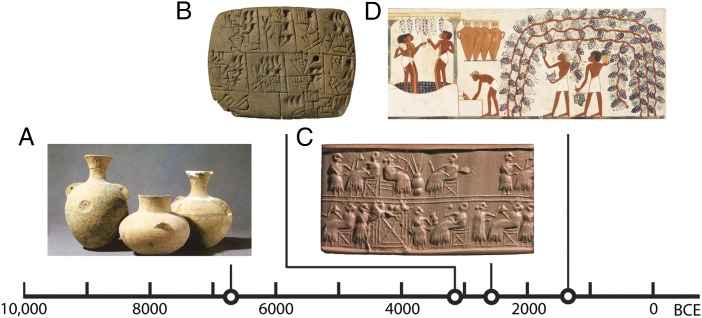
In 1953, botanist Jonathan D. Sauer suggested that our initial motivation to cultivate cereals was not for flour or bread, but for beer (1). The implications of this idea—that a preference for dietary ethanol, or alcohol, sparked the Neolithic Revolution (2)—are profound. No stage of human evolution has left a larger global footprint than the domestication of plants, animals, and landscapes (3). However, there is scant evidence of directed fermentation before the onset of the Neolithic, approximately 10,000 B.C.E. (4). The earliest archaeological evidence of alcohol is associated with the cultivation (5) and initial domestication (6) of cereals during the early Neolithic (Fig. 1), which suggests that fermentation was the happy outcome, rather than the cause, of grain storage and consumption.
Fig. 1.
Alcohol production in antiquity. (A) Early Neolithic jars, with flaring necks and rims, from Phase 2–3 of Jiahu (Henan Province, China), ca. 6500–5500 B.C.E. Chemical analyses (6) indicate a fermented mix of rice, honey, and fruit. Image courtesy of Juzhong Zhang (Institute of Cultural Relics and Archaeology of Henan Province, University of Science and Technology of China, Hefei, China). (B) A Sumerian tablet reports the allocation of beer, Late Uruk period, ca. 3100–3000 B.C.E. (British Museum accession no. 140855; photo © Trustees of the British Museum). (C) Impression of a Sumerian cylinder seal from the Early Dynastic IIIa period, ca. 2600 B.C.E. (27). The upper row depicts the use of long straws to drink unfiltered beer from a globular vessel (British Museum accession no. 121545; photo © Trustees of the British Museum). (D) Facsimile painting from the tomb of Nakht (Theban Tomb 52, Egypt), ca. 1400–1390 B.C.E. The scene depicts early viticulture and wine production. Image courtesy of Metropolitan Museum of Art www.metmuseum.org, artists Norman de Garis Davies, Lancelot Crane, and Francis Unwin; Rogers Fund, 1915.
Any gene involved in the alcohol metabolic pathway is therefore an exemplary candidate for testing the concept of gene-culture coevolution (7), a branch of theoretical population genetics that integrates Neolithic cultural shifts into models of genetic inheritance (8). This approach has been rewarding (9); however, the coevolutionary process is usually preceded with “cultural selection” (8), wherein cultural traits, such as dietary preference, impel the evolution of novel phenotypes. The reverse sequence is seldom considered, but a recent study in PNAS (10) raises new and alluring questions about the genetic adaptations that enabled our shift from foraging to producing societies.
In PNAS, Carrigan et al. (10) report the protein sequences and corresponding kinetic activities of alcohol dehydrogenase class IV (ADH4), the first enzyme to encounter and metabolize dietary alcohol. The authors focused on 18 primate species and resurrected nine ancestral proteins to better understand the evolution and functional ecology of ADH4. This innovative approach revealed three key results. First, the ADH4 enzymes of most primates are essentially inactive against ethanol. Second, a single amino acid change (A294V) causes a dramatic 40-fold increase in ethanol-catalyzing activity. Third, this mutation arose independently in two distantly related primates, the aye-aye (Daubentonia madagascariensis) and the last common ancestor of African apes and humans.
Ethanol with an Aye to Ecology
In the hall of animal oddities, the aye-aye is an exemplar of dietary specialization. It is a peculiar lemur that uses percussive foraging to prey on the larvae of cerambycid (longhorn) beetles. Given that beetle larvae are an improbable source of alcohol, the A294V transition of aye-ayes is very likely a spurious mutation: except that aye-ayes appear to have an enduring mutualism with the traveler’s tree (Ravenala madagascariensis; Strelitziaceae) (11, 12). Aye-ayes probably pollinate R. madagascariensis when they probe the large (30-cm) inflorescences for nectar (Fig. 2A). Carrigan et al.’s (10) speculation that aye-ayes are ingesting fermented nectar invites immediate testing. A diet of fermented floral nectar is not unknown among primates (e.g., Nycticebus coucang) (13).
Fig. 2.
(A) Inflorescense of Ravenala madagascariensis. The large bracts contain pooled, possibly fermented, nectar. Image courtesy of Rolf P. Kudritzki (University of Hawaii at Manoa, Honolulu, HI). (B) Ground-sourced fruits exhibit a range of developmental stages; here the fruits of Stemmadenia donnell-smithii (Apocynaceae) illustrate decomposition. For African apes, overripe fruits could have a calorically optimal combination of sugar and ethanol. Image courtesy of Jim Marden (Pennsylvania State University, University Park, PA). (C) A recumbent chimpanzee (Pan troglodytes) consumes the fermented fruit of an undetermined species in Kibale National Park, Uganda (image courtesy of Mike Knoche). (D) A mantled howling monkey (Alouatta palliata) consumes the fruit of Astrocaryum standleyanum (Arecaceae), a species in which ripe fruit can have an ethanol content ranging from 0.52 to 0.61% (25) (image courtesy of Greg Willis).
A Happy Hour for the Miocene
Perhaps the most striking outcome of Carrigan et al.’s (10) study is the evidence for enhanced ethanol-catalyzing activity in the last common ancestor of gorillas (Gorilla), chimpanzees and bonobos (Pan), and humans (Homo). This ancestor lived in Africa about 10 million y ago and, by inference, it traveled terrestrially between patches of arboreal resources. This view is based on the postcranial anatomies of Gorilla and Pan, which reflect a compromise between the competing demands of arboreal and terrestrial locomotion. Even still, terrestrial travel is energetically costly for any ape with a flexed hindlimb (14), suggesting an ancestral incentive to exploit energy-rich resources on the forest floor, including, possibly, fermented fruits (Fig. 2B).
There are at least two reasons for an ape to consume fermented fruit in moderation (Fig. 2C). First, the reward is substantial: the caloric value of ethanol (7.1 kcal/g) is nearly twice that of carbohydrates (4.1 kcal/g) (15). Second, the taste could be appealing. Fermentation releases glutamate and the savory taste of umami (16). Paul Breslin (16) has suggested that our preference for glutamate evolved in tandem with a diet based on fermented products. This tantalizing idea raises the possibility of coevolution between ADH4 and the glutamate taste receptors T1R1 and T1R3.
Ethanol and the Rise of Homo imbibens
If the last common ancestor of Gorilla, Pan, and Homo turned to fermented fruit to partly offset the energetic costs of terrestrial travel, then a 40-fold increase in the ethanol-catalyzing activity would have conferred selective advantages. For Carrigan et al. (10), this surge in ADH4 activity was a preadapation that improved our fitness “only after humans developed the process and tools for directing fermentation.” Quite inexplicably, given Sauer’s suggestion (1), there was no consideration for another scenario: that we evolved fermentative technologies because of our adaptive predilection for ethanol. In this light, directed fermentation is perhaps best viewed as cultural exaptation.
Recent evidence from Mozambique speaks to the processing of wild Sorghum grains 105,000 y ago (17). Sorghum grains can be malted to produce beer (4, 5), but the process requires inoculation with yeast (Saccharomyces cerevisiae). Middle Stone Age people could have added fermented fruit to the malted grains; or, insects (e.g., bees, Drosophila) could have landed on and inoculated the mix with yeast from their bodies. In any case, S. cerevisiae is a species complex that diversified into several strains approximately 12,000 y ago (18–20). The two oldest strains—those involved with grape and rice wine—show evidence of domestication (18), suggesting that the antiquity of directed fermentation is coincident with the early Neolithic, if not much earlier.
The origins of directed fermentation, or “Homo imbibens” (21), are therefore uncertain, but it is clear that the evolution of agriculture and dairy farming revolved, in part, around the production of fermented foods and beverages; indeed, fermented products account for about one-third of contemporary diets worldwide (22). This idiosyncrasy of human behavior is an enduring topic of interest within anthropology (23), and Carrigan et al.’s commendable paleogenetic analysis (10) sheds new light on how and why the behavior evolved.
Future Directions
The ADH4 enzyme of most primates is essentially inactive against ethanol (10). This result is puzzling in light of Robert Dudley’s drunken monkey hypothesis (24), which reasons that all ripe fruit must contain some ethanol (Fig. 2D). Accordingly, all fruit-eating primates face the challenge of metabolizing ethanol, and it is tempting to suggest that natural selection has acted on other ethanol-metabolizing enzymes (e.g., ADH1, ADH2, MEOS) or those involved in the metabolism of ethanol-induced byproducts (e.g., ALDH2, which oxidizes the acetaldehyde created from ethanol).
The hypothesized link between terrestrial travel and a diet of fermenting fruit (10) is compelling, but also unblemished by data. The ethanol contents of fruits in primate diets are scarcely known; only a handful of fruits have been analyzed in Panama (25) and Singapore (26), and none in tropical Africa. Moreover, the proportions of fermented fruits (and the yeasts within) are unknown in the diets of Gorilla and Pan. These empirical voids are telling and command attention.
Footnotes
The author declares no conflict of interest.
See companion article on page 458.
References
- 1.Braidwood RJ, et al. Did man once live by beer alone? Am Anthropol. 1953;55(4):515–526. [Google Scholar]
- 2.Katz SH, Voigt MM. Bread and beer: The early use of cereals in the human diet. Expedition. 1986;28(2):23–34. [Google Scholar]
- 3.Kareiva P, Watts S, McDonald R, Boucher T. Domesticated nature: Shaping landscapes and ecosystems for human welfare. Science. 2007;316(5833):1866–1869. doi: 10.1126/science.1140170. [DOI] [PubMed] [Google Scholar]
- 4.Hayden B, Canuel N, Shanse J. What was brewing in the Natufian? An archaeological assessment of brewing technology in the Epipaleolithic. J Archaeol Method Theory. 2013;20(1):102–150. [Google Scholar]
- 5.Haaland R. Porridge and pot, bread and oven: Food ways and symbolism in Africa and the Near East from the Neolithic to the present. Camb Archaeol J. 2007;17(2):165–182. [Google Scholar]
- 6.McGovern PE, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA. 2004;101(51):17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: Bringing genetics and the human sciences together. Nat Rev Genet. 2010;11(2):137–148. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien MJ, Laland KN. Genes, culture, and agriculture: An example of human niche construction. Curr Anthropol. 2012;53(4):434–470. [Google Scholar]
- 9.Peng Y, et al. The ADH1B Arg47His polymorphism in East Asian populations and expansion of rice domestication in history. BMC Evol Biol. 2010;10(1):15. doi: 10.1186/1471-2148-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrigan MA, et al. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc Natl Acad Sci USA. 2014;112:458–463. doi: 10.1073/pnas.1404167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kress WJ, Schatz GE, Andrianifahanana M, Morland HS. Pollination of Ravenala madagascariensis (Strelitziaceae) by lemurs in Madagascar: Evidence for an archaic coevolutionary system? Am J Bot. 1994;81(5):542–551. [Google Scholar]
- 12.Melin AD, Moritz GL, Fosbury RAE, Kawamura S, Dominy NJ. Why aye-ayes see blue. Am J Primatol. 2012;74(3):185–192. doi: 10.1002/ajp.21996. [DOI] [PubMed] [Google Scholar]
- 13.Wiens F, et al. Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci USA. 2008;105(30):10426–10431. doi: 10.1073/pnas.0801628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pontzer H, Raichlen DA, Rodman PS. Bipedal and quadrupedal locomotion in chimpanzees. J Hum Evol. 2014;66:64–82. doi: 10.1016/j.jhevol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Dudley R. Evolutionary origins of human alcoholism in primate frugivory. Q Rev Biol. 2000;75(1):3–15. doi: 10.1086/393255. [DOI] [PubMed] [Google Scholar]
- 16.Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol. 2013;23(9):R409–R418. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercader J. Mozambican grass seed consumption during the Middle Stone Age. Science. 2009;326(5960):1680–1683. doi: 10.1126/science.1173966. [DOI] [PubMed] [Google Scholar]
- 18.Fay JC, Benavides JA. Evidence for domesticated and wild populations of Saccharomyces cerevisiae. PLoS Genet. 2005;1(1):66–71. doi: 10.1371/journal.pgen.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legras J-L, Merdinoglu D, Cornuet J-M, Karst F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol Ecol. 2007;16(10):2091–2102. doi: 10.1111/j.1365-294X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 20.Sicard D, Legras J-L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. C R Biol. 2011;334(3):229–236. doi: 10.1016/j.crvi.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 21.McGovern PE. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages. Univ of California Press; Berkeley, CA: 2010. [Google Scholar]
- 22.Campbell-Platt G. Fermented foods—A world perspective. Food Res Int. 1994;27(3):253–257. [Google Scholar]
- 23.Dietler M. Alcohol: Anthropological/archaeological perspectives. Annu Rev Anthropol. 2006;35(1):229–249. [Google Scholar]
- 24.Dudley R. The Drunken Monkey: Why We Drink and Abuse Alcohol. Univ of California Press; Berkeley, CA: 2014. [Google Scholar]
- 25.Dudley R. Fermenting fruit and the historical ecology of ethanol ingestion: Is alcoholism in modern humans an evolutionary hangover? Addiction. 2002;97(4):381–388. doi: 10.1046/j.1360-0443.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 26.Dominy NJ. Fruits, fingers, and fermentation: The sensory cues available to foraging primates. Integr Comp Biol. 2004;44(4):295–303. doi: 10.1093/icb/44.4.295. [DOI] [PubMed] [Google Scholar]
- 27.Damerow P. Sumerian beer: The origins of brewing technology in Ancient Mesopotamia. Cuneiform Digital Library Journal. 2012;2012:2. Available at cdli.ucla.edu/pubs/cdlj/2012/cdlj2012_002.html. [Google Scholar]