SUMMARY
The aim of this study was to verify if hyoid myotomy without hyoid suspension is effective in surgical treatment of obstructive sleep apnoea syndrome (OSAS). We recruited six patients with OSAS, aged between 34 to 60 years, with retropalatal and retrolingual upper airway obstruction, non-obese (BMI < 27) and non-compliant to continuous positive airway pressure therapy. Pre-surgical clinical and instrumental evaluations included clinical examination, cephalometry, polysomnography (PSG) and sleep endoscopy. Surgical treatment included nasal surgery, uvulopalatopharyngoplasty, tonsillectomy and hyoid myotomy without hyoid suspension. Follow-up evaluations were performed with serial PSGs, performed early (one week after surgery), and at 1, 6 and 18 months after surgery. We observed that surgery was followed by immediate normalisation of breathing parameters evaluated by PSG that persisted after 18 months. Thus, hyoid myotomy without suspension combined with nasal and palatal surgery may be considered a valid treatment of non-obese OSAS patients with retrolingual and retropalatal collapse. Furthermore, we suggest that hyoid bone suspension, binding it to mandibular or to thyroid cartilage, might be unnecessary in selected cases.
KEY WORDS: Obstructive sleep apnoea syndrome, Hyoid myotomy, Uvulopalatopharyngoplasty, Sub-hyoid muscles
RIASSUNTO
Obiettivo di questo studio è stato verificare se la miotomia ioidea senza sospensione è efficace nel trattamento chirurgico della sindrome delle apnee ostruttive del sonno (OSAS) nell'ambito del primo step della chirurgia multilivello. Abbiamo reclutato sei pazienti affetti da OSAS, di età compresa tra i 34 e i 60 anni, con un'ostruzione delle alte vie aeree a livello retropalatale e retrolinguale, non obesi (BMI <27) e che mostravano scarsa tolleranza nei confronti della terapia con ventilazione meccanica a pressione positiva continua delle alte vie aeree. Durante la valutazione clinica pre-intervento i pazienti sono stati sottoposti a esame clinico otorinolaringoiatrico, cefalometria, polisonnografia (PSG) e sleep-endoscopy. Tutti i pazienti sono stati sottoposti nella stessa seduta a chirurgia nasale, uvulopalatofaringoplastica, tonsillectomia e miotomia ioidea senza sospensione. Nel corso del follow-up i pazienti sono stati sottoposti a PSG seriali, all'inizio (una settimana dopo l'intervento chirurgico), ed a 1, 6 e 18 mesi dopo l'intervento chirurgico. I dati polisonnografici hanno messo in evidenza un significativo miglioramento dei parametri respiratori nell'immediato post operatorio che si è mantenuto stabile per almeno 18 mesi. Alla luce della nostra analisi preliminare l'intervento di miotomia ioidea senza sospensione in combinazione con la chirurgia nasale e palatale può essere considerato un valido trattamento per i pazienti affetti da OSAS, non obesi, con collasso delle alte vie aeree a livello retrolinguale e retropalatale. Per tale motivo la sospensione ioidea, con ancoraggio alla mandibola o alla cartilagine tiroidea, in alcuni casi selezionati, potrebbe non essere necessaria.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is a multifactorial pathology consequent to upper airway obstruction and often associated with cardiovascular and metabolic comorbidities 1 2. Treatment of OSAS must be based on the exact determination of the site of upper airway collapse obtained through careful clinical evaluation including endoscopy of the upper airway during awake status and during drug-induced sleep endoscopy 3-6.
Several surgical and non-surgical procedures have been proposed as an alternative to continuous positive airway pressure (CPAP) for treatment of patients with OSAS. Among the non-surgical techniques, good results have been obtained with the use of oral devices, especially when there is evidence of improvement of the breathing space after mandibular advancement manoeuvres during sleep endoscopy 7. Surgical approaches have been described to treat OSAS patients and include nasal surgery, pharyngeal surgery, tongue surgery, hyoid bone surgery 8-11 and maxillary surgery with several variations and personalisations 12-15 with the aim of achieving good and safe results 16. Riley et al. 8 9 proposed a multilevel surgical approach consisting of two phases. The first includes nasal surgery, uvulopalatopharyngoplasty (UPPP), genioglossus advancement and hyoid bone suspension; the second phase (applied to patients not responding to the first phase of treatment) includes bi-maxillary advancement and tongue base surgery.
In the present report, we describe a multilevel surgical approach to the treatment of adult, non-obese patients with severe OSAS and both retropalatal and retrolingual collapse in agreement with the protocol of Riley et al. 8 9 combining nasal surgery, UPPP and hyoid myotomy. In contrast to other surgical procedures, we do not perform hyoid suspension. The hyoid bone is freed from its lower connections (by cutting the sub-hyoid muscles), but it is not suspended to nearby anatomical structures. Herein, we present our surgical technique and the results achieved.
Clinical techniques and technology
We enrolled 6 patients (5 males/1 female; mean age 44.3 ± 8.8) with a body mass index < 27 kg/m2, cephalometric parameters consistent with retropalatal and retrolingual obstruction confirmed by sleep endoscopy, absence of major cranio-facial abnormalities, apnoea-hypopnoea index (AHI) > 20 events per hour of sleep documented by full-night laboratory-based polysomnography. The study conformed with the Declaration of Helsinki and was approved by the local Ethics Committee. All patients gave written informed consent for surgical treatment.
According to standard protocols 17, pre-surgery evaluation included clinical otorhinolaryngoiatric examination, cephalometric evaluation [in particular the posterior airway space (PAS) measured as the minimal distance between the base of the tongue and the posterior pharyngeal wall, and the mandibular planus–hyoid bone distance (MP-H)], nasal and oro-pharyngeal endoscopy in awake status and during drug-induced sleep.
Four full-night laboratory nocturnal polysomnographies (PSGs) were performed: the first before surgical treatment (PSG#1), the second at one week after surgery (PSG#2), the third after 6 months follow-up (PSG#3) and the fourth at 18 months after treatment (PSG#4). PSGs were recorded with a Micromed System '98 digital polygraph with electroencephalographic electrodes, electro-oculographic electrodes, electromyography of submental and intercostal muscles, airflow measured by oronasal thermocouple, thoracic and abdominal effort, EKG (V2 modified derivation) and peripheral haemoglobin saturation. Sleep registration lasted from 11:00 pm to 7:00 am. A trained technician was present during data acquisition. Sleep stages were visually classified according to the criteria of Rechtschaffen and Kales 18. The scoring and classification of sleep-related respiratory events was made visually. Apnoeas were defined as a decrease of the airflow signal up to >= 90% from the baseline for more than 10 sec. Hypopnoea events were defined by a decrease of amplitude of the airflow signal by 30% or more and the event lasts at least 10 sec and the blood oxygenation drops by 4% or more or the event is associated with an EEG arousal (3 sec or more of fast, desynchronised activity). AHI was the ratio of apnoeas plus hypopnoea per hour of sleep (excluding central events). PSG features of patients are summarised in Table I.
Table I.
PSG features of patients before and after surgery.
| Before surgery | ||||||||
| Breathing abnormalities | 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD |
| All Apnoeas | 82 | 69 | 23 | 29 | 44 | 117 | 60.67 | 35.77 |
| All Hypopnoeas | 62 | 319 | 178 | 89 | 55 | 420 | 187.17 | 151.29 |
| Central Apnoeas | 6 | 1 | 3 | 0 | 0 | 0 | 1.67 | 2.42 |
| Central Hypopnoeas | 3 | 6 | 2 | 9 | 6 | 54 | 13.33 | 20.08 |
| Mixed Apnoeas | 2 | 3 | 1 | 0 | 0 | 0 | 1.00 | 1.26 |
| Mixed Hypopnoeas | 7 | 70 | 36 | 23 | 29 | 29 | 32.33 | 20.90 |
| Obstructive Apnoeas | 16 | 65 | 19 | 29 | 44 | 117 | 48.33 | 38.19 |
| Obstructive Hypopnoeas | 52 | 243 | 140 | 57 | 20 | 337 | 141.50 | 125.25 |
| Apnea + Hypopnea Index | 24.0 | 54.6 | 28.5 | 24.0 | 25.1 | 66.3 | 37.09 | 18.56 |
| Desaturations | ||||||||
| Total number | 63 | 296 | 139 | 103 | 130 | 425 | 192.67 | 214.28 |
| Desaturation index | 26.3 | 46.3 | 17.8 | 25.1 | 19.2 | 50.1 | 30.80 | 13.92 |
| Baseline SaO2 | 98 | 97 | 98 | 98 | 98 | 97 | 97.33 | 0.82 |
| Minimum SaO2 | 80 | 71 | 82 | 76 | 82 | 64 | 75.83 | 7.17 |
| Time with SaO2 < 90% | 14 | 38 | 32 | 18 | 29 | 47 | 29.67 | 12.31 |
| 6 months after surgery | ||||||||
| Breathing abnormalities | 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD |
| All Apnoeas | 33 | 53 | 24 | 28 | 43 | 61 | 40.33 | 14.61 |
| All Hypopnoeas | 22 | 19 | 8 | 22 | 30 | 30 | 21.83 | 8.16 |
| Central Apnoeas | 2 | 5 | 0 | 0 | 5 | 3 | 2.50 | 2.26 |
| Central Hypopnoeas | 3 | 0 | 0 | 0 | 5 | 6 | 2.33 | 2.73 |
| Mixed Apnoeas | 0 | 1 | 0 | 9 | 0 | 0 | 1.67 | 3.61 |
| Mixed Hypopnoeas | 0 | 6 | 0 | 3 | 5 | 0 | 2.33 | 2.73 |
| Obstructive Apnoeas | 31 | 47 | 24 | 19 | 38 | 58 | 36.17 | 14.61 |
| Obstructive Hypopnoeas | 19 | 13 | 8 | 19 | 20 | 24 | 17.17 | 5.71 |
| Apnea + Hypopnea Index | 5.9 | 9.9 | 3.5 | 3.8 | 8.6 | 11.9 | 7.27 | 3.41 |
| Desaturations | ||||||||
| Total number | 47 | 59 | 21 | 39 | 65 | 78 | 51.50 | 20.24 |
| Desaturation index | 3.6 | 10.5 | 4.6 | 3.9 | 9.6 | 9.9 | 7.02 | 3.30 |
| Baseline SaO2 | 98 | 99 | 98 | 98 | 98 | 98 | 98.17 | 0.41 |
| Minimum SaO2 | 83 | 74 | 91 | 83 | 92 | 85 | 84.67 | 6.53 |
| Time with SaO2 < 90% | 3 | 3 | 1 | 1 | 5 | 7 | 3.33 | 2.34 |
| 1 week after surgery | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD | |
| 25 | 70 | 24 | 19 | 56 | 77 | 45.17 | 25.64 | n° |
| 17 | 29 | 8 | 20 | 26 | 32 | 22.00 | 8.83 | n° |
| 0 | 3 | 3 | 0 | 4 | 4 | 2.33 | 1.86 | n° |
| 2 | 1 | 2 | 0 | 1 | 5 | 1.83 | 1.72 | |
| 1 | 4 | 1 | 5 | 2 | 8 | 3.50 | 2.74 | n° |
| 0 | 10 | 1 | 5 | 2 | 4 | 3.68 | 3.61 | n° |
| 24 | 63 | 20 | 14 | 50 | 65 | 39.33 | 22.73 | n° |
| 15 | 18 | 5 | 15 | 23 | 23 | 16.50 | 6.69 | n° |
| 5.1 | 12.3 | 3.2 | 4.8 | 9.3 | 12.8 | 7.92 | 4.12 | Per hour |
| 33 | 74 | 17 | 28 | 71 | 79 | 50.33 | 27.27 | |
| 4.1 | 9.6 | 2 | 3.4 | 8.5 | 10.1 | 6.28 | 3.52 | Per hour |
| 97 | 98 | 98 | 98 | 98 | 97 | 97.67 | 0.52 | |
| 85 | 76 | 90 | 86 | 91 | 83 | 85.17 | 5.42 | |
| 3 | 4 | 1 | 2 | 4 | 8 | 3.67 | 2.42 | % of SPT |
| 12 months after surgery | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD | |
| 40 | 51 | 27 | 27 | 35 | 54 | 39.00 | 11.61 | n° |
| 29 | 27 | 18 | 17 | 14 | 36 | 23.50 | 8.50 | n° |
| 4 | 4 | 6 | 8 | 2 | 4 | 4.67 | 2.07 | n° |
| 6 | 5 | 2 | 1 | 3 | 4 | 3.50 | 1.87 | n° |
| 2 | 6 | 2 | 3 | 3 | 4 | 3.33 | 1.51 | n° |
| 2 | 5 | 4 | 1 | 0 | 4 | 2.67 | 1.97 | n° |
| 34 | 41 | 19 | 16 | 30 | 46 | 31.00 | 11.87 | n° |
| 21 | 17 | 12 | 15 | 11 | 28 | 17.33 | 6.35 | n° |
| 4.6 | 12.1 | 5.2 | 4.3 | 7.8 | 10.4 | 7.40 | 3.27 | Per hour |
| 40 | 65 | 32 | 35 | 48 | 77 | 49.50 | 17.92 | |
| 4.1 | 9.6 | 5.0 | 3.8 | 7.0 | 9.3 | 6.52 | 2.64 | Per hour |
| 98 | 98 | 98 | 97 | 98 | 98 | 97.83 | 0.41 | |
| 81 | 80 | 89 | 82 | 91 | 95 | 84.67 | 4.50 | |
| 2 | 2 | 1 | 1 | 4 | 2 | 2.00 | 1.10 | % of SPT |
All patients, after diagnostic assessment, were prescribed a nocturnal ventilator treatment (nasal CPAP or BiLevel) and underwent a titration night. None of the 6 patients were compliant to ventilator therapy and voluntarily chose to undergo upper airway surgery.
Turbinate ablation with radiofrequency and septoplasty was performed in all patients. Palatoplasty and tonsillectomy were also performed in all patients according to the technique described by Riley et al. 8 9 Successively, subhyoid myotomy was performed via a 3 cm transversal cut, including total sectioning of the sub-hyoid muscles. In contrast to the other techniques proposed, the hyoid bone was not anchored to either thyroid cartilage or the mandibular bone. Surgery was well tolerated by all patients, and all patients were able to swallow normally at 7 to 10 days after surgery.
The results of PSG studies are detailed in Table I (for sleeprelated breathing abnormalities). Before surgery, baseline PSG results were consistent with severe OSAS. The AHI ranged from 27.0 to 54.6 with a mean of 35.4 ± 13.7. Immediately after surgery, all PSG parameters showed improvement relative to sleep breathing abnormalities: in particular, the AHI decreased from 35.4 ± 13.7 events/ hour before surgery to 7.9 ± 4.1 events/hour after surgery (Student's t-test p = 0.0054). After 18 months of followup, the AHI was 7.4 ± 3.2.
At cephalometry before surgery, the posterior airways space (PAS) ranged from 3 mm to 9 mm (mean 6.0 ± 2.4 mm). After surgery, a dramatic increase in PAS was seen, ranging from 12 mm to 16 mm (mean 14.8 ± 1.5 mm). This increase was statistically significant (Student's t-test p = 0.00079). The Mandibular Planus – Hyoid bone distance (MP-H) before surgery ranged from 10 mm to 16 mm (mean 12.8 ± 1.9 mm). After surgery the MP-H ranged from 6.8 mm to 13 mm (mean 9.0 ± 2.1 mm) thus showing a significant reduction (Student's t-test p = 0.00026). The values were unchanged after 18 months of follow-up.
Discussion
Hyoid bone suspension can be achieved by several surgical procedures 4-10. All of these interventions aim to displace the hyoid complex forward and upward, and to fix it to a nearby anatomical structure, either the mandible or thyroid cartilage. This movement increases the PAS, both in its posterior and, mainly, in its lateral portions 4.
The choice to leave the hyoid bone free was suggested by the hypothesis that, by fixing the hyoid to another anatomical structure as in classical surgery, this could induce passive lengthening of the supra-hyoid muscles (upper airway dilators). Therefore, this might impair the physiological action of these muscles in keeping the upper airways open during sleep. In this view, hyoid suspension might abolish one of the physiological mechanisms aimed at preventing upper airways collapse during sleep. Moreover, when the hyoid bone is fixed to thyroid cartilage, supra-hyoid muscle is subject to chronic stretching induced by inspiration; such stretching could possibly worsen OSAS 19. Hyoid myotomy without suspension could theoretically prevent such mechanical stress.
The most relevant finding in our study was that the employed surgical approach was effective in non-obese patients with severe OSAS. Furthermore, these positive results persisted after 18 months. The improvement was not associated with significant variation in the BMI. The omohyoid, the sternohyoid and thyrohyoid muscles were sectioned. All these muscles act by lowering the hyoid bone and opposing the upward movement of the larynx. Hyoid myotomy induces a spontaneous movement upward and forward of the hyoid bone. We did not perform hyoid suspension following hyoid myotomy: in this view, the proposed technique differs from the most widely applied techniques 4 8-11. Although commonly employed in literature, hyoid bone suspension and fixation was not necessary for clinical improvement in our sample of OSAS patients. In our opinion, several factors can explain this result:
After the described intervention, the respiratory mechanics of upper airway muscle appear to be modified: during inspiration, the decrease of thoracic pressure cannot be transmitted to the hyoid bone and tongue base, thus preventing the downward stretching of hypopharyngeal structures. In this way, the supra-hyoid (involved in digestive functions) and sub-hyoid muscular systems (involved in respiration) act "in parallel" rather than "serially".
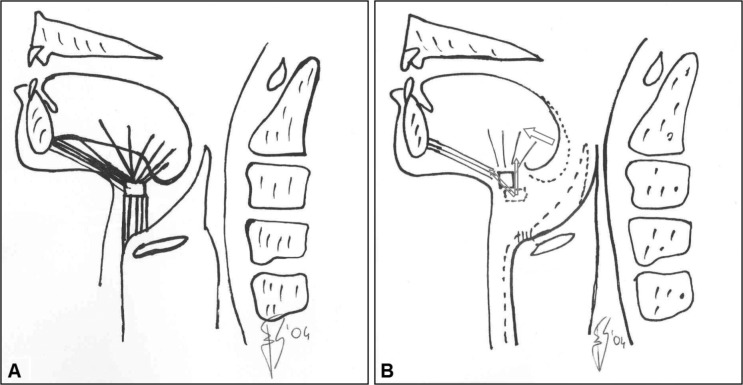
Following sectioning of sub-hyoid muscles, the hyoid is pulled forward and upward (Fig. 1). Accordingly, the supra-hyoid muscles (pharyngeal dilators) are consequently stretched upwards 4 10 19. It is well known that the force generated by muscle contraction largely depends on muscle length at rest 20. Thus, it can be hypothesised that, by fixing the hyoid to another anatomical structure, classical surgery induces passive stretching of the supra-hyoid muscles (upper airway dilators). Such stretching modifies the operating length of supra-hyoid muscles, and it might therefore interfere with the ability of these muscles to keep the upper airways open during sleep 20, and by abolishing one of the physiological mechanisms aimed at preventing upper airway collapse in sleep.
Fig. 1.
(A) Hyoid muscles before surgery. (B) Hyoid bone and tongue displacement after surgery. Arrows indicate strength vectors. Following the sectioning of the sub-hyoid muscles, the hyoid is pulled forward and upward, and the supra-hyoid muscles (pharyngeal dilators) are consequently stretched upwards. The tongue base moves forward, inducing an increase in the posterior airway space.
Nonetheless, it must be stressed that all our patients were affected by not extremely severe OSAS. Moreover, all were young, and with relatively short clinical history of snoring and sleep apnoea. In fact, it has been reported that the natural history of non-treated OSAS induces morphological, physiological, biochemical and histochemical modifications in the upper airway muscles. It is conceivable that, in our group of patients, early diagnosis allowed surgical treatment before the appearance of such modifications. Therefore, the integrity of the upper airway dilator (supra-hyoid) muscles did not require fixation to the hyoid bone.
In conclusion, the present data, although collected from a small sample, suggests that hyoid myotomy is an effective, minimally-invasive and well-tolerated surgical technique that may be associated with nasal and palatal surgery in a multilevel approach for patients with OSAS and retrolingual and retropalatal collapse. For these reasons, we suggest that hyoid bone suspension to mandibular or thyroid cartilage might be unnecessary in selected cases. Future studies with a larger number of patients are necessary to confirm our preliminary observations.
References
- 1.Fusetti M, Fioretti AB, Valenti M, et al. Cardiovascular and metabolic comorbidities in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:320–325. [PMC free article] [PubMed] [Google Scholar]
- 2.Passali D, Caruso G, Arigliano LC, et al. Database application for patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:252–255. [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita S. Midline laser glossectomy with linguoplasty: a treatment of sleep apnea syndrome. Op Tech Otolaryngol HNS. 1991;2:127–131. [Google Scholar]
- 4.Sher AE, Schechtman KB, Piccirillo F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. An American sleep disorders association review. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 5.Corso E, Fiorita A, Rizzotto G, et al. The role of druginduced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital. 2013;33:405–413. [PMC free article] [PubMed] [Google Scholar]
- 6.Salamanca F, Costantini F, Bianchi A, et al. Identification of obstructive sites and patterns in obstructive sleep apnoea syndrome by sleep endoscopy in 614 patients. Acta Otorhinolaryngol Ital. 2013;33:261–266. [PMC free article] [PubMed] [Google Scholar]
- 7.Milano F, Mondini S, Billi MC, et al. The impact of a multidisciplinary approach on response rate of mandibular advancing device therapy in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:337–342. [PMC free article] [PubMed] [Google Scholar]
- 8.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg. 1993;51:742–747. doi: 10.1016/s0278-2391(10)80412-4. discussion 748-9. [DOI] [PubMed] [Google Scholar]
- 9.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea and the hyoid: a revised surgical procedure. Otolaryngol Head Neck Surg. 1994;111:717–721. doi: 10.1177/019459989411100604. [DOI] [PubMed] [Google Scholar]
- 10.Sher AE. Upper airway surgery for obstructive sleep apnea. Sleep Med Rev. 2002;6:195–212. doi: 10.1053/smrv.2002.0242. [DOI] [PubMed] [Google Scholar]
- 11.Passali D, Tatti P, Toraldo M, et al. OSAS and metabolic diseases: Round Table, 99(th) SIO National Congress, Bari 2012. Acta Otorhinolaryngol Ital. 2014;34:158–166. [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani M, Minetti A, Torretta S, et al. The "Barbed Roman Blinds" technique: a step forward. Acta Otorhinolaryngol Ital. 2013;33:128–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani M, Minetti A, Torretta S, et al. The velo-uvulopharyngeal lift or "roman blinds" technique for treatment of snoring: a preliminary report. Acta Otorhinolaryngol Ital. 2012;32:48–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Tarsitano A, Marchetti C. Unusual presentation of obstructive sleep apnoea syndrome due to a giant mandible osteoma: case report and literature review. Acta Otorhinolaryngol Ital. 2013;33:63–66. Review. [PMC free article] [PubMed] [Google Scholar]
- 15.Mesolella M, Cimmino M, Cantone E, et al. Management of otolaryngological manifestations in mucopolysaccharidoses: our experience. Acta Otorhinolaryngol Ital. 2013;33:267–272. [PMC free article] [PubMed] [Google Scholar]
- 16.Giarda M, Brucoli M, Arcuri F, et al. Efficacy and safety of maxillomandibular advancement in treatment of obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:43–46. [PMC free article] [PubMed] [Google Scholar]
- 17.Shepard JW, Jr, Gefter WB, Guilleminault C, et al. Evaluation of the upper airway in patients with obstructive sleep apnea. Sleep. 1991;14:361–371. doi: 10.1093/sleep/14.4.361. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, technique and scoring system for sleep stages of human subjects. Los Angeles (UCLA): Brain Information Service / Brain Research Institute, University of California; 1968. [Google Scholar]
- 19.Partinen M, Guilleminault C, Quera-Salva MA, et al. Obstructive sleep apnea and cephalometrci roentgenograms. The role of anatomic upper airway abnormalities in the definition of abnormal breathing during sleep. Chest. 1998;93:1199–1205. doi: 10.1378/chest.93.6.1199. [DOI] [PubMed] [Google Scholar]
- 20.Series F. Upper airway muscles awake and asleep. Sleep Med Rev. 2002;6:229–242. doi: 10.1053/smrv.2001.0163. [DOI] [PubMed] [Google Scholar]