Abstract
Aim of the present study was to investigate far field R-wave sensing (FFRS) timing and characteristics in 34 Myotonic Dystrophy type 1 (DM1) patients undergoing dual chamber pacemaker implantation, comparing Bachmann's bundle (BB) stimulation (16 patients) site with the conventional right atrial appendage (RAA) pacing site (18 patients). All measurements were done during sinus rhythm and in supine position, with unipolar (UP) and bipolar (BP) sensing configuration. The presence, amplitude threshold (FFRS trsh) and FFRS timing were determined. There were no differences between both atrial sites in the Pmin and Pmean values of sensed P-wave amplitudes, as well as between UP and BP sensing configurations. The FFRS trsh was lower at the BB region in comparison to the RAA site. The mean BP FFRS trsh was significantly lower than UP configuration in both atrial locations. There were no significant differences in atrial pacing threshold, sensing threshold and atrial lead impedances at the implant time and at FFRS measurements. Bachmann's bundle area is an optimal atrial lead position for signal sensing as well as conventional RAA, but it offers the advantage of reducing the oversensing of R-wave on the atrial lead, thus improving functioning of standard dual chamber pacemakers in DM1 patients.
Key words: far field, oversensing, far field R-wave sensing, myotonic dystrophy type 1, atrial lead, Bachmann's bundle
Introduction
Myotonic Dystrophy type 1 (DM1), or Steinert's disease, is the most common muscular dystrophy in adult life, with an incidence of 1:8000 births (1, 2). It is an autosomal dominant disorder caused by an abnormal expansion of an unstable trinucleotide repeat in the 3-prime untranslated region of DMPK gene on chromosome 19 (3, 4). The phenotype is characterized by myotonia and muscle weakness, but multisystem involvement is frequent. Cardiac involvement is noticed in about 80% of cases, and it often precedes the skeletal muscle one. Paroxysmal atrial arrhythmias (atrial fibrillation, atrial flutter, atrial tachycardia) frequently occur in DM1 patients (5, 6), but the atrioventricular block is the first and most clinically significant cardiac disease in this group of patients (7). To prevent cardiac sudden death, implantation of a pacemaker (PM) is required in 3-22% of cases (8, 9). Considering the high risk of supraventricular arrhythmias in this particular class of patients, optimal atrial sensing is an important prerequisite for proper pacemaker function. During conventional right atrial appendage (RAA) stimulation, the bipolar (BP) atrial electrogram amplitudes were shown to be lower in AF and atrial flutter (10); this aspect requires higher atrial programmed sensitivity, thereby increasing the risk of sensing of ventricular depolarization in the atrial channel (FFRS). It has been shown that Bachmann's bundle (BB) stimulation is a safe and feasible procedure with low rate of sensing and pacing defects (11, 12). However, BB pacing does not provide significant benefit for the prevention of paroxysmal atrial fibrillation in DM1 population (13-15). No data are available concerning the effects of the different atrial lead placement on the far field R-wave sensing (FFRS) characteristics in DM1 patients. Aim of the present study was to investigate FFRS timing and characteristics in 34 DM1 patients undergoing dual chamber pacemaker implantation, comparing Bachmann's bundle stimulation (16 patients) with the conventional right atrial appendage (RAA) pacing site (18 patients).
Methods
Study population
The study involved 34 patients (age 51.4 years ± 8.5; 23M:11F), with a genetic established diagnosis of Myotonic Dystrophy type 1, undergoing dual chamber PM implantation from January 2007 to December 2013, in the Arrhythmologic Unit of Department of Cardio-Thoracic and Respiratory Sciences, Second University of Naples. The indications for PM implantation were: a)first-degree atrioventricular blocks with a pathological infra-Hissian conduction (16 patients); b) symptomatic second- or third-degree atrioventricular blocks, respectively in 14 and 4 patients. Before PM implantation a comprehensive cardiac examination including physical examination, 12-lead electrocardiogram (ECG), 24-h ECG Holter monitoring, echocardiogram and invasive electrophysiological study (EPS) was performed. No statistically significant differences in the electrical parameters (P wave amplitude, pacing threshold and lead impedance) were observed at implantation.
Device characteristics and programming
Standard techniques for implantation of a dualchamber PM system (Medtronic Kappa D901, or Adapta ADDR01, Medtronic Inc., Minneapolis, MN, USA) were used. Percutaneous subclavian vein cannulation was performed in all cases;the right ventricular lead was first positioned in the apex, under fluoroscopic guidance. All patients received the bipolar atrial screw-in pacing lead CapSureFixw 5076 (Medtronic Inc., Minneapolis, MN, USA). The electrode surface material consisted of titanium– nitride alloy with an electrode surface area of 4.2 mm2 for the helix and of 22 mm2 for the ring electrode. There was 1-mg of dexamethasone in the electrode tip, which eluted after lead implantation. The distance between the two electrodes was 10 mm. The atrial pacing lead was positioned in the right atrial appendage or on the right side of the interatrial septum, in the region of Bachmann's bundle. All the devices were programmed in DDDR mode. The lower rate was set to 60 bpm. Rate adaptive pacing was used with a maximum rate of 130 bpm. Mode switches were programmed to occur for atrial rates > 200 bpm, persisting for more than 8 ventricular beats. The devices used in this study were programmed to detect episodes of atrial tachycardia, and to record summary and detailed data, including atrial and ventricular electrogram.
Study protocol
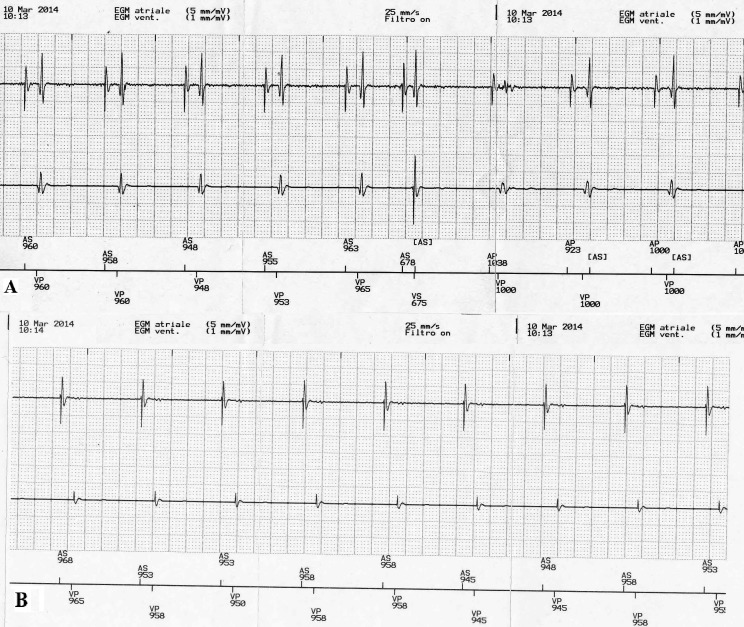
The study population was retrospectively subdivided into 2 groups according to the location of the atrial lead: right atrial appendage (18 patients) or Bachmann's bundle region (16 patients). Patients with foramen ovale, atrial septal aneurysm, severe mitral stenosis, left atrial enlargement or receiving prior surgery (coronary bypass or valvular heart surgery) that involved the right atrium (RA) or taking anti-arrhythmic medications were excluded from the study. All measurements were performed with patients in the supine position. The minimum (Pmin), maximum (Pmax) and mean (Pmean) sensed P-wave amplitude were established in each patient, both in unipolar (UP) and bipolar (BP) atrial lead sensing configuration, by the automatic P-wave amplitude test. Pmean is the result of the arithmetic average between Pmax and Pmin values. The results between the two atrial stimulation sites were statistically compared. Using the same test, the FFRS after sensed R-waves was evaluated, at the highest available testing atrial sensitivity (0.1 mV), with the simultaneous recording of RA intracardiac electrogram (IEGM), marker channels and surface ECG. During the test the presence of the atrial sense marker coincident with the R-wave was monitored, and if FFRS occurred, the IEGM was frozen and displayed at the programmer screen, at a sweep speed of 100 mm/s. Then, using an electronic calliper system (with an accuracy of 3 ms) the interval between the beginning of the QRS complex on the surface ECG and the first atrial FFRS marker was measured to determine FFRS timing. Subsequently, the automatic P-wave amplitude test was repeated with the atrial sensitivity gradually decreasing step by step, until no FFRS was seen (as indicated by the absence of atrial sense marker coincident with the R-wave). The FFRS threshold (FFRS trsh) was defined as the minimal atrial sensitivity at which no FFRS occurred (Figure 1). If FFRS was not present at the most sensitive setting of 0.1 mV, FFRS trsh was assumed to be 0.1 mV.
Figure 1.
A) At the programmed sensitivity of 0.1 mV with BP sensing configuration the FFRS after sensed and paced R-wave is present during the automatic P-wave amplitude test, as indicated by the atrial sense marker corresponding to the R-wave. B) With the atrial sensitivity setting of 1.2 mV FFRS is no longer observed during the test.
Statistical analysis
The χ2-test was used to analyze differences between categorical variables. For normally distributed continuous variables, Student's t-test was applied. If variables did not follow normal distribution, the Mann–Whitney U-test was performed for comparisons of independent variables. Wilcoxon signed rank test was used for comparisons of related variables. STATISTICA software (version 7.1, StatSoft, Inc.) was used to calculate statistics. P < 0.05 was set as statistically significant.
Results
Data are presented as means and standard deviations, or medians and ranges, when appropriate. Table 1 shows the pacing and sensing parameters, at the time of FFRS measurements in the 2 study groups: Group RAA (n:18; age 54.1 ± 6 years; 12 M:6 F) with atrial lead positioned in the right atrial appendage, and Group BB (n:16; age 48.5 + 6.8 years; 11 M:5 F) with atrial lead positioned in the Bachmann's bundle region. There were no statistically significant differences between age and sex composition of the 2 groups and the medications intake. No differences were observed between the two atrial stimulation sites regarding the Pmin and Pmean values of sensed P-wave amplitudes, as well as between UP and BP sensing configurations. Table 2 shows the FFRS characteristics in both right atrial stimulation sites. Significant differences were observed in terms of FFRS trsh, between the two study groups, but not in terms of FFRS timing. The FFRS trsh was lower at the BB region compared to RAA site. The mean BP FFRS trsh was significantly lower than UP configuration in both atrial locations (P < 0.02).
Table 1.
Electrical measurements of the atrial lead in the two study groups.
| Sensing configuration | Parameters | RAA group | BB group | P-value |
|---|---|---|---|---|
| UP | Pacing threshold (V) | 0.7 + 0.2 (0.4 – 1.5) | 0.9 + 0.3(0.3 – 1.7) | ns |
| Pacing impedance (Ohm) | 602 + 235 (227 –984) | 676 + 288 (275 – 1001) | ns | |
| P min (mV) | 2.1 + 1.3(0.6 – 4.6) | 2.8 + 1.0 (0.5 – 4.9) | ns | |
| P mean (mV) | 3.4 + 1.3 (0.6 – 5.1) | 3.2 + 1.1 (0.8 – 5.1) | ns | |
| BP | Pacing threshold (V) | 0.8 + 0.4 (0.3 – 2.6) | 0.6 + 0.5 (0.2 – 3.0) | ns |
| Pacing impedance (Ohm) | 721 + 233 (315 –1080) | 751 + 304 (301 – 1099) | ns | |
| P min (mV) | 2.3 + 1.2 (0.5 – 4.3) | 2.5 + 1.6 (0.6 – 4.8) | ns | |
| P mean (mV) | 3.3 + 1.5 (0.8 – 4.9) | 3.0 + 1.4 (0.9 – 5.2) | ns |
Data are presented as mean ± standard deviation and range.
Legenda: Pmin: the minimal, and Pmean: the mean amplitude of sensed P-wave; UP: unipolar; BP: bipolar; RAA: right atrial appendage; BB: Bachmann's bundle.
Table 2.
Far field R-wave sensing (FFRS) characteristics at both right atrial stimulation sites.
| Sensing configuration | Parameters | RAA group | BB group | P-value |
|---|---|---|---|---|
| UP | FFRS trsh (mV) | 0.9 ± 0.4 (0.5 – 3.1) | 0.5 ± 0.1(0.1 – 1.3) | P < 0.05 |
| R-T1 (msec) | 33 ± 21 (16 – 88) | 30 ± 19 (12 – 77) | ns | |
| BP | FFRS trsh (mV) | 0.6 ± 0.3 (0.4 – 2.0) | 0.3 ± 0.1 (0.1 – 1.1) | P < 0.05 |
| R-T1 (msec) | 39 ± 18 (28 – 76) | 44 ± 22 (24 – 85) | ns |
Data are presented as mean ± standard deviation and range
Legenda FFRS trsh: FFRS threshold; R-T1: FFRS timing; UP: unipolar; BP: bipolar.
Discussion
Optimal atrial sensing is an important prerequisite for proper pacemaker functions. It is especially important in DM1 patients with frequent paroxysmal atrial tachyarrhythmias, in whom automatic mode switch algorithms are involved in the prevention and termination therapies for atrial fibrillation. This aspect requires a higher atrial programmed sensitivity with the risk of sensing of ventricular depolarization in the atrial channel (FFRS), that interferes with advanced diagnostic and therapeutic functions of modern dual chamber devices, including ICD (16-18). However, with the ability to program the post ventricular atrial blanking (PVAB) time in modern devices, FFRS-related consequences can be easily solved. Nevertheless, it should be emphasized that long atrial blanking periods may decrease the sensitivity of arrhythmias detection, especially atrial flutter, if every other flutter wave will not be sensed. Thus, for reliable AF detection, the programming of short PVAB time is recommended as well as a higher atrial sensitivity setting is necessary to detect low AF amplitude. In the heart conduction system, Bachmann's bundle is a branch of the anterior internodal tract that resides on the inner wall of the left atrium. It is a broad band of cardiac muscle that passes from the right atrium, between the superior vena cava and the ascending aorta (19). During the normal sinus rhythm, BB is the preferential path for the conduction of cardiac impulse from right to left atrium. We have previously shown (11, 12) that in DM1 patients undergoing dual chamber pacemaker implantation, the insertion of the atrial lead in the interatrial septum is a safe procedure, presenting a low rate of sensing and pacing defects. However, it seems not to be able to prevent the onset of paroxysmal atrial fibrillation in this population (13-15). Lewicka-Nowak et al. showed that the BB stimulation is affected by a low rate of FFRS compared to conventional RAA pacing, with a high atrial programmed sensitivity (20). These results were explained by Kirchoff's law, which states that the electrical potential, at any location, is inversely related to its distance from the current source (21). It has also been reported that the BP atrial sensing configuration is clearly superior in rejecting FFRS compared with the UP configuration, both in BB region and RAA site (20-24). Furthermore, it has been observed that atrial oversensing is more frequent and the amplitude of far field R-waves greater with a longer tip-to-ring spacing; reducing the inter-electrode distance, it decreases the incidence of FFRS and increases the ratio between the P-wave and FFRS amplitudes. DM1 patients are a higharrhythmic risk population (9, 25-28), probably related to the heterogeneity of ventricular repolarization (6), proven by the increase of QTc and JTc dispersion, as reported in congenital (29-32) or acquired (33-35) heart diseases and in other neuromuscular disorders (36-40). Considering the high risk of supraventricular arrhythmias in DM1 patients, an optimal atrial sensing is an important prerequisite for proper pacemaker functions.
At our knowledge, this is the first paper investigating the effects of the different atrial lead positioning on the FFRS characteristics in DM1 population. We observed that the atrial lead positioning in BB region significant decreases the FFRS threshold compared to RAA atrial lead placement, and that BP sensing configuration significantly improves FFRS threshold compared to UP sensing configuration, in both placements.
A possible explanation for these results is that FFRS is only related to the distance from the atrial lead placement and the point of ventricular activation, and to the inter-electrode distance; furthermore it does not depend on the degree of fibrosis, hypertrophy of the atrial myocytes and fatty acid infiltration, which are generally the histopathological cardiac pattern observed in patients with DM1. In conclusion, we report that in DM1 patients, Bachmann's bundle area is an optimal atrial lead position for signal sensing as the conventional RAA. Furthermore based on our experience, it offers the advantage of reducing the oversensing of R-wave on the atrial lead, thus improving the functions of standard dual chamber pacemakers in DM1 patients.
Acknowledgements
DNA samples for the genetic analysis are stored at the Naples Human Mutation Gene Biobank. NHMGB is member of the Telethon Network of Genetic Biobanks (project no. GTB12001), funded by Telethon Italy, and of the EuroBioBank network.
References
- 1.Phillips MF, Harper PS. Cardiac disease in Myotonic Dystrophy. Cardiovasc Res. 1997;33:13–22. doi: 10.1016/s0008-6363(96)00163-0. [DOI] [PubMed] [Google Scholar]
- 2.Harley HG, Brook JD, Rundle SA, et al. Families with Myotonic Dystrophy with and without cardiac involvement. Arch Intern Med. 1983;143:2134–2136. [PubMed] [Google Scholar]
- 3.Rotundo IL, Faraso S, Leonibus E, et al. Worsening of cardiomyopathy using deflazacort in an animal model rescued by gene therapy. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancioni A, Rotundo IL, Kobayashi YM, et al. Combined deficiency of alpha and epsilon sarcoglycan disrupts the cardiac dystrophin complex. Hum Mol Genet. 2011;20:4644–4654. doi: 10.1093/hmg/ddr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo AD, Mangiola F, Della Bella P, et al. Risk of arrhythmias in Myotonic Dystrophy: trial design of the RAMYD study. J Cardiovasc Med (Hagerstown) 2009;10:51–58. doi: 10.2459/jcm.0b013e328319bd2c. [DOI] [PubMed] [Google Scholar]
- 6.Cudia P, Bernasconi P, Chiodelli R, et al. Risk of arrhythmia in type I Myotonic Dystrophy: the role of clinical and genetic variables. J Neurol Neurosurg Psychiatry. 2009;80:790–793. doi: 10.1136/jnnp.2008.162594. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HH, Wolfe JT, III, Holmes DR, Jr, et al. Pathology of the cardiac conduction system in Myotonic Dystrophy: A study of 12 cases. J Am Coll Cardiol. 1988;11:662–671. doi: 10.1016/0735-1097(88)91547-1. [DOI] [PubMed] [Google Scholar]
- 8.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmya devices: Summary article: A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 9.Russo V, Rago A, D'Andrea A, et al. Early onset "electrical" heart failure in Myotonic Dystrophy type 1 patient: the role of ICD biventricular pacing. Anadolu Kardiyol Derg. 2012;12:517–519. doi: 10.5152/akd.2012.161. [DOI] [PubMed] [Google Scholar]
- 10.Wood MA, Moskovljevic P, Stambler BS, et al. Comparison of bipolar atrial electrogram amplitude in sinus rhythm, atrial fibrillation, and atrial flutter. PACE. 1996;19:150–156. doi: 10.1111/j.1540-8159.1996.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 11.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann's bundle stimulation: a two-year comparative study of electrical parameters in Myotonic Dystrophy type-1 patients. Pacing Clinical Electrophysiol. 2009;32:1191–1196. doi: 10.1111/j.1540-8159.2009.02464.x. [DOI] [PubMed] [Google Scholar]
- 12.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in Myotonic Dystrophy patients: the role of Bachmann's Bundle stimulation. Pacing Clin Electrophysiol. 2008;31:1463–1466. doi: 10.1111/j.1540-8159.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 13.Nigro G, Russo V, Politano L, et al. Does Bachmann's bundle pacing prevent atrial fibrillation in Myotonic Dystrophy type 1 patients? A 12 months follow-up study. Europace. 2010;12:1219–1223. doi: 10.1093/europace/euq170. [DOI] [PubMed] [Google Scholar]
- 14.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in Myotonic Dystrophy type 1 patients: a long term follow-up study. Acta Myol. 2012;31:139–143. [PMC free article] [PubMed] [Google Scholar]
- 15.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: a prospective, randomized, single-bind cross-over study. Europace. 2012;14:486–489. doi: 10.1093/europace/eur373. [DOI] [PubMed] [Google Scholar]
- 16.Brandt J, Worzewski W. Far-field QRS complex sensing: prevalence and timing with bipolar atrial leads. PACE. 2000;23:315–320. doi: 10.1111/j.1540-8159.2000.tb06755.x. [DOI] [PubMed] [Google Scholar]
- 17.Vitiello C, Faraso S, Sorrentino NC, et al. Disease rescue and increased lifespan in a model of cardiomyopathy and muscular dystrophy by combined AAV treatments. PLoS One. 2009;4:e5051–e5051. doi: 10.1371/journal.pone.0005051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Andrea A, Salerno G, Scarafile R, et al. Right ventricular myocardial function in patients with either idiopathic or ischemic dilated cardiomyopathy without clinical sign of right heart failure: effects of cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2009;32:1017–1029. doi: 10.1111/j.1540-8159.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- 19.James T.N. The connecting pathways between the sinus node and A-V node and between the right and the left atrium in the human heart. American Heart Journal. 1963;66:498–508. doi: 10.1016/0002-8703(63)90382-x. [DOI] [PubMed] [Google Scholar]
- 20.Lewicka-Nowak E, Kutarski A, Dabrowska-Kugacka A, et al. Atrial lead location at the Bachmann's bundle region results in a low incidence of far field R-wave sensing. Europace. 2008;10:138–146. doi: 10.1093/europace/eum277. [DOI] [PubMed] [Google Scholar]
- 21.Bakker JMT, Hauer RNW, Simmers TA. Activation mapping, unipolar versus bipolar recording. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology –From Cell to Bedside. 2nd ed. Philadelphia: WB Saunders Company; 1995. pp. 1068–1078. [Google Scholar]
- 22.Brouver J, Nagelkerke D, Heijer P, et al. Analysis of atrial sensed far-field ventricular signals: a reassessment. PACE. 1997;20:916–922. doi: 10.1111/j.1540-8159.1997.tb05494.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffin JC. Sensing characteristic of the right atrial appendage electrode. PACE. 1983;6:22–25. doi: 10.1111/j.1540-8159.1983.tb06576.x. [DOI] [PubMed] [Google Scholar]
- 24.Cools F, Twembeke W, Hemelhof H, et al. Feasibility of using atrial sensitivities below 0,5 mV in DDD(R) pacemaker with mode switching algorithm. Prog Biomed Res. 1999;4:303–306. [Google Scholar]
- 25.Dello Russo A, Pelargonio G, Parisi Q, et al. Widespread electroanatomic alterations of right cardiac chambers in patients with Myotonic Dystrophy type 1. J Cardiovasc Electrophysiol. 2006;17:34–40. doi: 10.1111/j.1540-8167.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 26.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in Myotonic Dystrophy type 1 patients? Kardiol Pol. 2013;71:1147–1153. doi: 10.5603/KP.2013.0295. [DOI] [PubMed] [Google Scholar]
- 27.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: a long term follow-up study. Acta Myol. 2012;31:139–143. [PMC free article] [PubMed] [Google Scholar]
- 28.Politano L, Nigro G. Treatment of dystrophinopathic cardiomyopathy: review of the literature and personal results. Acta Myol. 2012;31:24–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Nigro G, Russo V, Rago A, et al. Heterogeneity of ventricular repolarization in newborns with severe aortic coarctation. Pediatr Cardiol. 2012;33:302–306. doi: 10.1007/s00246-011-0132-4. [DOI] [PubMed] [Google Scholar]
- 30.Nigro G, Russo V, Rago A, et al. The effect of aortic coarctation surgical repair on QTc and JTc dispersion in severe aortic coarctation newborns: a short term follow up study. Physiol Res. 2014;63:27–33. doi: 10.33549/physiolres.932491. [DOI] [PubMed] [Google Scholar]
- 31.Russo V, Rago A, Pannone B, et al. Dispersion of repolarization and beta-thalassemia major: the prognostic role of QT and JT dispersion for identifying the high-risk patients for sudden death. Eur J Haematol. 2011;86:324–331. doi: 10.1111/j.1600-0609.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 32.Nigro G, Russo V, Rago A, et al. Regional and transmural dispersion of repolarisation in patients with Emery-Dreifuss muscular dystrophy. Kardiol Pol. 2012;70:1154–1159. [PubMed] [Google Scholar]
- 33.Nigro G, Russo V, Salvo G, et al. Increased heterogenity of ventricular repolarization in obese nonhypertensive children. Pacing Clin Electrophysiol. 2010;33:1533–1539. doi: 10.1111/j.1540-8159.2010.02889.x. [DOI] [PubMed] [Google Scholar]
- 34.Russo V, Ammendola E, Crescenzo I, et al. Effect of weight loss following bariatric surgery on myocardial dispersion of repolarization in morbidly obese patients. Obes Surg. 2007;17:857–865. doi: 10.1007/s11695-007-9160-9. [DOI] [PubMed] [Google Scholar]
- 35.Santangelo L, Ammendola E, Russo V, et al. Influence of biventricular pacing on myocardial dispersion of repolarization in dilated cardiomyopathy patients. Europace. 2006;8:502–505. doi: 10.1093/europace/eul054. [DOI] [PubMed] [Google Scholar]
- 36.Russo V, Rago A, Politano L, et al. Increased dispersion of ventricular repolarization in Emery Dreifuss muscular dystrophy patients. Med Sci Monit. 2012;18:CR643–CR647. doi: 10.12659/MSM.883541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nigro G, Russo V, Rago A, et al. Regional and transmural dispersion of repolarisation in patients with Emery-Dreifuss muscular dystrophy. Kardiol Pol. 2012;70:1154–1159. [PubMed] [Google Scholar]
- 38.Nigro G, Nigro G, Politano L, et al. Is the value of QT dispersion a valid method to foresee the risk of sudden death? A study in Becker patients. Heart. 2002;87:156–157. doi: 10.1136/heart.87.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigro G, Russo V, Ventriglia VM, et al. Early onset of cardiomyopathy and primary prevention of sudden death in X-linked Emery- Dreifuss muscular dystrophy. Neuromuscul Disord. 2010;20:174–177. doi: 10.1016/j.nmd.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Ammendola E, Russo V, Politano L, et al. Is heart rate variability a valid parameter to predict sudden death in patients with Becker's muscular dystrophy? Heart. 2006;92:1686–1687. doi: 10.1136/hrt.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]