Significance
The work described here was motivated by our previous discovery of a connection between Rho GTPase activation and the up-regulation of mitochondrial glutaminase C (GAC), which is responsible for satisfying the glutamine addiction of cancer cells. This connection was originally established by our identification of a lead compound, 968, for a new class of inhibitors of oncogenic transformation. Although GAC was identified as the putative target for 968, how it regulated GAC was poorly understood. Here we provide important insights into the actions of 968, through the development of novel assays for its direct binding to GAC and its effects on enzyme activity. These findings offer exciting new strategies for interfering with the metabolic reprogramming critical for malignant transformation.
Keywords: glutaminase, glutaminolysis, FRET, benzophenanthridines, Rho GTPases
Abstract
The mitochondrial enzyme glutaminase C (GAC) catalyzes the hydrolysis of glutamine to glutamate plus ammonia, a key step in the metabolism of glutamine by cancer cells. Recently, we discovered a class of allosteric inhibitors of GAC that inhibit cancer cell growth without affecting their normal cellular counterparts, with the lead compound being the bromo-benzophenanthridinone 968. Here, we take advantage of mouse embryonic fibroblasts transformed by oncogenic Dbl, which hyperactivates Rho GTPases, together with 13C-labeled glutamine and stable-isotope tracing methods, to establish that 968 selectively blocks the enhancement in glutaminolysis necessary for satisfying the glutamine addiction of cancer cells. We then determine how 968 inhibits the catalytic activity of GAC. First, we developed a FRET assay to examine the effects of 968 on the ability of GAC to undergo the dimer-to-tetramer transition necessary for enzyme activation. We next demonstrate how the fluorescence of a reporter group attached to GAC provides a direct read-out of the binding of 968 and related compounds to the enzyme. By combining these fluorescence assays with newly developed GAC mutants trapped in either the monomeric or dimeric state, we show that 968 has the highest affinity for monomeric GAC and that the dose-dependent binding of 968 to GAC monomers directly matches its dose-dependent inhibition of enzyme activity and cellular transformation. Together, these findings highlight the requirement of tetramer formation as the mechanism of GAC activation and shed new light on how a distinct class of allosteric GAC inhibitors impacts the metabolic program of transformed cells.
Recently, the mitochondrial enzyme glutaminase (GLS1) has gained significant attention as a therapeutic target for cancer (1–3). GLS1 catalyzes the hydrolysis of glutamine to glutamate, which is used in the citric acid cycle (TCA) of cancer cells undergoing an aberrant glycolytic flux (i.e., the Warburg effect) as a non–glucose-derived source for anaplerosis. The elevation in glutamine metabolism exhibited by many cancer cells (“glutamine addiction”) is critical for sustaining their proliferative capacity, as well as for other aspects of their transformed phenotypes (4–9). Work from our laboratory has shown that a specific GLS1 splice variant, glutaminase C (GAC), plays an essential role in the transformation of NIH 3T3 fibroblasts by Rho GTPases, as well as in the proliferative and invasive activities of various cancer cells (10, 11). Thus, given the importance of GAC expression and activation for oncogenic transformation, the identification of inhibitors that target this metabolic enzyme offers new opportunities for the development of anticancer drugs.
Because glutamine is necessary for a range of biochemical reactions, including nucleotide and protein synthesis, glutamine analogs like the GLS1 inhibitor diazo-O-norleucine (DON) (12, 13) are not ideal candidates for cancer drugs (14). However, two classes of allosteric inhibitors of GAC have been identified and offer more promising options as lead compounds for the development of cancer therapeutics. One of these consists of analogs of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES), a reversible GAC inhibitor that has been extensively characterized (15, 16). X-ray crystal structures of the GAC–BPTES complex show that BPTES effectively traps GAC as an inactive tetramer (16–18).
A second, more recently identified, class of allosteric GAC inhibitors that is highly specific for inhibiting cancer cell growth while having little effect on normal (nontransformed) cells is represented by the benzophenanthridinone 968 (11). However, until now, very little was known regarding how this class of molecules functions. Here, we establish that the novel GAC inhibitor, 968, negatively impacts glutaminolysis in transformed cells, as well as determine how it influences GAC activity. First, we demonstrate through 13C-isotopic labeling studies that 968 inhibits the elevation in glutamine metabolism that accompanies Rho GTPase-dependent transformation. We go on to determine how 968 regulates GAC activity in vitro. By developing a FRET assay to read out the ability of GAC to undergo a dimer-to-tetramer transition necessary for enzyme activation (15, 19, 20), we show that, although the binding of BPTES induces a stable high-affinity GAC tetramer, 968 neither inhibits nor promotes tetramer formation. However, we discovered that the binding of 968 to the FRET donor-labeled GAC caused a significant dose-dependent quenching of the donor fluorescence, even in the absence of a FRET acceptor, which directly correlated with the ability of 968 to inhibit GAC activity. Using this novel 968-binding assay, in combination with recently developed GAC oligomerization-defective mutants, we show that 968 preferentially interacts with the monomeric form of GAC. Moreover, the dose–response for the binding of 968 to monomeric GAC matches that for its inhibition of oncogenic transformation. These findings provide important insights into the mechanism by which 968 blocks GAC activation and glutamine metabolism, as well as open the way toward developing novel therapeutics targeting glutamine-dependent cancer cells.
Results
The GAC Inhibitor 968 Blocks Glutaminolysis in Transformed Cells.
Previous work by our laboratory identified a potential connection between glutamine metabolism and Rho GTPase-dependent oncogenic transformation through the discovery of the small molecule inhibitor 968 (11). We showed that 968 specifically inhibited the growth of transformed cells and various cancer cells by blocking GAC activation, although the detailed mechanism was unclear. Here, we have set out to better understand how 968 functions by first examining its effects on glutamine metabolism in a well-defined model system for oncogenic transformation, in which the stable expression of the Dbl oncogene in mouse embryonic fibroblasts (MEFs) is controlled by the removal of doxycycline (Dox).
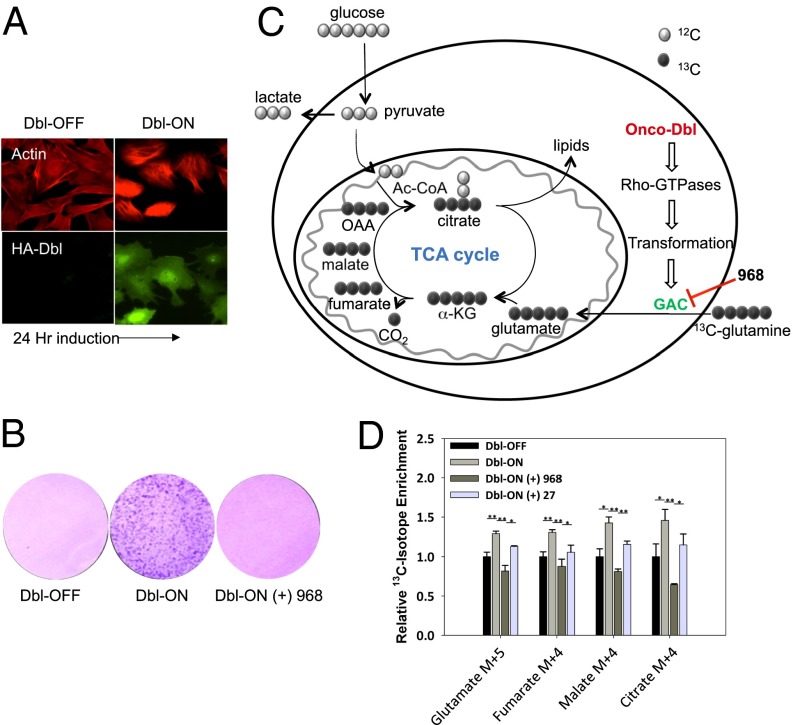
Induction of oncogenic Dbl in MEFs results in marked changes in cell morphology, as a result of cytoskeletal rearrangements caused by the activation of Rho GTPases (Fig. 1A) (10, 21–23). These morphological changes accompany the ability of oncogenic Dbl-expressing cells to overcome contact inhibition to form foci. Consistent with our previous results (11), treatment of MEFs expressing oncogenic Dbl with 968 blocked focus formation (Fig. 1B). We examined whether these effects were accompanied by an inhibition of glutaminolysis. The induction of oncogenic Dbl expression in MEFs increased glutaminolysis and glutamine-dependent anaplerosis, as monitored by 13C enrichment in TCA cycle intermediates derived from [U-13C]-glutamine (Fig. 1 C and D). Treatment of Dbl-expressing cells with 968 caused significant reductions in the glutamine-derived 13C isotopic enrichment within TCA cycle intermediates, but did not result in the depletion of relative pool sizes of each metabolite, with the exception of an almost twofold reduction in intracellular glutamate (Fig. S1A, histograms labeled glutamate, compare red and yellow vs. black, green, and blue). The reduction in intracellular glutamate along with the reduced 13C labeling would be an expected result if 968 were indeed inhibiting glutaminase activity. To further corroborate these observations, we also measured the absolute rates of secreted ammonia and glutamate (i.e., the products of the glutaminase reaction) in the growth medium and found that in fact 968 potently inhibited the accumulation of the direct products of glutaminolysis (Fig. S1B). A modest inhibition of glutaminolysis by 968 was also observed in MEFs not expressing Dbl (see Fig. S1C for the 13C enrichment diagram, and the M+5 histograms for Dbl-OFF, ±968, in Fig. S1D, and the M+4 histograms in Fig. S1 E–G). However, previous studies by Wang et al. (11) have reported no significant decrease in cell proliferation in nontransformed NIH 3T3 (Fig. 1 D and E) and human mammary epithelial cells (HMECs; Fig. 4 F and G), suggesting that glutamine metabolism is critical for supporting the transformed phenotypes accompanying oncogenic Dbl expression but not for the proliferative capability of normal MEFs. Treatment with the less potent 968-analog, compound 27, caused a weaker inhibition of the 13C enrichment of metabolites (Fig. 1D), consistent with its reduced potency to inhibit enzymatic activity (see below).
Fig. 1.
Dbl-induced transformation and increased glutaminolysis are inhibited by 968. (A) Fluorescent staining before (+ Dox) and after (− Dox) a 24-h induction of Dbl-inducible MEFs with anti-actin (red) and anti-HA (green) antibodies. (B) Expression of Dbl confers the ability of MEFs to form foci, which is blocked by treatment with 10 μM 968. (C) Diagram showing 13C enrichment from [U-13C]-glutamine into TCA cycle intermediates, where GAC activation downstream from Dbl is highlighted. 13C-carbons are shown as dark-filled circles and 12C-carbons as light-filled circles. (D) Glutamine-derived metabolites (glutamate M+5, fumarate M+4, malate M+4, citrate M+4) were normalized to 13C enrichment observed for MEFs not expressing Dbl. Comparisons were made between treatment with 968, its less potent analog 27 (see Fig. 3D for molecular structures), and untreated cells. Bars represent the mean (±SD) of triplicate determinations. P values were determined by the Student t test (*P < 0.05, **P < 0.005).
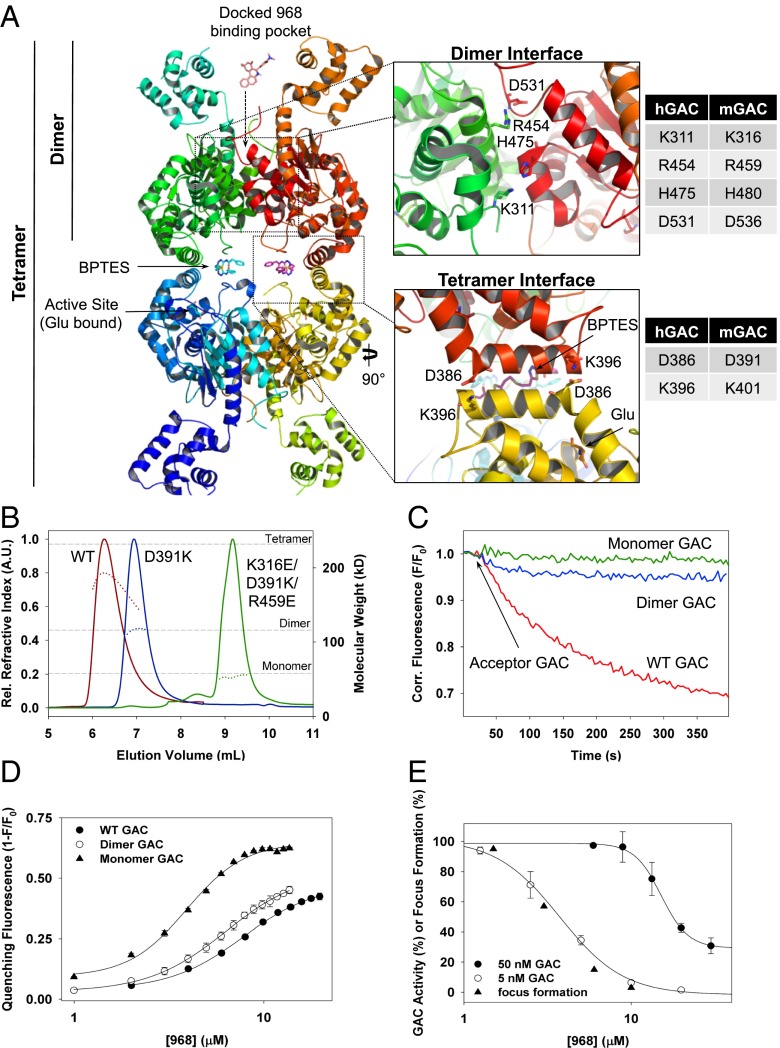
Fig. 4.
Examination of 968 binding to monomeric and dimeric GAC mutants. (A) Crystal structure of the GAC tetramer (human isoform) in complex with both BPTES and glutamate (PDB 3UO9), with the proposed 968-binding pocket indicated by the arrow pointing toward the C-terminal monomer-monomer interface. Insets highlight critical monomer-monomer (Upper) and dimer-dimer (Lower) contacts, with the corresponding human and mouse GAC isoform residue numbering. (B) Multiangle light scattering profiles of WT GAC (red), D391K-GAC (blue), and K316E-D391K-R459E-GAC (green), 250 μg (each), where the solid line represents the elution of each species by monitoring refractive index (R.I.), and the broken line designates the calculated molecular weight for the species eluted at that time. Reference lines for the molecular weights of the monomeric, dimeric, and tetrameric forms of the enzyme are included at 58, 116, and 232 kDa, respectively. (C) FRET assays on addition of 200 nM WT QSY9-labeled GAC (red), the dimeric QSY9-GAC (D391K) (blue), and monomeric QSY9-GAC (K316E, D391K, R459E) (green) to 20 nM WT 488-labeled GAC. (D) 968 binding monitored by its quenching of the fluorescence of WT 488-labeled GAC, dimeric 488-GAC (D391K), and the monomeric GAC (K316E, D391K, and R459E) (10 nM total monomer in each sample). Data points represent the mean (±SD) of three independent experiments and were fit as in Fig. 3C. (E) In vitro inhibition curves of 50 nM (●) and 5 nM WT GAC (○) preincubated with increasing concentrations of 968. Data points represent the mean (±SD) of three independent experiments and were fit to a logistic four parameter curve. Overlaid is the dose-dependent inhibition by 968 of Dbl-induced focus formation (triangles).
Oncogenic Dbl induction did not cause marked increases in glucose-fueled anaplerosis, as measured by 13C enrichment in citrate, when using [U-13C]-glucose as a tracer (see the M+2 histograms in Fig. S2A), demonstrating that a highly specific stimulation of glutamine metabolism accompanies Rho GTPase-dependent transformation. However, 968 was observed to inhibit glucose labeling of citrate isotopologues (see M+2 histogram in Fig. S2B). This presumably was due to the inhibition of glutamate flux by 968. Overall, these results show how 968 attenuates cellular glutamine metabolism and restores a normal growth phenotype in cells expressing oncogenic Dbl, thus highlighting the role of glutamine as a critical source for anaplerosis during cellular transformation.
Examining the Effects of 968 on the Dimer-to-Tetramer Transition of GAC.
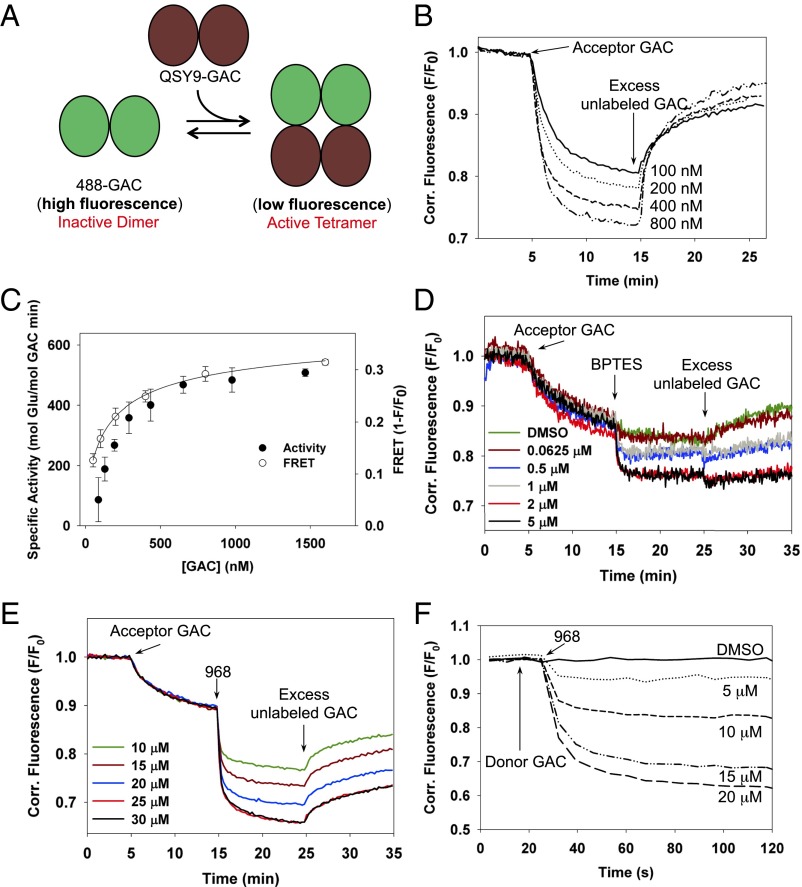
The transition of GAC from a dimer to a tetramer has been suggested to be essential for enzyme activity (15, 19, 20). A well-established allosteric inhibitor of GAC, BPTES, has been shown to stabilize an inactive tetrameric state of the enzyme (15). Thus, we examined whether 968 acted in a similar manner by developing a real-time read out for the GAC dimer-to-tetramer transition. Fig. 2A depicts the proposed FRET assay, where oligomer formation is monitored between two populations of purified recombinant GAC, labeled with either the highly fluorescent AlexaFluor 488 (donor) probe or with the nonfluorescent QSY9 (acceptor) chromophore. A major advantage of using FRET as a direct read out for GAC tetramer formation is the ability to monitor oligomer formation at the low concentrations of GAC commonly used for assaying its enzymatic activity.
Fig. 2.
Real-time fluorescence assay detecting GAC tetramer formation. (A) Schematic depiction of the FRET assay. (B) 25 nM 488-GAC (donor) fluorescence is quenched on addition of QSY9-GAC (acceptor) in a dose-dependent manner and reversed with the addition of a 10-fold excess of unlabeled GAC. (C) FRET resulting from the titration of 25 nM 488-GAC with increasing amounts of QSY9-GAC (○) overlaid with concentration-dependent in vitro activation of GAC (●). FRET data were fit to a quadratic binding isotherm. Points represent the mean ± SD of three independent experiments. (D) Increasing amounts of BPTES were added to 25 nM 488-GAC and 25 nM QSY9-GAC to examine the effects of the inhibitor on GAC tetramer formation. A 10-fold excess of unlabeled GAC was added to attempt to reverse tetramer formation. (E) 968 induces a dose-dependent quenching of 488-GAC fluorescence that is distinct from the quenching induced by the addition of QSY9-GAC. (F) Fluorescence quenching on addition of different concentrations of 968 to 10 nM 488-GAC in the absence of QSY9-GAC.
The addition of QSY9-GAC to 488-GAC yielded a dose-dependent quenching of the donor 488 emission due to FRET, which was reversible on the addition of unlabeled GAC, demonstrating that GAC tetramer formation is a dynamic process (Fig. 2B). The dose-dependent binding isotherm obtained from the QSY9-GAC titration profile directly correlated with the basal activation of GAC that occurs at increasing protein concentrations (i.e., due to tetramer formation through mass action), yielding an apparent KD of 164 nM (±20 nM) for tetramer formation (Fig. 2C), supporting the contention that the GAC tetramer is the minimal unit for enzymatic activity.
We then compared the effects of 968, vs. BPTES, on the GAC dimer-to-tetramer transition. Consistent with previous findings that BPTES stabilizes GAC as an inactive tetramer (16), we found that it caused an immediate quenching of 488-GAC fluorescence emission when added to a mixture of 488-GAC and QSY9-GAC (Fig. 2D), i.e., due to the ability of BPTES to promote the formation of 488-GAC:QSY9-GAC (donor:acceptor) tetramers. These stable GAC:BPTES tetrameric complexes were less susceptible to reversal by the addition of unlabeled GAC (Fig. 2D, compare the green trace for the addition of unlabeled GAC in the presence of the vehicle control DMSO vs. the black trace that represents the addition of unlabeled GAC in the presence of 5 μM BPTES). Interestingly, 968 elicited a markedly different response, causing a significant change in the fluorescence emission of 488-GAC, followed by a partial fluorescence recovery on the addition of excess unlabeled GAC (Fig. 2E). The recovery of 488 fluorescence when adding excess unlabeled GAC was due to the elimination of FRET between 488-GAC and QSY9-GAC, following the formation of mixed tetramers between 488-GAC or QSY9-GAC and unlabeled GAC. Thus, 968 does not appear to interfere with GAC tetramer formation. However, the inability to achieve a full recovery of the fluorescence emission suggested that 968 binding was directly affecting 488-GAC donor fluorescence emission. Indeed, we found that 968 caused a dose-dependent quenching of 488-GAC emission (in the absence of the FRET acceptor QSY9-GAC) that matched the 968-mediated inhibition of GAC activity (Fig. 2F and Fig. S3). Taken together, these findings show that 968 does not mimic the actions of BPTES by trapping GAC in an inactive tetrameric state but instead regulates GAC activity through a distinct allosteric mechanism.
Real-Time Monitoring of 968 Binding to GAC and Its Inhibition of Enzyme Activity.
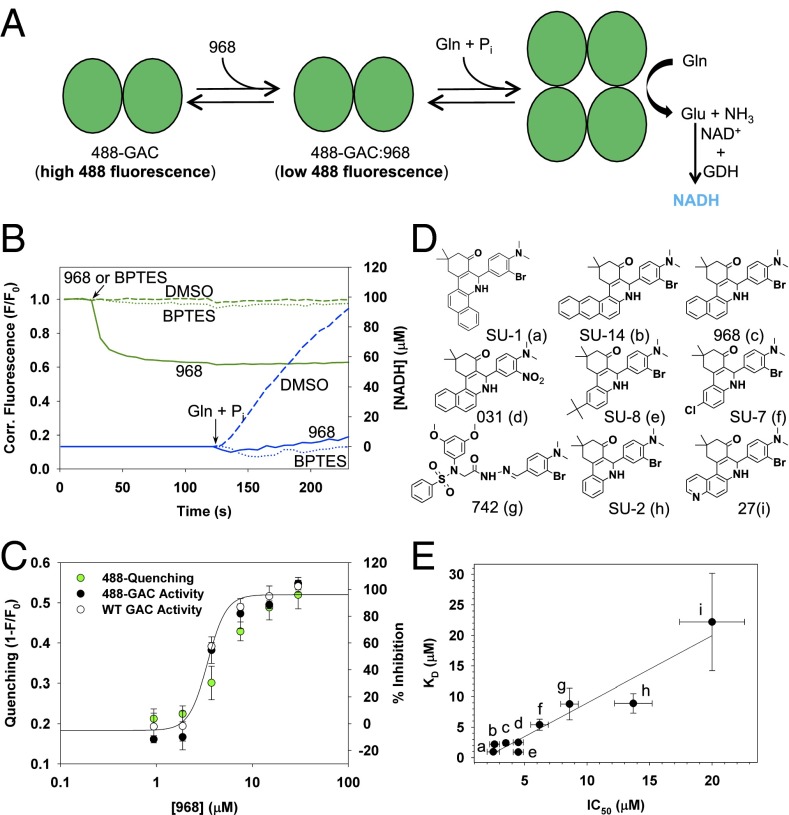
We developed a real-time enzyme activity assay to simultaneously monitor both the binding of 968 to GAC and its effects on enzyme activity. The enzymatic activity of 488-GAC is assayed by monitoring NADH production (i.e., fluorescence emission at 460 nm) that accompanies the conversion of glutamate (the product of the GAC-catalyzed reaction) to α-ketoglutarate, catalyzed by glutamate dehydrogenase. Fig. 3A depicts the coupling of these two fluorescence assays and Fig. 3B shows the results of an experiment simultaneously monitoring the direct binding of 968 to GAC (green solid line) and its inhibition of enzyme activity (blue solid line; the blue dashed line represents the control enzyme activity treated with the solvent vehicle DMSO). Unlike 968, BPTES does not directly affect 488-GAC fluorescence, under conditions where it strongly inhibits GAC activity (Fig. 3B, see the green and blue dotted traces, respectively).
Fig. 3.
Development of real-time 968 binding and inhibition assays. (A) Schematic model of real-time 968 binding and inhibition assays. Monitoring 488-GAC fluorescence quenching serves as a read-out for 968 binding, and enzymatic activity is monitored through the generation of NADH fluorescence on addition of 20 mM glutamine and 50 mM phosphate to an assay incubation containing labeled GAC together with 10 units of glutamate dehydrogenase (GDH) and 2 mM NAD+. (B) Fluorescence of 10 nM 488-GAC (520-nm emission, green curves) was monitored on addition of 20 μM 968 (–), 10 μM BPTES (•••), or DMSO (—) at the indicated time. Simultaneously, NADH fluorescence (460-nm emission, blue curves) was monitored following the addition of 20 mM glutamine and 50 mM phosphate at 120 s. (C) Real-time 968 binding and inhibition assays adapted to a 96-well plate format show overlapping inhibition and fluorescence quenching profiles for 10 nM 488-GAC and 10 nM WT unlabeled GAC. Data points are the average ± SD of three independent experiments. The solid line shows the semilog plot of the binding isotherm with KD = 3 μM. (D) Structures of 968 and 968-like analogs used in real-time binding and inhibition assays. (E) Plotted IC50 (±SD) values from inhibition data and measured KD (±SD) values from fluorescence quenching data for a representative group of 968 analogs (depicted in D). The compounds a–i correspond to the letter designations shown in D. Values obtained from inhibition data and quenching data were fit to a ligand binding equation for a biomolecular interaction. The line represents a linear regression fit with the following values (R2 = 0.92, slope = 1.10).
We then adapted these assays to a 96-well plate format and showed that 968 exhibited an overlapping dose-dependent inhibition of both 488-GAC and unlabeled GAC activity (Fig. 3C, black closed and open circles, respectively), as well as an overlapping dose–response for its direct binding to 488-GAC (Fig. 3C, green closed circles). We tested the robustness of these high-throughput binding and enzymatic assays by examining a group of newly synthesized 968 derivatives (compounds SU-1, SU-2, SU-7, SU-8, and SU-14 in Fig. 3D), together with molecules 031, 27, and 742 that were previously characterized and shown to be GAC inhibitors (11, 24). A direct correlation exists between the ability of different 968 analogs to bind to GAC and to inhibit its enzymatic activity (Fig. 3E). The results of these analyses, and particularly the finding that substituting the napthyl group of 968 with a quinoline moiety (e.g., compound 27) markedly affected both binding and inhibitory activity, suggests that hydrophobicity at this position is required for maximal efficacy.
Previous studies of the 968-mediated inhibition of recombinant GAC activity showed that 968 was much more effective when it was added before glutamine and inorganic phosphate (the latter being an allosteric activator that stimulates GAC tetramer formation and GAC activity) compared with when it was added after the addition of phosphate (24). Therefore, we examined whether the ability of 968 to bind to GAC was compromised under conditions where the enzyme was pretreated with inorganic phosphate and assumed an activated tetrameric state. In fact, we found that 968 was capable of binding to a tetrameric GAC species comprised of 488-labeled GAC and QSY9-labeled GAC dimers, as read out by the quenching of 488 fluorescence emission (Fig. S4 A and B). Moreover, 968 was able to bind to GAC that had been preincubated with inorganic phosphate (Fig. S4C, closed vs. open circles). However, under these conditions, 968 was much less effective at inhibiting enzyme activity (Fig. S4D, closed vs. open circles). Thus, phosphate induces an activated state that is less sensitive to 968 inhibition, even though 968 is able to bind to phosphate-activated GAC. In contrast, when GAC was preincubated with 968 before adding phosphate, the enzyme activity was strongly inhibited and directly correlated with the binding of 968 (Fig. S4 C and D, open circles).
968 Preferentially Binds to the Monomeric State of GAC.
Docking analyses using the X-ray structure of the GAC tetramer, together with mutagenesis studies, suggested that 968 binds in a cove between the monomer-monomer interface (24). To examine the ability of 968 to bind to different oligomeric states of GAC, we took advantage of recently solved X-ray structures of GAC (16) to design mutants trapped as either monomers or dimers. Fig. 4A depicts the BPTES-binding sites within the GAC tetramer interface and the proposed 968-binding pocket at the C-terminal region of the monomer-monomer interface. Residue contacts that were mutated to create constitutive monomeric and dimeric GAC mutants are highlighted at the GAC-tetramer helical interface (Lower Inset), as well as at the GAC dimer interface (Upper Inset). When a point mutation was incorporated at the tetramer interface of mouse GAC (D391K), tetramer formation was disrupted with the resulting GAC mutant being trapped in a dimeric state, as determined by multiangle light scattering (MALS) (Fig. 4B, blue trace). Introducing point mutations at the dimer interface of mouse GAC (K316E and R459E), within the background of the dimeric GAC mutant, resulted in a monomeric GAC (D391K, K316E, and R459E) species (Fig. 4B, green trace). As expected, the monomeric and dimeric GAC mutants showed neither a concentration-dependent basal enzymatic activity nor phosphate-stimulated activity (Fig. S5A and B). Although the addition of WT QSY9-labeled GAC to WT 488-labeled GAC resulted in the expected FRET due to tetramer formation (Fig. 4C, red trace), the addition of either the QSY9-labeled GAC (D391K) dimer or the GAC (D391K, K316E, and R459E) monomer to WT 488-labeled GAC failed to result in a significant quenching of the 488 donor fluorescence (Fig. 4C, blue and green traces, respectively). The addition of the dimeric QSY9-GAC (D391K) to WT 488-GAC did induce a minor quenching of the 488-GAC emission; however, this was most likely due to the formation of mixed donor and acceptor labeled dimers, which result from a relatively minor exchange of the monomeric GAC units.
We found that 968 was capable of binding to WT 488-GAC, as well as to both the dimeric GAC (D391K) and the monomeric GAC (D391K, K316E, and R459E), with the monomeric GAC having the highest affinity for 968 (Fig. 4D). These results suggested that 968 should be most effective at inhibiting WT GAC at relatively low enzyme concentrations, i.e., where equilibrium conditions favor GAC initially existing as a monomer. Fig. 4E shows that when the concentration of GAC was decreased from 50 to 5 nM, 968 was able to inhibit GAC activity with greater potency. Furthermore, the 968-mediated inhibition of GAC activity at these low enzyme concentrations correlated well with its inhibition of oncogenic transformation (Fig. 4E).
Discussion
Previous work from our laboratory aimed at identifying inhibitors that specifically block Rho GTPase-dependent transformation led to the discovery of the benzophenanthridinone 968 (11). Unexpectedly, the protein target for 968 appeared to be a specific splice variant (GAC) of a family of enzymes collectively called glutaminase that catalyzes the hydrolysis of glutamine to glutamate with the production of ammonia. This highlighted a previously unappreciated connection between the roles of Rho GTPases in driving oncogenic transformation and the regulation of glutamine metabolism. Given the striking specificity that 968 exhibited in its ability to inhibit transformed cells and cancer cells, with little or no effect on their normal cellular counterparts, it was of interest to better understand how 968 functions.
We took advantage of an inducible expression system for oncogenic Dbl that allowed us to temporally control the expression of this upstream activator of Rho GTPases in a well-defined manner. Using this system, we were able to establish a direct correlation between the ability of 968 to prevent a key outcome of Dbl-induced transformation, namely focus formation, and to specifically inhibit glutaminolysis. Thus, the inhibitory actions of 968 on oncogenic transformation appear to be a direct outcome of its ability to interfere with glutamine metabolism.
We then set out to understand how 968 inhibits the activity of a key enzyme in glutamine metabolism, GAC. Because BPTES, a well characterized allosteric inhibitor of GAC, has been shown to bind and stabilize an inactive tetrameric form of the enzyme, one possibility was that 968 had a similar effect. Previous studies used analytical ultracentrifugation, gel filtration, and electron microscopy to investigate the oligomeric transitions of GAC; however, these analyses were performed at GAC concentrations above the KD for tetramer formation reported here (15, 19, 20, 25, 26). Thus, we made use of a real-time FRET assay for monitoring GAC tetramer formation. The highly sensitive FRET assay enabled us to directly monitor GAC tetramer formation and show that it correlates with enzyme activation, as well as to compare the effects of 968 and BPTES on the dimer-to-tetramer transition. We found that unlike BPTES, 968 does not stabilize an inactive tetrameric state of GAC. However, during the course of these FRET experiments, we discovered that the binding of 968 to GAC resulted in a quenching of the reporter group fluorescence, thus providing us with a direct spectroscopic read out for the ability of this inhibitor and various analogs to bind to the enzyme.
By taking advantage of a direct binding assay for 968, together with the recent development of GAC mutants that exist as monomers or dimers, we discovered that 968 has a marked preference for binding to the monomeric form of the enzyme. Although 968 is able to bind, albeit more weakly, to a GAC dimer, as well as to a GAC tetramer that has been activated by the allosteric regulator inorganic phosphate, it is unable to inhibit the activity of the activated enzyme tetramer. Therefore, 968 preferentially binds to an inactive, monomeric state of GAC and prevents it from undergoing activating conformational changes, whereas, if GAC reaches an activated state before 968 binding, then 968 is unable to inhibit enzyme activity.
These findings highlight the distinction between the two classes of allosteric GAC inhibitors for which BPTES and 968 are the prototypes. BPTES is able to bind and inhibit activated GAC, whereas 968 binds preferentially to and stabilizes an inactive state of the enzyme. In addition, these results shed light on the reason for previous discrepancies when comparing the 968 dose dependencies for the inhibition of recombinant GAC activity vs. oncogenic transformation (24). Specifically, in those earlier experiments, the concentrations of recombinant GAC routinely being assayed represented a mixture of dimers and tetramers. Consequently, the IC50 values for 968 reflected its weaker binding to these oligomeric GAC species. Indeed, when the binding of 968 to GAC, together with its ability to inhibit enzyme activity, is assayed at GAC concentrations where it initially exists predominantly as a monomer, the dose–response profiles for these binding assays match the dose-dependent inhibition of transformation in cell culture.
In conclusion, we show that 968 functions as a highly specific inhibitor of oncogenic transformation by blocking a key step in glutamine metabolism necessary for sustaining the transformed state. We demonstrate that 968 is capable of directly binding to GAC, a key enzyme responsible for elevated glutamine metabolism in transformed cells and cancer cells, and that 968 preferentially binds to a monomeric, inactive state of the enzyme. Although an X-ray crystal structure of 968 bound to GAC has not yet been achieved, these findings provide rationale as to why this has been so challenging, given that crystallization trials have been routinely performed at GAC concentrations where it exists as a tetramer, i.e., the least favorable species for binding 968 (13, 16–18). Our ability to generate monomeric GAC mutants now provides new opportunities for achieving such a structure. Moreover, the availability of a direct binding read out adapted for plate reader assays offers exciting possibilities for the identification of 968-like allosteric inhibitors that could yield new therapeutic strategies against cancer.
Methods
Recombinant GAC, prepared as described in ref. 11, was labeled with 50 μM of the fluorescent probes and resolved from unreacted probe using a PD10 desalting column. Additional details of experimental methods, including cell culture, transformation assays, metabolic tracing experiments, GAC preparation, fluorescence labeling and analyses, enzymatic activity and FRET assays, and synthesis of 968 analogs, are presented in SI Methods.
Supplementary Material
Acknowledgments
We thank Cindy Westmiller for excellent secretarial assistance. This study was supported by grants from the National Institutes of Health (to R.A.C.) and Award T32GM008500 from the National Institute of General Medical Sciences (to C.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414056112/-/DCSupplemental.
References
- 1.DeBerardinis RJ, Cheng T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng T, et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci USA. 2011;108(21):8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le A, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15(1):110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seltzer MJ, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Heuvel AP, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13(12):1185–1194. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem. 1999;274(33):23633–23641. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- 11.Wang JB, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18(3):207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro RA, Clark VM, Curthoys NP. Inactivation of rat renal phosphate-dependent glutaminase with 6-diazo-5-oxo-L-norleucine. Evidence for interaction at the glutamine binding site. J Biol Chem. 1979;254(8):2835–2838. [PubMed] [Google Scholar]
- 13.Brown G, et al. Functional and structural characterization of four glutaminases from Escherichia coli and Bacillus subtilis. Biochemistry. 2008;47(21):5724–5735. doi: 10.1021/bi800097h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46(2):243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 15.Robinson MM, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem J. 2007;406(3):407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLaBarre B, et al. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry. 2011;50(50):10764–10770. doi: 10.1021/bi201613d. [DOI] [PubMed] [Google Scholar]
- 17.Thangavelu K, et al. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci USA. 2012;109(20):7705–7710. doi: 10.1073/pnas.1116573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassago A, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA. 2012;109(4):1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfrey S, Kuhlenschmidt T, Curthoys P. Correlation between activation and dimer formation of rat renal phosphate-dependent glutaminase. J Biol Chem. 1977;252(6):1927–1931. [PubMed] [Google Scholar]
- 20.Kenny J, et al. Bacterial expression, purification, and characterization of rat kidney-type mitochondrial glutaminase. Protein Expr Purif. 2003;31(1):140–148. doi: 10.1016/s1046-5928(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 21.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 23.Olivo C, et al. Distinct involvement of cdc42 and RhoA GTPases in actin organization and cell shape in untransformed and Dbl oncogene transformed NIH3T3 cells. Oncogene. 2000;19(11):1428–1436. doi: 10.1038/sj.onc.1203440. [DOI] [PubMed] [Google Scholar]
- 24.Katt WP, Ramachandran S, Erickson JW, Cerione RA. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Mol Cancer Ther. 2012;11(6):1269–1278. doi: 10.1158/1535-7163.MCT-11-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira AP, et al. Active glutaminase C self-assembles into a supratetrameric oligomer that can be disrupted by an allosteric inhibitor. J Biol Chem. 2013;288(39):28009–28020. doi: 10.1074/jbc.M113.501346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Møller M, et al. Small angle X-ray scattering studies of mitochondrial glutaminase C reveal extended flexible regions, and link oligomeric state with enzyme activity. PLoS ONE. 2013;8(9):e74783. doi: 10.1371/journal.pone.0074783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.