Significance
Methodological constraints can limit our ability to quantify potential impacts of climate warming. We assessed the consistency of three approaches in estimating warming effects on plant community composition: manipulative warming experiments, repeat sampling under ambient temperature change (monitoring), and space-for-time substitution. The three approaches showed agreement in the direction of change (an increase in the relative abundance of species with a warmer thermal niche), but differed in the magnitude of change estimated. Experimental and monitoring approaches were similar in magnitude, whereas space-for-time comparisons indicated a much stronger response. These results suggest that all three approaches are valid, but experimental warming and long-term monitoring are best suited for forecasting impacts over the coming decades.
Keywords: thermophilization, space-for-time substitution, climate change, warming experiment, tundra
Abstract
Inference about future climate change impacts typically relies on one of three approaches: manipulative experiments, historical comparisons (broadly defined to include monitoring the response to ambient climate fluctuations using repeat sampling of plots, dendroecology, and paleoecology techniques), and space-for-time substitutions derived from sampling along environmental gradients. Potential limitations of all three approaches are recognized. Here we address the congruence among these three main approaches by comparing the degree to which tundra plant community composition changes (i) in response to in situ experimental warming, (ii) with interannual variability in summer temperature within sites, and (iii) over spatial gradients in summer temperature. We analyzed changes in plant community composition from repeat sampling (85 plant communities in 28 regions) and experimental warming studies (28 experiments in 14 regions) throughout arctic and alpine North America and Europe. Increases in the relative abundance of species with a warmer thermal niche were observed in response to warmer summer temperatures using all three methods; however, effect sizes were greater over broad-scale spatial gradients relative to either temporal variability in summer temperature within a site or summer temperature increases induced by experimental warming. The effect sizes for change over time within a site and with experimental warming were nearly identical. These results support the view that inferences based on space-for-time substitution overestimate the magnitude of responses to contemporary climate warming, because spatial gradients reflect long-term processes. In contrast, in situ experimental warming and monitoring approaches yield consistent estimates of the magnitude of response of plant communities to climate warming.
Because of polar amplification, both current warming trends and projected future warming in high-latitude environments are among the highest on earth (1–3). Thus, knowledge of plant responses to warming in tundra environments is critical to understanding the changes that have occurred and likely will occur in the near future. Ecologists typically have used one of three primary approaches for forecasting ecological responses to climate warming: experiments, long-term observational records, and space-for-time substitutions. The pros and cons of each approach are well-recognized. However, few studies have directly tested congruency among the approaches, despite sustained and in some cases renewed interest in supporting primary research on climate change effects, including synthetic assessments of climate change impacts on biodiversity and coupling observations with models to assess likely impacts of change on ecosystem processes and feedbacks (4–6). Arguably, the robustness of these derived analyses could be increased by understanding the strengths and limitations of each approach.
Long-term experiments are a popular technique for assessing the effects of rapid environmental change because they provide an explicit control. They can be quite effective in simulating climate warming (7), but may introduce unintended artifacts that compromise mimicry of real climate warming (8); for example, open-top chambers, commonly used in passive warming experiments of tundra ecosystems, reduce wind speed and typically modify light availability, snow accumulation, and winter temperatures (9). As a result, the degree to which global change manipulations adequately represent real-world conditions is a subject of active debate (7, 10, 11). The largest-scale comparative analysis of experimental warming effects conducted to date (contrasting effects of experimental vs. interannual temperature on plant phenology), concluded that warming experiments systematically underestimate climate change impacts (12).
Evaluating responses to changes in the biophysical environment through observational records (monitoring) also has advantages and disadvantages. Time series data have proved fruitful in evaluating climate effects on biological phenomena that are tightly linked to yearly weather patterns, such as phenology (13); however, if interannual temperature variations are correlated with interannual variations in other environmental drivers (e.g., irradiance, precipitation), observational data may overestimate climate warming effects (1). In addition, responses to interannual variability may provide a poor proxy for biological phenomena that require accumulated responses to multiyear, sustained changes in climate to exhibit a detectable shift. Where the observational record encompasses a long-term environmental change trend, the combination of short- and long-term (e.g., annual and decadal) variations in responses provides a stronger basis for forecasts of future change (14, 15).
Space-for-time substitutions use extant spatial patterns between biota and climate to project impacts of future changes in climate on species, functional groups, or communities (16–19). Inferring impacts of contemporary climate change based on spatial relationships that were established over much longer time scales can be problematic as well (20). This mode of extrapolation fails to account for temporal lags in biotic responses, including migration, soil organic matter development, and other covarying biophysical limitations (16, 21).
In response to the previously described critiques, researchers have called for combined studies that integrate multiple modes of inference to predict both short- and long-term responses to climate warming (21, 22). Here we compare the thermophilization response of tundra plant communities to climate warming using spatial gradients, long-term observations, and experiments. Thermophilization describes a particular pattern of species turnover wherein more cold-adapted species decline in relative abundance and more warm-adapted species increase (23). We amassed records of plant community composition from repeated sampling of historical studies and warming experiments conducted in tundra sites distributed across the alpine to high Arctic of North America and Europe (Fig. 1 and Table S1). We assessed compositional changes in response to temperature using the community temperature index (CTI). This index, a synthetic indicator of the thermal niche of a suite of species found a given location and time, has been adopted as a common metric of how taxonomic groups respond to climate change over space and time (23, 24). Higher CTI values are indicative of communities dominated by species with ranges centered in warmer environments, and lower values are indicative of communities dominated by species with ranges centered in cooler environments. If all three methods yield valid inferences for near-term climate change impact predictions, then both the direction and magnitude of changes in CTI with changes in temperature should be consistent across approaches.
Fig. 1.
Map of study sites, grouped by region. Red triangles indicate regions used in the experimental warming analyses only (n = 5), blue circles indicate monitoring studies only (n = 19), and purple squares indicate regions with data in both analyses (n = 9). The regions (each indicated by a single dot) often contained multiple experiments or monitoring sites.
Results
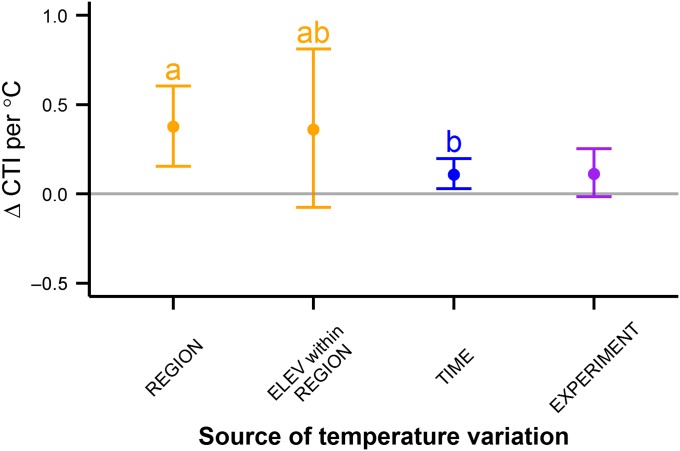
Over all monitoring sites, CTI increased slowly over time (estimated rate of increase in CTI per year from mixed models, 0.0199; 95% CI, 0.0004–0.0382). This result is consistent with numerous studies showing a trend toward a greater abundance of species with a warmer thermal niche, a process referred to as thermophilization of ecological communities over time (23–25). Using hierarchical models, we found higher CTI values associated with regions with higher summer temperatures (Fig. 2), as well as with time periods with relatively warm summers. Similarly, experimental warming caused an increase in the relative abundance of species with a warmer thermal niche, an effect that increased with the duration of experimental manipulation (Fig. 3 and Fig. S2). Although the changes in CTI with temporal and experimental changes in temperature were in the same direction as those occurring over spatial temperature gradients, the magnitude of change was significantly larger for the spatial gradients (Fig. 2).
Fig. 2.
Thermophilization of plant communities in response to variability in temperature over space (orange) or time (blue) or induced by experimental warming (purple). The y-axis shows the change in CTI per 1 °C. Points are the predicted magnitude of effect; error bars encompass 95% CI based on the parametric bootstrap. Bars with different letters (a, b) are significantly different (95% CI of difference in effect size >0, based on the parametric bootstrap of differences in effect size of the plots not experimentally warmed). Parametric bootstrapping across datasets (monitoring vs. experimental warming) was not feasible, so a formal statistical comparison of effect size between experiments and other sources is not presented. Shown are 85 studies in 28 regions for long-term monitoring (orange and blue bars) and 12 studies in 8 regions for experimental warming (purple bars).
Fig. 3.
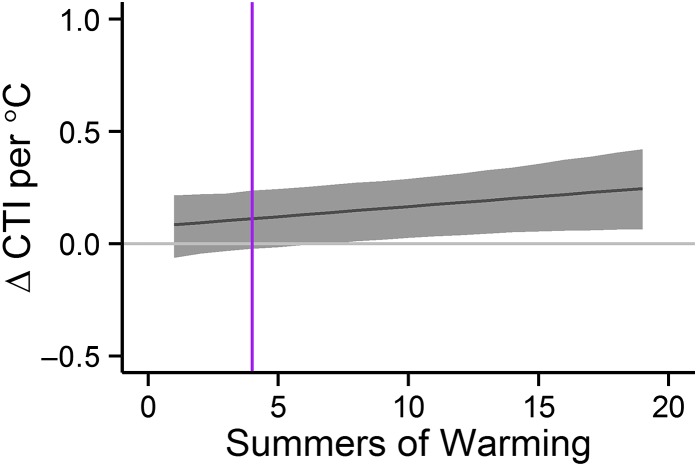
Thermophilization of plant communities in response to multiple years of experimental warming scaled to a 1 °C increase in temperature. The purple reference line shows effect size after four summers of warming, for direct comparison with Fig. 2 (8 regions, 12 studies, 320 plots).
Discussion
As climate warming continues to accelerate, there is an increasing urgency to understand the impacts of current and future temperatures on ecological communities. This is particularly the case in the Arctic, one of only a few ecosystems that have already entered a regime shift in response to contemporary climate change (6). This urgency has catalyzed a surge in climate change manipulations, space-for-time projections, and reanalysis of historical data.
Despite the litany of potential caveats accompanying each methodology, our analyses demonstrate a remarkable degree of correspondence for the direction of tundra plant community change predicted in response to summer temperature warming. Specifically, we found that the CTI increased with summer temperature, regardless of whether variation in summer temperatures reflected natural spatial temperature gradients (latitudinal/elevational), warm years within a temporal sequence of varying weather, or experimentally warmed conditions. These results lend credence to a broad collection of contemporary climate change research that relies on the ability of experimental manipulations, time series data, and/or space-for-time substitutions to simulate future conditions.
One potential cause of the congruence of comparisons vis-à-vis some recent assessments of space-for-time substitution in species distribution models (26) is that we chose to model a synthetic community property (i.e., CTI) rather than individual species responses. This was a practical choice, given that few species were well represented in multiple study sites. It also may have improved the precision of the overall estimate of the climate response by averaging over both sampling error and potentially idiosyncratic responses of some species. Ferrier (27) observed that models of climate change impacts on emergent community properties (e.g., biodiversity) tend to yield more robust results than single-species approaches or the combined results from many, individual data-poor species. It stands to reason that thermophilization may be another emergent community property with a relatively strong sensitivity to climate. Although results tend to be more similar when examining synthetic community properties, in situ temporal variability does not always alter community properties in the same direction as predicted by geographic gradients; for example, the effect of rainy years on species richness does not generally mimic the positive relationship between grassland species richness and precipitation across latitudinal gradients (28, 29).
The greater magnitude of the thermophilization response to natural temperature gradients suggests that the lag in biological response to climate change may be restricting the response detected with experimental warming and monitoring approaches. This result is consistent with the expectation of delayed responses of vegetation composition to climate warming (21, 30), and suggests that predictions of vegetation migration with climate change based solely on the correspondence between current range and current temperature likely overestimate decadal scale vegetation change (31). In contrast to the results from decadal-scale studies, over millennia there seems to be broad congruence between forecasts of community composition based on space-for-time substitution and those based on repeat observations. Using fossil pollen records, Blois et al. (32) concluded that predictions of contemporary community composition based on space-for-time substitution were nearly as accurate as those based on time-series data; however, even Blois et al. found that the magnitude of community turnover associated with a particular environmental driver over space vs. time differed substantially. We also found important differences in the magnitude of change associated with a given increment of environmental change when contrasting space and temporal projections.
We hypothesize that differences in response magnitude could be related to lags in species responses to temperature, as well as to slower changes to biophysical conditions (e.g., soil development) with warming (21, 33). Comparing the estimated effects of 1 °C of warming after 10+ years with those seen over latitudinal gradients (compare Fig. 2 and Fig. 3) suggests that several decades of experimental warming could reproduce effects similar to those observed over latitudinal gradients; however, this interpretation requires extrapolating results from linear models outside the range of measurement, which could be an inappropriate interpretation of the longer-term trend. Linear approximation provided the best fit to the data within the date range contained in our study set, but it is implausible to expect warming effects to have increasing effects indefinitely. Maintenance and continued resampling of experimental plots will help clarify the changing magnitude of these longer-term effects over time.
Effects of elevational gradients within regions were in the same direction as broad-scale spatial effects, but much more variable. This variability could be a result of our specific dataset, which did not target broad elevational transects within regions. Instead, most sites within regions were in relatively close proximity for logistical reasons, and typically selected to cover a diversity of plant communities based on soil and microtopographic conditions. The variability among sites within a region, independent of elevation, likely introduced noise into the elevation effects seen here.
The magnitude of the estimated effect size was remarkably similar for the experimental warming and monitoring studies. Our results contradict the main conclusions of Wolkovich et al. (12), and indicate that warming experiments do not always underpredict climate change impacts. Differences between the present study and that of Wolkovich et al. (12) in both the focal response (in plant community composition and plant phenology, respectively) and geographic distribution and vegetation type (tundra only vs. global) might have contributed to the disparity in results. Our previous work also showed many similarities, along with some differences, in which growth forms increased or decreased in response to ambient (15) or experimental (34) air temperature warming. Here we used a condensed and comparable dataset to directly evaluate results on units of change in CTI (based on percent cover of vascular plants) per 1 °C, and demonstrate how analyses using a combination of experimental warming and observational studies can be complementary for a synthetic community-level property.
In summary, our results suggest that space-for-time approaches can be useful for gauging the direction, but not the magnitude, of climate change impacts occurring over decadal scales. With this understanding, our results support the application of any of the three techniques described in climate impact assessment studies, particularly when used to estimate the effect of climate change on a synthetic community property, such as the CTI. This intercomparability should facilitate the identification of hotspots of climate change vulnerability using a diverse array of impact assessment methodologies.
Materials and Methods
We estimated thermophilization based on changes in CTI value. The CTI of each plot was calculated at each sampling as the mean of each species’ thermal niche weighted by each species’ total cover. Plant cover was measured by repeated sampling of both historical and experimental warming studies in alpine, sub-Arctic, low Arctic, and high Arctic tundra plant communities (35). The relative thermal niche of each species was determined by overlaying occurrence data obtained from the Global Biodiversity Information Facility (GBIF; www.gbif.org) on a gridded estimate of the mean temperature of the warmest month using climate data from Worldclim (reference period 1950–2000; www.worldclim.org). Each species’ relative thermal niche was defined as the median value of the warmest monthly temperature over all GBIF occurrence records. We omitted sites located outside of North America or Europe, as well as all nonvascular taxa, because they are poorly represented in the GBIF.
To determine the temperatures of our study sites, we used the monthly data provided at 0.5° resolution based on the University of East Anglia Climate Research Unit (CRU; www.cru.uea.ac.uk) TS 3.21 dataset (36), with a lapse rate adjustment of −6°C/km of elevation to account for differences in elevation between the actual study sites and the gridded data (37). Lapse rates can vary based on slope exposure, wind, and moisture, but because we did not have site-specific information on these parameters, we used a common lapse rate for all sites. For each site in each year, we estimated the temperature of the warmest summer month. We used CRU data here because it provides monthly time series, and used Worldclim to calculate thermal niche (above) because it has a finer spatial resolution.
We conducted experimental warming using passive warming chambers, as part of the International Tundra Experiment (ITEX; www.geog.ubc.ca/itex/). To determine the magnitude of experimental warming, we calculated the mean daily temperature difference between warmed and control plots over all days with temperature logger data available between June 1 and August 31, and then calculated the mean warming effect over all years. This generated a single “warming effect” value per site. In studies where daily temperature values were not available, warming effects were derived from previous publications or metadata associated with archived site data.
Estimates of thermophilization in response to different sources of temperature variation were derived from two linear mixed models (38). To analyze the impact of changes in temperature over space and time on thermophilzation, we used plots with long-term repeated measurements that were not experimentally warmed. We used group means centering to separate the effects of temperature at different levels: level 1, annual/lagged annual temperature (in situ temperature effect); level 2, study within region (elevation effect); level 3, region effect (broad-scale geographic gradients). Specifically, the model contained fixed-effects terms for each of (i) the aggregate (mean) temperature of study sites per region, (ii) the difference in mean temperature (based on elevation) for each site within a region from the mean temperature of all sites sampled in that region, and (iii) the difference in temperature for each year within a site from the mean temperature over time. Study sites were defined by sampling layout, and generally included replicate survey plots within a single vegetation community type. Regions were defined by the CRU grid cell encompassing each study site.
To analyze the impact of experimental warming on thermophilzation, we used plots from the warming studies only. We used a linear mixed model that included fixed effects of duration of the warming experiment (years), treatment, interaction between duration and treatment, mean regional ambient temperature, and deviation of site temperature from mean regional temperature. We ran two variations of the experimental warming analyses, one analysis on a larger dataset in which treatment was coded as a factor (warmed vs. control) and a second analysis in which the warming effect was estimated based on the measured difference in temperature between warmed and control plots in a given study; the former is presented in SI Materials and Materials.
Random intercepts for plot (within site), site (within region), region, and year (within region) were included in both of the models. The models for the experimental data included an additional random effect for treatment within site to ensure that the warming effect entered the model at the level at which it was measured.
Supplementary Material
Acknowledgments
We thank Pat Webber for his vision and continued enthusiasm for the ITEX project, as well as Robert Bjork, Elisabeth Cooper, Diana Ebert-May, Felix Gugerli, David Johnson, Kari Klanderud, Julia Klein, Jeremy May, Joel Mercado, Marko Spasojevic, and Ørjan Totland, as well many researchers, postdoctoral and graduate students, and summer field technicians who contributed to plot maintenance and data collection. This work was made possible by the parks, wildlife refuges, research and field stations, and local and indigenous residents who provided access and support for research conducted on their land. Research funding was provided by the US National Science Foundation; US Fish and Wildlife Service; Icelandic Research Fund; Icelandic Centre for Research and Ministry of Agriculture; Research Council of Norway; Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning; International Polar Year Program of Canada; ArcticNet; Parks Canada; Northern Scientific Training Program; National Science and Engineering Research Council of Canada, US Forest Service, International Institute of Tropical Forestry, and University of Puerto Rico.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Plant abundance data from the unmanipulated and experimentally warmed plots have been deposited in the Polar Data Catalogue at www.polardata.ca (CCIN ref. no. 10786).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410088112/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 2.Diffenbaugh NS, Giorgi F. Climate change hotspots in the CMIP5 global climate model ensemble. Clim Change. 2012;114(3-4):813–822. doi: 10.1007/s10584-012-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pithan F, Mauritsen T. Arctic amplification dominated by temperature feedbacks in contemporary climate models. Nat Geosci. 2014;7:181–184. [Google Scholar]
- 4.Melillo JM, Richmond T, Yohe GW. Climate Change Impacts in the United States: The Third National Climate Assessment. US Global Change Research Program; Washington, DC: 2014. [Google Scholar]
- 5.Intergovernmental Panel on Climate Change . In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Field CB, et al., editors. Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 6.Intergovernmental Panel on Climate Change . In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Barros VR, et al., editors. Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 7.Hollister RD, Webber PJ. Biotic validation of small open-top chambers in a tundra ecosystem. Glob Change Biol. 2000;6:835–842. [Google Scholar]
- 8.Bokhorst S, et al. Microclimate impacts of passive warming methods in Antarctica: Implications for climate change studies. Polar Biol. 2011;34:1421–1435. [Google Scholar]
- 9.Kennedy AD. Simulated climate change: Are passive greenhouses a valid microcosm for testing the biological effects of environmental perturbations? Glob Change Biol. 1995;1:29–42. [Google Scholar]
- 10.Carlyle CN, Fraser LH, Turkington R. Tracking soil temperature and moisture in a multi-factor climate experiment in temperate grassland: Do climate manipulation methods produce their intended effects? Ecosystems (NY) 2011;14:489–502. [Google Scholar]
- 11.Fraser LH, et al. Coordinated distributed experiments: An emerging tool for testing global hypotheses in ecology and environmental science. Front Ecol Environ. 2012;11:147–155. [Google Scholar]
- 12.Wolkovich EM, et al. Warming experiments underpredict plant phenological responses to climate change. Nature. 2012;485(7399):494–497. doi: 10.1038/nature11014. [DOI] [PubMed] [Google Scholar]
- 13.Cook BI, et al. Sensitivity of spring phenology to warming across temporal and spatial climate gradients in two independent databases. Ecosystems (NY) 2012;15:1283–1294. [Google Scholar]
- 14.Hudson JMG, Henry GHR. Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology. 2009;90(10):2657–2663. doi: 10.1890/09-0102.1. [DOI] [PubMed] [Google Scholar]
- 15.Elmendorf SC, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Change. 2012;2:453–457. [Google Scholar]
- 16.La Sorte FA, Lee TM, Wilman H, Jetz W. Disparities between observed and predicted impacts of climate change on winter bird assemblages. Proc Biol Sci. 2009;276(1670):3167–3174. doi: 10.1098/rspb.2009.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukami T, Wardle DA. Long-term ecological dynamics: Reciprocal insights from natural and anthropogenic gradients. Proc Biol Sci. 2005;272(1577):2105–2115. doi: 10.1098/rspb.2005.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemoine N, Schaefer H-C, Böhning-Gaese K. Species richness of migratory birds is influenced by global climate change. Glob Ecol Biogeogr. 2007;16:55–64. [Google Scholar]
- 19.Pearson RG, et al. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat Clim Change. 2013;3:673–677. [Google Scholar]
- 20.Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc Natl Acad Sci USA. 2009;106(Suppl 2):19729–19736. doi: 10.1073/pnas.0901639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaver GR, et al. Global warming and terrestrial ecosystems: A conceptual framework for analysis. Bioscience. 2000;50:871. [Google Scholar]
- 22.Dunne JA, Saleska SR, Fischer ML, Harte J. Integrating experimental and gradient methods in ecological climate change research. Ecology. 2004;85:904–916. [Google Scholar]
- 23.Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nat Clim Change. 2012;2:111–115. [Google Scholar]
- 24.Devictor V, et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nat Clim Change. 2012;2:121–124. [Google Scholar]
- 25.De Frenne P, et al. Microclimate moderates plant responses to macroclimate warming. Proc Natl Acad Sci USA. 2013;110(46):18561–18565. doi: 10.1073/pnas.1311190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veloz SD, et al. No-analog climates and shifting realized niches during the late quaternary: Implications for 21st-century predictions by species distribution models. Glob Change Biol. 2012;18:1698–1713. [Google Scholar]
- 27.Ferrier S. Mapping spatial pattern in biodiversity for regional conservation planning: Where to from here? Syst Biol. 2002;51(2):331–363. doi: 10.1080/10635150252899806. [DOI] [PubMed] [Google Scholar]
- 28.Adler PB, Levine JM. Contrasting relationships between precipitation and species richness in space and time. Oikos. 2007;116:221–232. [Google Scholar]
- 29.Cleland EE, et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology. 2013;94(8):1687–1696. doi: 10.1890/12-1006.1. [DOI] [PubMed] [Google Scholar]
- 30.Epstein HE, Calef MP, Walker MD, Stuart Chapin F, Starfield AM. Detecting changes in arctic tundra plant communities in response to warming over decadal time scales. Glob Change Biol. 2004;10:1325–1334. [Google Scholar]
- 31.Dullinger S, et al. Post-glacial migration lag restricts range filling of plants in the European Alps. Glob Ecol Biogeogr. 2012;21:829–840. [Google Scholar]
- 32.Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc Natl Acad Sci USA. 2013;110(23):9374–9379. doi: 10.1073/pnas.1220228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodkinson ID, Coulson SJ, Webb NR. Community assembly along proglacial chronosequences in the high Arctic: Vegetation and soil development in northwest Svalbard. J Ecol. 2003;91:651–663. [Google Scholar]
- 34.Elmendorf SC, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol Lett. 2012;15(2):164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 35.Elmendorf S. Global tundra vegetation change: 30 years of plant abundance data from unmanipulated and experimentally warmed plots. CCIN ref. no. 10786. Available at www.polardata.ca. Accessed December 16, 2014.
- 36.University of East Anglia Climatic Research Unit 2013 CRU TS3.21: Climatic Research Unit (CRU) time series (TS) version 3.21 of high-resolution gridded data of month-by-month variation in climate (January 1901–December 2012). NCAS British Atmospheric Data Centre, 2013. Available at badc.nerc.ac.uk/view/badc.nerc.ac.uk. Accessed July 10, 2013.
- 37.Nagy L, Grabherr G. The Biology of Alpine Habitats. Oxford Univ Press; New York: 2009. [Google Scholar]
- 38.Bates D, Maechler M, Bolker B, Walker S. 2014 lme4: Linear mixed-effects models using Eigen and S4. Available at CRAN.R-project.org/package=lme4. Accessed September 12, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.