Abstract
Background
Acute kidney injury (AKI) following heart surgery is associated with long-term risk of heart failure. It is not known if AKI following valvular heart surgery is associated with early changes in cardiac function or structure.
Methods
A cohort study was conducted on 201 patients with AKI and 201 patients without AKI after valvular heart surgery, who were matched for age, sex, left ventricular function, and estimated glomerular filtration rate. AKI was defined as an increase in postoperative serum creatinine of ≥26 μmol/l (≥0.3 mg/dl) or a relative increase of ≥50%. The two primary outcomes were changes in post-compared with preoperative left ventricular ejection fraction (LVEF) and left ventricular end-diastolic diameter (LVEDD) assessed by echocardiography.
Results
The mean age was 72 years, and 33% were female. Aortic valve surgery was the most frequent procedure. The mean time from surgery to the postoperative echocardiographic examination was 4.9 days (SD 3.7). There was no significant change in postoperative mean LVEF (−3.6 vs. −4.3%; p = 0.58) or mean LVEDD (−4.7 vs. −3.9 mm; p = 0.31) in patients with AKI compared to those without AKI.
Conclusion
We found no acute changes in cardiac function or structure assessed by echocardiography in patients with AKI compared to those without AKI after valvular heart surgery.
Key Words: Acute kidney injury, Echocardiography, Valvular heart surgery, Cardiorenal syndrome
Introduction
Acute kidney injury (AKI) after heart surgery is common and associated with increased short- and long-term mortality and worsening of renal function [1,2,3]. A recent study showed that AKI after coronary artery bypass grafting increased the long-term risk of new-onset heart failure [4]. During a mean follow-up of 4.1 years, patients with a 44-μmol/l (>0.5 mg/dl) increase in postoperative serum creatinine values had an almost doubled risk of a first hospitalization for heart failure.
Chronic kidney disease is a well-known risk factor for myocardial infarction and heart failure [5,6]. However, little is known about AKI and its direct effects on the heart. An animal study found that rats with ischemia-induced AKI had changes in left ventricular dimension and fractional shortening within 48 h of injury [7]. The relationship between the heart and kidney dysfunction has been described as cardiorenal syndrome [8]. Cardiorenal syndrome type 3 is defined as acute worsening of the kidney function leading to heart injury and/or dysfunction [8]. Injuries and dysfunctions described are, among others, acute decompensated heart failure, acute myocardial infarction, pulmonary edema, and arrhythmia [9]. Several pathophysiological pathways have been suggested, such as indirect effects on the heart by fluid and electrolyte disturbances, uremia, hemodynamic alterations, neuroendocrine activation, and direct effects by inflammatory mediation [9]. However, evidence for the existence of cardiorenal syndrome type 3 is scarce. In a study in which AKI was associated with an increased risk of new-onset heart failure, the incidence was particularly increased the first few months after surgery [4].
The purpose of this study was to compare patients with and without AKI regarding left ventricular ejection fraction (LVEF) as a measure of cardiac function, and left ventricular end-diastolic diameter (LVEDD) as a measure of cardiac structure, assessed by echocardiography before and after valvular heart surgery.
Methods
Study Population
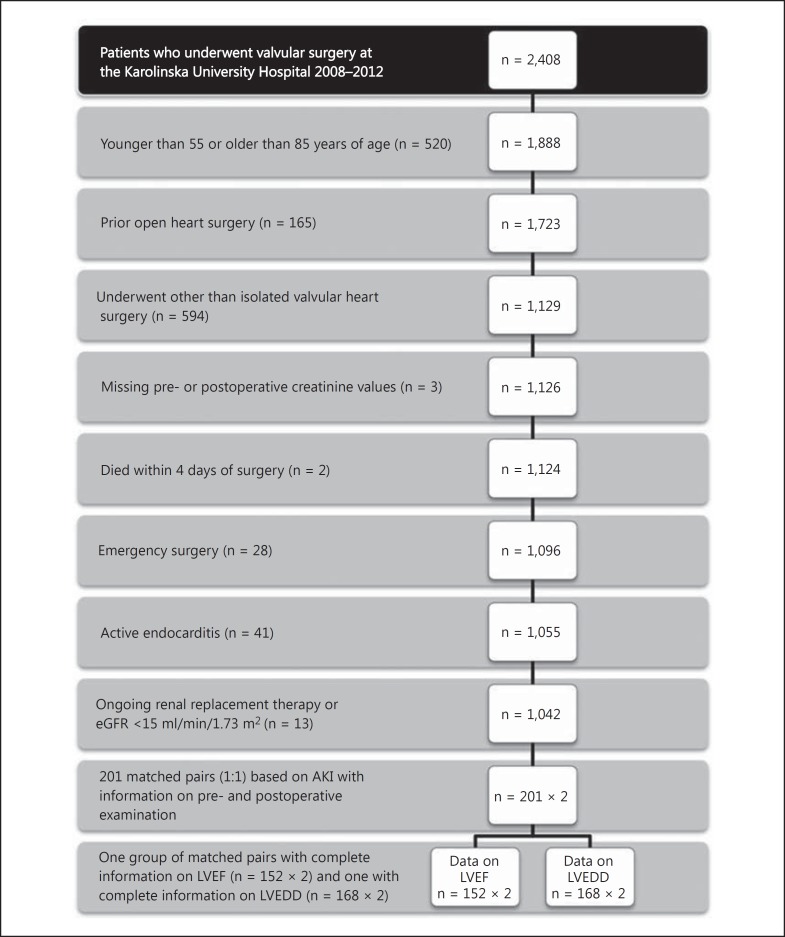
A cohort study was conducted; all patients who underwent valvular heart surgery at the Karolinska University Hospital from January 1, 2008, to December 31, 2012, were eligible for inclusion. As standard practice, all patients undergoing valvular heart surgery are examined with a pre- and postoperative echocardiographic examination. The patients were identified from the Swedish Heart Surgery Register, where all heart surgery performed in Sweden is recorded. The register is part of the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease according to Recommended Therapies (SWEDEHEART) register. The quality of the SWEDEHEART register has been reviewed, and it has been found to be a credible tool for researchers [10]. Exclusion criteria and the number of patients excluded are shown in figure 1. Patients excluded from the study were those older than 85 or younger than 55 years of age; those who had undergone previous heart surgery; those who underwent other procedures than isolated valvular surgery; those who had missing pre- or postoperative serum creatinine; those who died within 4 days after surgery; those who had surgery within 24 h from the decision to operate (emergency surgery); those who had active endocarditis, and those who had dialysis-dependent renal failure or an estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2. The study was performed in compliance with the Declaration of Helsinki and was approved by the regional ethics committee in Stockholm, Sweden.
Fig. 1.
All patients undergoing valvular heart surgery at the Karolinska University Hospital from January 1, 2008, to December 31, 2012, were eligible for inclusion. Exclusion criteria with the number of excluded patients in parentheses are shown.
Definitions
From the SWEDEHEART register, information on age, sex, preoperative LVEF, pre- and postoperative serum creatinine values, prior stroke, diabetes mellitus, chronic obstructive pulmonary disease, peripheral vascular disease, prior acute myocardial infarction, and operated heart valves was collected. Creatinine kinase-MB (CKMB) was collected from the patients' medical records. AKI was defined by comparing pre- and postoperative serum creatinine values. The preoperative value was normally collected within 24 h before surgery, and the postoperative value was the highest value during the index hospitalization. AKI was defined according to the Acute Kidney Injury Network (AKIN) criteria stage 1: an increase in serum creatinine of ≥26 μmol/l (≥0.3 mg/dl) or a relative increase in postoperative serum creatinine of ≥50% [11]. The reference group was defined as an increase of <26 μmol/l (<0.3 mg/dl), a relative increase of <50%, or a decrease in postoperative serum creatinine. GFRs were estimated using the simplified Modification of Diet in Renal Disease (MDRD) study equation: GFR = 186 · (serum creatinine in μmol/l/88.4)-1.154· age−0.203, multiplied by 0.742 in women. Hypertension, diabetes mellitus and chronic obstructive pulmonary disease were defined according to the patients' ongoing pharmacological treatment. Peripheral vascular disease was defined according to the European System for Cardiac Operative Risk Evaluation (EUROSCORE) definition of presence of claudication, occlusion or >50% stenosis of a carotid artery, amputation for arterial disease, previous or planned intervention on the abdominal aorta, limb arteries, or carotids. CKMB was sampled within the first 24 h after surgery. LVEF was categorized as normal (EF >50%), reduced (EF 30-50%) or severely reduced (EF <30%).
Matching
After exclusion, there were 1,042 patients, of whom 295 had developed AKI postoperatively. Patients with AKI were matched to patients without AKI in a 1:1 ratio on the following characteristics: age (3 groups: 55-65, 65-75 and 75-85 years), sex, preoperative LVEF (3 groups: <30, 30-50 or >50%) and eGFR (4 groups: 15-30, 30-45, 45-60, and >60 ml/min per 1.73 m2). We matched for LVEF, age and eGFR, since left ventricular systolic dysfunction, high age and chronic kidney disease are among the most important risk factors for AKI. Information on preoperative LVEF from the SWEDEHEART register was only used for the purpose of matching. Patients with missing pre- or postoperative echocardiographic examination protocols were excluded (24 AKI patients). Patients with echocardiographies >1 year before (13 AKI patients) or 1 month after surgery were excluded (15 AKI patients). If a match had either a missing pre- or postoperative echocardiography, another match was chosen. In a few cases, a suitable match could not be found (40 AKI patients). Three AKI patients were excluded since the echocardiographic examinations could not be dated. In total, 201 patients with AKI could be matched to a patient without AKI (fig. 1).
Outcome
The outcome variables under study were LVEF and LVEDD, which were extracted from pre- and postoperative echocardiographic examination protocols found in the patients' medical records. A standardized protocol was regularly used at echocardiographic examination, and the examinations closest in time before and after surgery were used. The preoperative examinations were performed at five different hospitals and the postoperative examination at the hospital where heart surgery was performed. In general, physicians or biomedical analysts specialized in echocardiography performed the echocardiographies. In most cases, the echocardiographist used visual examination to assess LVEF, and LVEDD was assessed by quantitative measurement. At the time when the echocardiographists performed the examination they had no information if the patients were exposed to AKI or not. The reported values were later extracted from echocardiography protocols for this study. If the values reported were in the range between two values, the mean value was calculated. Some of the echocardiographic examinations had no numerical reports. If LVEF was reported as ‘normal' (n = 8), we assigned a value of 55%, which is the lowest normal LVEF according to the current guidelines for chamber quantification [12]. If LVEDD was reported as ‘normal' (n = 95), we assigned a value of 59 mm in men and 53 mm in women, which is the highest normal LVEDD. If LVEDD was reported as ‘small' (n = 7), we assigned a value of 42 mm in men and 39 mm in women, which is the lowest normal LVEDD. Complete information on LVEF was available for 152 pairs, which were analyzed for changes in LVEF, and complete information on LVEDD was available for 168 pairs, which were analyzed for changes in LVEDD.
Statistical Analysis
Descriptive statistics were used to present baseline characteristics for the study group. Categorical variables were described with frequencies and percentages, and continuous variables were described with means and standard deviations (SDs). The time interval in days between the pre- and postoperative echocardiographic examinations and surgery was presented as mean, median and SD. Scatterplots and box plots were used to demonstrate changes in LVEF and LVEDD in patients with AKI compared to those without AKI. The data on changes in LVEF and LVEDD had a normal distribution. A paired two-sided t test was used for group comparisons of changes in pre- and postoperative LVEF and LVEDD according to AKI. Stata version 13.0 (StataCorp LP, College Station, Tex., USA) was used for all statistical analyses.
Results
The study consisted of 201 patients with AKI and 201 patients without AKI. The mean age was 71.8 years, and 33% were female (table 1). Patients with AKI were more likely to have an increased postoperative CKMB, prior stroke, chronic obstructive pulmonary disease, peripheral vascular disease, multiple valve surgery, and surgery on the tricuspid valve compared with patients without AKI. Aortic valve surgery was the most frequent procedure (table 1). The mean time interval between the preoperative echocardiographic examination and surgery was 100 days (SD 69, median 91), and the mean time interval from surgery to postoperative examination was 4.9 days (SD 3.7, median 4.0). Baseline characteristics in patients with complete information on LVEF and LVEDD, respectively, are presented in online supplementary tables 1 and 2 (for all online suppl. material, see www.karger.com/doi/10.1159/000368199). The characteristics were similar in patients with complete information on LVEF, LVEDD or the sum of the two groups as presented in table 1.
Table 1.
Characteristics of the study population in relation to the presence or absence of AKI
| All patients | No AKI | AKI | |
|---|---|---|---|
| Number of patients | 402 | 201 | 201 |
| Percent of study group | 100 | 50 | 50 |
| Age at operation, years | 71.8 ± 7.7 | 71.7 ± 7.2 | 71.9 ± 7.8 |
| Females | 134 (33) | 67 (33) | 67 (33) |
| LVEF | |||
| >50% | 266 (66) | 133 (66) | 133 (66) |
| 30 – 50% | 106 (26) | 53 (26) | 53 (26) |
| <30% | 30 (7.5) | 15 (7.5) | 15 (7.5) |
| eGFR, ml/min/1.73 m2 | 75 ± 21 | 76 ± 21 | 75 ± 21 |
| eGFR (CKD-EPI), ml/min/1.73 m2 | 71 ± 17 | 71 ± 17 | 70 ± 17 |
| eGFR | |||
| 15 – 30 ml/min | 0 (0) | 0 (0) | 0 (0) |
| 30 – 45 ml/min | 22 (5.5) | 11 (5.5) | 11 (5.5) |
| 45 – 60 ml/min | 66 (16) | 33 (16) | 33 (16) |
| >60 ml/min | 314 (78) | 157 (78) | 157 (78) |
| Postoperative CKMB, µg/l | 27 ± 27 | 23 ± 18 | 32 ± 32 |
| Prior stroke | 30 (7.5) | 10 (5.0) | 20 (10) |
| Diabetes mellitus | 65 (16) | 29 (14) | 36 (18) |
| COPD | 48 (12) | 19 (9.5) | 29 (14) |
| Peripheral vascular disease | 32 (8.0) | 13 (6.5) | 19 (9.5) |
| Prior AMI | 10 (2.5) | 4 (2.0) | 6 (3.0) |
| Valvular heart surgery | |||
| Multiple valve intervention | 43 (8.7) | 15 (5.9) | 28 (12) |
| Aortic valve | 298 (74) | 145 (72) | 153 (76) |
| Mitral valve | 118 (29) | 61 (30) | 57 (28) |
| Tricuspid valve | 23 (5.4) | 6 (3.0) | 17 (8.5) |
| Pulmonary valve | 0 (0) | 0 (0) | 0 (0) |
| Days between preoperative ECHO and surgery | 100 ± 69 [91] | 94 ± 60 [93] | 106 ± 77 [89] |
| Days between postoperative ECHO and surgery | 4.9 ± 3.7 [4] | 5.0 ± 4.0 [4] | 4.8 ± 3.5 [4] |
| First 24-hour postoperative urine production, ml | 2,540 ± 853 | 2,675 ± 766 | 2,405 ± 914 |
Data are n (%) or means ± SDs. Figures in brackets are medians. CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; COPD = chronic obstructive pulmonary disease; AMI = acute myocardial infarction; ECHO = echocardiography. AKI was defined as an increase of >26 mmol/l (>0.3 mg/dl) or a relative increase of >50% in postoperative serum creatinine.
Left Ventricular Ejection Fraction
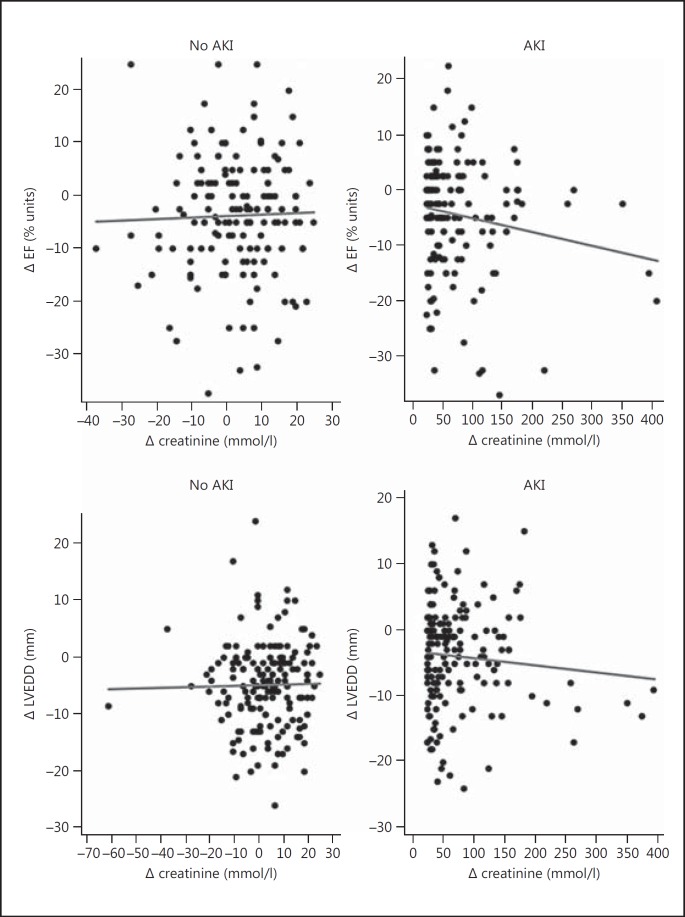
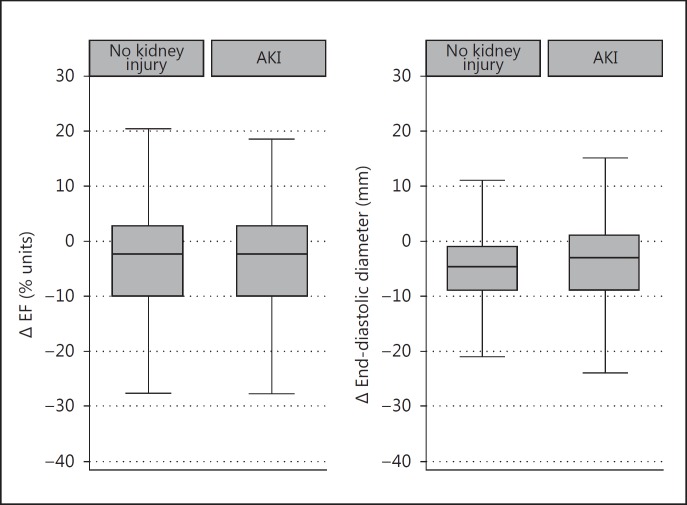
Complete information on pre- and postoperative LVEF was available for 152 matched pairs. There was a weak and nonsignificant correlation between an increase in Δ serum creatinine and a decrease in LVEF (Pearson's correlation coefficient = −0.10; p = 0.08) (fig. 2). There was also no significant difference in LVEF change between patients with and without AKI. The mean reduction in LVEF in AKI patients was −3.6% (95% CI −5.4 to −1.8) and −4.3% in patients without AKI (95% CI −6.0 to −2.6; p = 0.58) (table 2; fig. 3).
Fig. 2.
Change in LVEF and LVEDD, respectively, plotted against the change in postoperative creatinine in patients with or without AKI.
Table 2.
Changes in mean LVEF and LVEDD in relation to the presence or absence of AKI
| No AKI | AKI | p value | |
|---|---|---|---|
| Δ LVEF | |||
| Number of patients | 152 | 152 | |
| Mean | −3.6 (−5.4 to −1.8) | −4.3 (−6.0 to −2.6) | 0.58 |
| Δ LVEDD | |||
| Number of patients | 168 | 168 | |
| Mean | −4.7 (−5.8 to −3.6) | −3.9 (−5.1 to −2.7) | 0.31 |
Figures in parentheses are 95% CIs. AKI was defined as an increase of >26 µmol/l (>0.3 mg/dl) or a relative increase of >50% in postoperative serum creatinine.
Fig. 3.
Box plots of changes in LVEF and LVEDD in relation to the presence or absence of AKI. The median is represented by the central line inside the box. Upper and lower quartiles correspond with the ends of the boxes. Whiskers represent the extreme values and dots are the outliers.
Left Ventricular End-Diastolic Diameter
Complete information on pre- and postoperative LVEDD was available for 168 matched pairs. There was no correlation between Δ serum creatinine and increased LVEDD (Pearson's correlation coefficient = −0.01; p = 0.81) (fig. 2). There was no significant difference in LVEDD change between patients with and without AKI. The mean change in LVEDD was −4.7 mm (95% CI −5.8 to −3.6) in patients with AKI and −3.9 mm (95% CI −5.1 to −2.7; p = 0.31) in patients without AKI (table 2; fig. 2).
Discussion
We found no evidence for an association between AKI after valvular heart surgery and early changes in cardiac function or structure. AKI is a well-known complication to heart surgery and is associated with increased mortality, morbidity and hospitalization postoperatively [1,2]. However, few studies have focused on changes in cardiac structure and function. An animal study by Kelly [7] showed that LVEDD increased and fractional shortening decreased within 48 h after inducing bilateral renal ischemia to rats. Another study found that AKI following coronary artery bypass grafting was associated with long-term risk of a first hospitalization for heart failure [4]. Interestingly, the incidence of heart failure seemed to be highest the first few months after surgery, indicating that there may be acute changes to the heart in the immediate postoperative period [4]. However, it is uncertain if AKI after heart surgery is merely an indicator of more severe cardiovascular disease or increased vulnerability, rather than a direct causative risk factor of new-onset heart failure, myocardial infarction or end-stage renal disease.
There may be several reasons why we found no association between AKI and echocardiographic changes in cardiac structure or function. First, there might not be a causal association between AKI and functional or structural changes to the heart. However, the echocardiographic examinations might not be sensitive enough to identify these changes, if there are any. Other more sensitive methods have been developed recently to estimate cardiac function or structure. Echocardiographic two- or three-dimensional strain is a novel method to quantify left ventricular movement and function [13]. Another noninvasive method for the measurement of cardiac output is inert gas rebreathing techniques such as Innocor® [14,15]. We used a relatively low threshold of increase in postoperative serum creatinine to define AKI. In patients with the highest increases in serum creatinine, there were indications of a decrease in LVEF and an increase in LVEDD. There might be an association between AKI and changes in cardiac function and structure in patients with more severe AKI, and future studies might preferably focus on these patients.
One of the strengths of our study was the even distribution of confounding factors between patients with and without AKI, which were attributed to the matched study design. The median time to the postoperative echocardiographic examination was only 4 days, and all examinations were performed at the same laboratory at the Karolinska University Hospital. However, the time interval between the preoperative echocardiographic examination and surgery could vary between 1 day and 1 year, with a median of 91 days. In that time, cardiac changes may have developed. Also, the echocardiographic examination is highly user dependent, and estimations could vary between echocardiographers. Another limitation was that we had no information regarding postoperative diuretics administration. The postoperative use of diuretics might have prevented volume expansion and thereby prevented echocardiographic changes.
Conclusions
In conclusion, we found no evidence for acute changes in LVEF or LVEDD in patients with AKI after valvular heart surgery. Studies with more sensitive methods such as the noninvasive measurement of cardiac output or echocardiography-based strain imaging are needed.
Disclosure Statement
The authors have nothing to disclose.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.Rydén L, Ahnve S, Bell M, Hammar N, Ivert T, Holzmann MJ. Acute kidney injury following coronary artery bypass grafting: early mortality and postoperative complications. Scand Cardiovasc J. 2012;46:114–120. doi: 10.3109/14017431.2012.657229. [DOI] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsson D, Sartipy U, Braunschweig F, Holzmann MJ. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail. 2013;6:83–90. doi: 10.1161/CIRCHEARTFAILURE.112.971705. [DOI] [PubMed] [Google Scholar]
- 5.Meisinger C, Döring A, Löwel H KORA Study Group. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27:1245–1250. doi: 10.1093/eurheartj/ehi880. [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Gaziano JM, Djoussé L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4:138–144. doi: 10.1161/CIRCHEARTFAILURE.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuasuwan A, Kellum JA. Cardio-renal syndrome type 3: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32:31–39. doi: 10.1016/j.semnephrol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, Gorissen W, Houle H. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013;30:88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 14.Peyton PJ, Bailey M, Thompson BR. Reproducibility of cardiac output measurement by the nitrous oxide rebreathing technique. J Clin Monit Comput. 2009;23:233–236. doi: 10.1007/s10877-009-9187-7. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, Wasserman K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol. 2005;46:1779–1781. doi: 10.1016/j.jacc.2005.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data