al. Common tumors of the parapharyngeal space: refined imaging diagnosis. Radiology. 1988;169:81–85. doi: 10.1148/radiology.169.1.2843942. [DOI] [PubMed] [Google Scholar]
- 16.Olsen WL, Dillon WP, Kelly WM, et al. MR imaging of paragangliomas. Am J Roentgenol. 1987;148:201–204. doi: 10.2214/ajr.148.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Som PM, Braun IF, Shapiro MD, et al. Tumors of the parapharyngeal space and upper neck: MR imaging characteristics. Radiology. 1987;164:823–829. doi: 10.1148/radiology.164.3.3039571. [DOI] [PubMed] [Google Scholar]
- 18.Miller FR, Wanamaker JR, Lavertu P, et al. Magnetic resonance imaging and the management of parapharyngeal space tumors. Head Neck. 1996;18:67–77. doi: 10.1002/(SICI)1097-0347(199601/02)18:1<67::AID-HED9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Nour SG, Lewin JS. Parapharyngeal and Masticator spaces. In: Mafee MF, Valvassori GE, Becker M, editors. Imaging of the Head and Neck. 2nd ed. New York: Thieme; 2005. pp. 580–624. [Google Scholar]
- 20.Shirakura S, Tsunoda A, Akita K, et al. Parapharyngeal space tumors: anatomical and image analysis findings. Auris Nasus Larynx. 2010;37:621–625. doi: 10.1016/j.anl.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Lawson VG, LeLiever WC, Makerewich LA, et al. Unusual parapharyngeal lesions. J Otolaryngol. 1979;8:241–249. [PubMed] [Google Scholar]
- 22.Oliai BR, Sheth S, Burroughs FH, et al. "Parapharyngeal space" tumors: a cytopathological study of 24 cases on fineneedle aspiration. Diagn Cytopathol. 2005;32:11–15. doi: 10.1002/dc.20154. [DOI] [PubMed] [Google Scholar]
- 23.Fleming MV, Oertel YC, Rodríguez ER, et al. Fine-needle aspiration of six carotid body paragangliomas. Diagn Cytopathol. 1993;9:510–515. doi: 10.1002/dc.2840090508. [DOI] [PubMed] [Google Scholar]
- 24.Yu GH, Sack MJ, Baloch Z, et al. Difficulties in the fine needle aspiration (FNA) diagnosis of schwannoma. Cytopathology. 1999;10:186–194. doi: 10.1046/j.1365-2303.1999.00132.x. [DOI] [PubMed] [Google Scholar]
- 25.Beswick DM, Vaezi A, Caicedo-Granados E, et al. Minimally invasive surgery for parapharyngeal space tumors. Laryngoscope. 2012;122:1072–1078. doi: 10.1002/lary.23244. [DOI] [PubMed] [Google Scholar]