Significance
Effector CD8+ T-cell differentiation is essential for protective immunity. Investigating specific genes in this process by knockdown with RNAi is challenging because naive T cells are refractory to viral transduction. To overcome this obstacle, we developed a novel strategy to knock down genes in naive CD8+ T cells by creating bone marrow chimera from hematopoietic progenitors transduced with an inducible shRNA. This approach enabled inducible in vivo gene knockdown in any cell type developed from this progenitor pool. We applied this strategy to show that the transcription factor BATF is essential for initial commitment to effector differentiation but becomes dispensable by 72 h. This approach now enables the study of gene function in vivo in cells of hematopoietic origin otherwise refractory to viral transduction.
Keywords: CD8 T cell, RNAi, transcription factor, BATF
Abstract
The differentiation of effector CD8+ T cells is critical for the development of protective responses to pathogens and for effective vaccines. In the first few hours after activation, naive CD8+ T cells initiate a transcriptional program that leads to the formation of effector and memory T cells, but the regulation of this process is poorly understood. Investigating the role of specific transcription factors (TFs) in determining CD8+ effector T-cell fate by gene knockdown with RNAi is challenging because naive T cells are refractory to transduction with viral vectors without extensive ex vivo stimulation, which obscures the earliest events in effector differentiation. To overcome this obstacle, we developed a novel strategy to test the function of genes in naive CD8+ T cells in vivo by creating bone marrow chimera from hematopoietic progenitors transduced with an inducible shRNA construct. Following hematopoietic reconstitution, this approach allowed inducible in vivo gene knockdown in any cell type that developed from this transduced progenitor pool. We demonstrated that lentivirus-transduced progenitor cells could reconstitute normal hematopoiesis and develop into naive CD8+ T cells that were indistinguishable from wild-type naive T cells. This experimental system enabled induction of efficient gene knockdown in vivo without subsequent manipulation. We applied this strategy to show that the TF BATF is essential for initial commitment of naive CD8+ T cells to effector development but becomes dispensable by 72h. This approach makes possible the study of gene function in vivo in unperturbed cells of hematopoietic origin that are refractory to viral transduction.
Following activation by antigen, costimulation, and inflammation, naive CD8+ T cells initiate a differentiation program resulting in massive changes in gene expression and cell function, which leads to the formation of effector and memory T cells (1). This differentiation program is critical for the development of effective tumor immunity (2) and the control of pathogens (3). Although the development of effector CD8+ T cells occurs over a period of days (4), early events in the life history of CD8+ T cells are critical in determining their fate (5–10), suggesting that investigating the events that occur in the hours following initial antigen encounter will be essential for defining the mechanisms that regulate the fate of effector CD8+ T cells.
The AP-1 family transcription factor (TF) BATF is absolutely required for effector CD8+ T-cell differentiation and coordinates the program of gene expression essential for this process (11). The role of specific TFs in regulating CD8+ T-cell effector differentiation has generally been investigated using germ-line or conditional KOs. However, these approaches are restricted to studying a small number of candidate genes (12). In contrast, perturbing genes with RNAi could permit the study of many more candidate regulators in parallel (13), but techniques to deliver shRNAs to T cells are limited by the need to stimulate cells to divide using T-cell receptor (TCR) cross-linking (14), infection (15, 16), or cytokine stimulation (17) to achieve meaningful transduction frequencies with viral vectors encoding shRNA constructs. The need to activate T cells for delivery of shRNAs raises concerns about whether this activation alters these T cells at a critical phase of time when even subtle perturbations of TFs can profoundly influence T-cell fate (10).
To address these limitations, we have developed an experimental system to knock down gene expression in T cells in vivo using shRNA without the need to transduce T cells directly. We generated bone marrow (BM) chimera from hematopoietic stem cells (HSCs) transduced with an inducible shRNA vector. Following hematopoietic reconstitution, this strategy allows inducible gene knockdown in any cell type that developed from this transduced progenitor pool, including resting naive CD8+ T cells in vivo. We have applied this system to show that BATF is essential for initial commitment of naive CD8+ T cells to effector cell development, but becomes dispensable after 72 h.
Results
Lentivirus-Transduced Stem Cells Reconstitute Blood Immune Lineages and Give Rise to Effector CD8+ T Cells with Unaltered Functionality.
Resting T cells are refractory to lentiviral transduction, but HSCs are more readily transduced. We therefore generated bone marrow chimeric animals using lentivirus-transduced hematopoietic progenitor cells in which hematopoietic lineages (including T cells) are reconstituted with transduced cells (Fig. 1A). We isolated lineage–/sca-1+/c-kit+ (LSK) cells (which include HSCs and multipotent progenitors), from the bone marrow of P14 TCR transgenic (Tg) mice in which most CD8+ T cells express a TCR specific for lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP)33–41 peptide presented on H-2Db (Fig. 1A), and transduced them with a lentivirus carrying a GFP expression cassette so that the fate of transduced cells could be tracked. We used congenic markers to distinguish transplanted cells from recipient cells in bone marrow chimeras.
Fig. 1.

Transduced bone marrow progenitor populations efficiently reconstitute myeloid and lymphoid compartments and develop normally into functional CD8+ T cells. (A) Schematic diagram of transduction strategy. (B) Fraction of LSK cells (Left) transduced with GFP-expressing lentivirus at the time of transplant, and in lymphoid (Middle) and myeloid (Right) cell populations following engraftment. (C) Quantitation of fractions of transduced (GFP+) and untransduced (GFP–), donor-derived T cells following engraftment as in B. (D) Fraction of transduced (GFP+) and untransduced (GFP–), donor-derived naive P14 CD8+ T cells (Left) before adoptive transfer, and of effector (Right) P14 CD8+ T cells in tissues indicated 10 d following transfer and subsequent host infection with PR8-GP33 influenza virus. Representative data are shown from two to three experiments with four to five mice per group.
To test whether lentivirus-transduced LSK cells could be used to generate fully functional CD8+ T cells, we first transduced the LSK cells with lentivirus encoding only GFP under a human phosphoglycerate kinase (PGK) promoter (PGK-eGFP) and transplanted them into lethally irradiated animals (50,000 cells per animal). Following reconstitution (8–12 wk later), analyses of major lineages in the immune system showed that the frequencies of GFP+ B cells (B220+), CD4+ and CD8+ T cells, dendritic cells (CD11c+), and monocytes (CD11b+) were similar to that of the LSK inoculum (Fig. 1B), suggesting efficient engraftment of transduced cells. CD4+ and CD8+ T, total T, B, and myeloid lineages developed from transplanted GFP+ (transduced) and GFP– (untransduced) LSK with equal efficiency (Fig. 1C and Fig. S1). We next compared effector CD8+ T-cell differentiation of naive CD8+ T cells derived from transplanted PGK-eGFP transduced (GFP+) LSK cells with differentiation of naive CD8+ T cells derived from transplanted but untransduced (GFP–) LSK cells. We transferred equal ratios of GFP+ and GFP– naive P14 CD8+ T cells to naive wild-type recipients (10,000 cells per animal) and infected them with H1N1 influenza PR8 engineered to express GP33 (PR8-GP33) (Fig. 1D). We found equal expansion and persistence of GFP+ and GFP– effector CD8+ T cells at 10 d postinfection (p.i.).
We next compared the phenotype and function of effector CD8+ T cells arising from naive CD8+ T cells that developed from transduced LSKs with effector CD8+ T cells differentiating from untransduced naive CD8+ T cells. We analyzed the proliferative capacity, expression of cell surface molecules, key transcription factors, and production of cytokines upon restimulation and found no difference between untransduced and transduced effector CD8+ T cells at d 8 postinfection (p.i.) (Fig. S2). Thus, lentiviral transduction of LSK neither impairs the development of lymphoid and myeloid lineages following transplantation nor alters effector CD8+ T-cell generation, proliferative capacity, or survival following transfer of naive CD8+ T cells.
To compare the persistence and phenotype of effector CD8+ T cells derived from untransduced naive CD8+ T cells, or naive CD8+ T cells generated from bone marrow chimeras, we transferred either unmodified naive P14 CD8+ T cells or naive P14 CD8+ T cells carrying the 1xLacO–shLacZ construct into congenically distinct LCMV-infected wild-type recipients. We analyzed the fraction of transferred cells at d28 p.i. and observed no difference in the frequency of unmodified CD8+ T cells and those carrying the 1xLacO–shLacZ construct (Fig. S3 A and B). Additionally, 1xLacO and WT P14 memory cells were indistinguishable in their expression of cell surface molecules and production of cytokines upon restimulation (Fig. S3 C and D). This finding suggests that the persistence of CD8+ T cells following effector differentiation is not altered by the presence of the lentiviral vector.
Naive T Cells That Develop from Transduced LSK Are Indistinguishable from Wild-Type Naive T Cells.
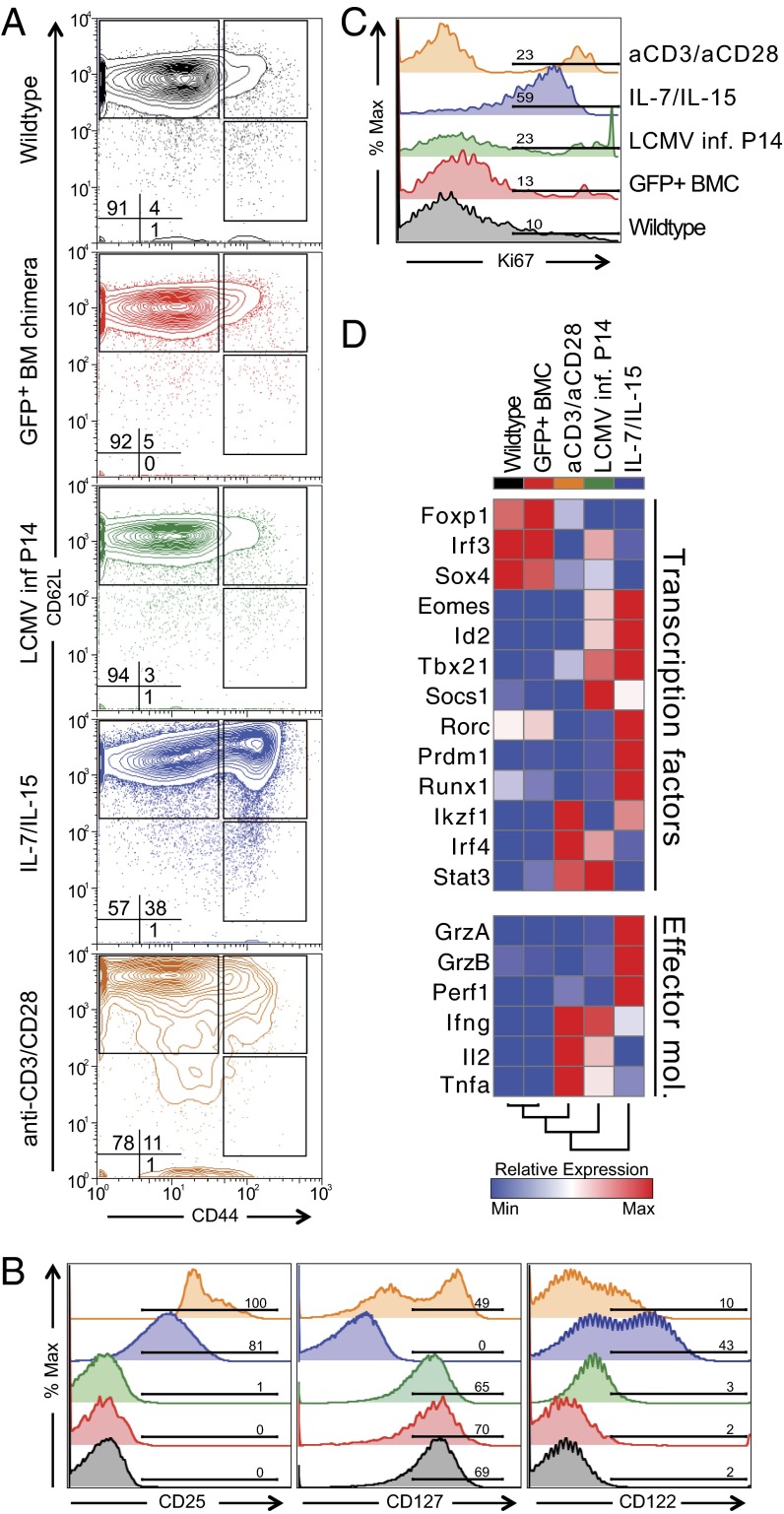
We next examined whether naive CD8+ T cells that developed from transduced LSK cells show any alterations of surface phenotype, proliferative status, or gene expression that might obscure analysis of early differentiation events. We compared naive CD8+ T cells that were derived from transduced LSK cells with control wild-type P14 CD8+ T cells. We also studied P14 CD8+ T cells cultured in conditions used in previous studies to facilitate direct viral transduction of T cells (14–18) to compare CD8+ T cells generated by our approach and previously reported methods: (i) activation of CD8+ T cells in vivo by infecting P14 mice with 2 × 105 p.f.u. LCMV Armstrong, (ii) activation in vitro by stimulation with anti-CD3 and anti-CD28, or (iii) incubation in vitro with a combination of IL-7 and IL-15 cytokines.
The proportions of naive (CD62L+ CD44–), central memory (CD62L+ CD44+), and effector memory (CD62L– CD44+) cells were similar in the GFP+ naive CD8+ T cells from the transduced BM chimeras and in naive CD8+ T cells from wild-type mice, but were markedly altered by the other stimulation conditions, particularly with cytokine treatment (Fig. 2A). The expression of cytokine receptors including CD25, CD127, and CD122 was not different in GFP+ naive and wild-type naive CD8+ T cells, but was altered in naive CD8+ T cells treated with anti-CD3/CD28 or cytokines (Fig. 2B). The GFP+ naive CD8+ T cells also showed a low rate of homeostatic turnover that was similar to wild-type naive CD8+ T cells (Fig. 2C). In contrast, all of the other stimulation conditions induced varying degrees of cell proliferation (Fig. 2C).
Fig. 2.
CD8+ T cells derived from transduced LSK cells are indistinguishable from untransduced naive CD8+ T cells. Expression of (A) naive surface markers, (B) cytokine receptors, and (C) Ki-67 by wild-type P14 CD8+ T cells (black); P14 CD8+ T cells derived from transduced LSKs (red); P14 CD8+ T cells stimulated by LCMV infection (green); by the cytokines IL-7 and IL-15 (blue); or by anti-CD3 and anti-CD28 antibodies (orange). (D) Relative transcript abundance of transcriptional regulators and effector molecules changes measured by quantitative RT-PCR in examined groups.
We measured transcript abundance for TFs and effector molecules that change during CD8+ effector T-cell differentiation. Important regulators of effector differentiation such as T-bet (Tbx21), Eomesodermin (Eomes), and Blimp1 (Prdm1), as well as effector molecules including granzyme A and B, perforin1, and IFNγ and TNFα were unchanged in GFP+ naive CD8+ T cells relative to wild-type naive CD8+ T cells, but were up-regulated in the other stimulation conditions (Fig. 2D). Thus, GFP+ naive CD8+ T cells that had developed from transduced LSK cells were indistinguishable from untransduced naive CD8+ T cells. In contrast, existing protocols used to achieve viral transduction of naive CD8+ T cells were associated with marked perturbation of the T-cell state.
Lac Operon-Regulated shRNA Allows Inducible, Efficient, and Transient Gene Knockdown in Vivo at Low Concentrations of Isopropyl β-d-1-Thiogalactopyranoside.
Because constitutive gene knockdown in LSK could compromise the development of immune lineages, we used an inducible shRNA expression vector that uses the Lac operon system to regulate the shRNA promoter following addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) (Fig. S4A). We confirmed the inducibility of gene knockdown by targeting a control gene in a Jurkat cell line. Target gene (GFP) expression was only minimally affected in the uninduced state (Fig. 3A). However, gene knockdown following IPTG induction of shRNA expression was as efficient as that achieved by a constitutive shRNA expressing vector (Fig. 3A) even at low concentrations of IPTG (Fig. S4B).
Fig. 3.
Novel shRNA vector enables efficient, inducible, and transient gene knockdown in vitro and in vivo. (A) Fraction of GFP-expressing Jurkat cells transduced with lentivirus expressing an shRNA targeting GFP under constitutive (white symbols) or inducible (black) promoters, cultured with 100 μM IPTG (gray box) for times indicated. BATF transcript levels (B) in anti-CD3/CD28-stimulated shBATF-naive CD8+ T cells cultured in vitro with (dark gray or black bars) or without (light gray) IPTG starting at the day indicated. Cells were continuously exposed to IPTG by in vivo exposure in bone marrow chimeric mice 3 d before T-cell sort (d −3) or 1 d following activation (d +1) and for the remainder of the experiment. (C) BATF protein abundance in anti-CD3/CD28-stimulated wild-type and shBATF–naive CD8+ T cells exposed to IPTG d −3 or incubated in medium alone. Numbers represent BATF densiometry values normalized to β-actin in shBATF relative to the wild-type cells. (D) Cells treated as in B were transferred into recipient mice that were also infected with LCMV and IPTG exposure was maintained by treating mice with 20 mM IPTG in drinking water starting 3 d prior to transfer (in bone marrow chimeras) or 1 d following transfer until 3 d following transfer. Batf mRNA level was normalized to Hprt and 2-ΔCt values reported. Significance was assessed with one-way ANOVA; *P < 0.05, ***P < 0.001, ****P < 0.0001. Representative data are shown from two experiments.
To test knockdown efficiency in primary CD8+ T cells, we generated bone marrow chimeras with an IPTG-inducible vector encoding an shRNA targeting BATF (shBATF) and a GFP expression cassette to create GFP+ naive T cells that carried the inducible shRNA vector (hereafter “shBATF–naive T cells”). We first tested inducible knockdown in vitro by stimulating the cells with anti-CD3/CD28 and assessing the Batf transcript levels 3 d following activation. IPTG was administered to the bone marrow chimeras 3 d before activation (d −3) or 1 d following activation (d +1). Decreased target gene expression was apparent in both transcript and protein abundance as early as 2 d following IPTG addition in vitro (Fig. 3 B and C) and was comparable to knockdown with the constitutive vector (mean = 80.4%, SD = 7.8%). To test inducible knockdown in vivo, we transferred shBATF–naive P14 CD8+ T cells into mice that were at the same time infected with LCMV-infected mice treated with IPTG, and measured BATF expression after 3 d. Initiating IPTG induction 1 d following cell activation resulted in modest (18%) gene knockdown but treating bone marrow chimera 3 d before transfer resulted in a significantly greater degree of gene silencing (68% knockdown) when it was measured 3 d following transfer and infection (Fig. 3C). Thus, efficient, inducible gene knockdown can be achieved in following activation of shBATF–naive CD8+ T cells in vivo.
BATF Knockdown Impairs the Development of CD8+ Effector T Cells Following Acute Viral Infection.
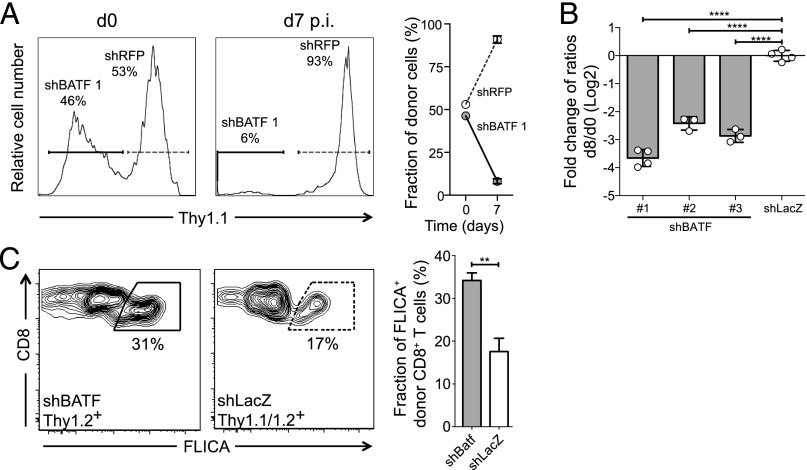
We have recently shown that Batf−/− CD8+ T cells show profoundly impaired effector CD8+ T-cell differentiation (11). To test whether BATF knockdown in wild-type CD8+ T cells also impaired CD8+ effector T-cell development, we adoptively transferred naive P14 CD8+ T cells from bone marrow chimeras transduced with either an inducible shBATF vector or a control shRNA vector targeting LacZ in a 1:1 ratio with naive P14 CD8+ T cells from a bone marrow chimera transduced with a second control shRNA (shRFP) into wild-type recipients (Fig. S5A). Endogenous, shBATF– (or control shLacZ–) and shRFP–naive CD8+ T cells were distinguished by the use of congenic markers. Comparison of the ratios of numbers shBATF– or shLacZ–effector T cells to shRFP–effector T cells was used to determine the effect of BATF knockdown, while controlling for any effect of shRNA expression on differentiation. We found markedly reduced numbers of P14 shBATF–effector CD8+ T cells at days 7–9 p.i. relative to shRFP P14 CD8+ T cells, when the cells were exposed to IPTG from d −3 until the end of the experiment. In contrast, the ratio of shLacZ–effectors to shRFP–effectors remained constant (Fig. 4 A and B). This reduction in shBATF–effector cell numbers was seen with three different BATF shRNAs designed with different seed regions, making this unlikely to be due to off-target effects (Fig. 4B) (19).
Fig. 4.
BATF knockdown in vivo in primary CD8+ T cells impairs effector differentiation. (A) Relative fraction of P14 shBATF– (solid gates/lines) or shRFP– (dotted gates/lines) CD8+ T cells at the time of transfer or at d 7 p.i. with LCMV Armstrong in IPTG-treated animals (from d −3 on). Representative plots (Left and Middle) from a single animal and summary data from five mice (Right). (B) Ratio of P14 shBATF– or control shLacZ–effector CD8+ T cells to shRFP–effector CD8+ T cells at d 8 p.i. with IPTG exposure. Ratio at d 8 p.i. was normalized to ratio at d 0 and shown for three different shRNAs targeting BATF. (C) Apoptosis of shBATF– or shLacZ–effector CD8+ T cells measured by active caspase staining (FLICA) at d 5 p.i. Representative plots (Left) and summary data (Right). Significance was assessed with Student’s t test; **P < 0.01, ****P < 0.0001. Representative data are shown from three (A and B) or two (C) experiments with three to five mice per group.
To identify the reason for the reduced population size of effector CD8+ T cells following BATF knockdown, we measured both cell death and proliferation in shBATF–effector CD8+ T cells at d 5 p.i. Analysis of active caspase abundance showed significantly higher apoptosis in shBATF–effector CD8+ T cells (Fig. 4C). In addition, there was a modest increase in the proliferation of the fraction of remaining shBATF–effector CD8+ T cells compared with shLacZ–effector CD8+ T cells (Fig. S5B). Thus, knockdown of BATF impairs the development of an effector CD8+ T-cell response primarily by increasing cell death during early differentiation. These findings are consistent with previous studies using germ-line deletion of BATF, which have demonstrated that naive Batf−/− T cells undergo massive cell death at 72–96 h after stimulation (11).
BATF Is Required to Initiate but Not Maintain Effector CD8+ T-Cell Development.
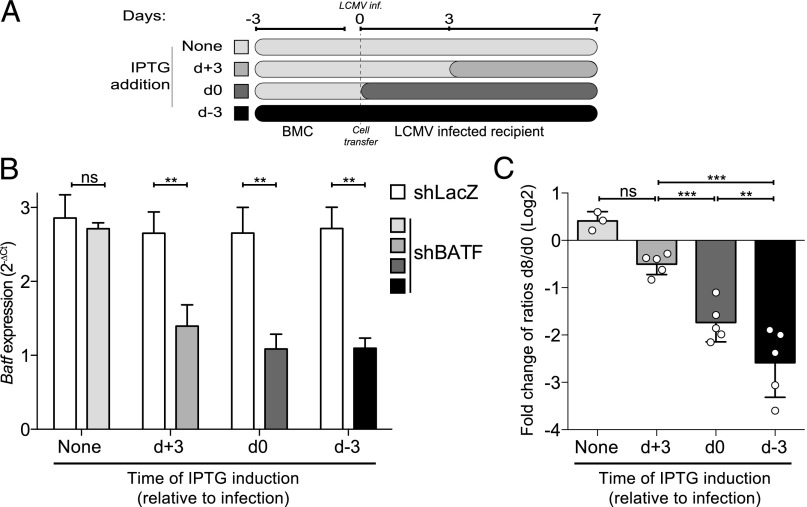
Because previous studies of the role of BATF in effector CD8+ T-cell differentiation have been carried out using T cells with constitutive germ-line deletion, it is not known whether BATF is required only to initiate the development of CD8+ effector T cells (i.e., at the time of initial antigen encounter) or whether BATF is also needed to maintain CD8+ effector T-cell development once underway. To address this question, we adoptively transferred 1:1 mixtures of congenically distinguishable P14 shBATF– and shLacZ–CD8+ T cells into recipient wild-type animals, which were then infected with LCMV Armstrong. IPTG was administered to induce BATF knockdown either before infection, at the time of infection, or 72 h p.i. (Fig. 5A). We assessed BATF knockdown at d 8 p.i. and found that BATF transcript abundance was significantly reduced in shBATF compared with shRFP–effector CD8+ T cells, regardless of when IPTG was initiated (Fig. 5B), and was not significantly different between any of the shBATF–effector cell conditions.
Fig. 5.
BATF is required to initiate but not maintain effector differentiation. (A) Schematic diagram of timing of IPTG administration. (B) BATF transcript abundance in shBATF– and shRFP–effector CD8+ T cells on d 8 p.i. as described in A. (C) Ratio of shBATF– or shLacZ–effector CD8+ T cells to shRFP–effector CD8+ T cells at d 8 p.i. with continuous IPTG exposure initiated at the times indicated. Day 8 p.i. ratios were normalized to the d 0 ratio and Log2 transformed. Significance was assessed with one-way ANOVA; **P < 0.01, ***P < 0.001, ****P < 0.0001. Representative data are shown from three experiments with three to five mice per group.
We observed profound differences in the ratio of shBATF:shLacZ–CD8+ T cells at d 8 p.i., depending on the time at which BATF knockdown had been initiated. BATF knockdown initiated 3 d before infection or at the time of infection was associated with a significant reduction in the numbers of d 8 p.i. effector CD8+ T cells compared with controls with no IPTG induction. In contrast, inducing BATF knockdown 72 h postinfection did not significantly change the numbers of effector CD8+ T cells d 8 p.i. (Fig. 5C). These finding show that, whereas BATF is required for effector CD8+ T-cell development at the time of initial antigen encounter, by 72 h p.i., BATF becomes largely dispensable, at least through d 8 of CD8+ effector T-cell differentiation.
Discussion
We have developed a strategy to inducibly silence gene expression in unperturbed hematopoietic cells in vivo using RNAi. We used this system to show that BATF is initially required for the development of effector CD8+ T cells, but becomes dispensable after 72 h. Our findings suggest that this experimental approach can be used to accelerate the understanding of how effector and memory T-cell responses are regulated.
Viral vectors expressing shRNA molecules have been used to investigate gene function in the immune system (13, 15–17, 20). However, this approach is limited by the inability to deliver viral vectors to quiescent cells. To study gene function in T cells, most approaches have used T-cell activation in vitro (14, 17) or in vivo (15, 16) to achieve efficient transduction. We show that both of these approaches profoundly alter the underlying transcriptional and functional state of naive T cells, suggesting that many aspects of effector differentiation are initiated by these manipulations before gene knockdown can occur. Our experimental system permits the inducible knockdown of genes in quiescent, unperturbed naive T cells, allowing the events that occur during the initial minutes to hours after T-cell stimulation to be interrogated.
We used this system to show that BATF is required at or immediately after antigen encounter but rapidly becomes dispensable for subsequent development of effector CD8+ T cells until at least d 8 p.i. These findings are consistent with several prior observations that support a role for BATF in the initial commitment to an effector state. First, the defects in effector CD8+ T cells that lack BATF are evident within 72–96 h of antigen encounter (11), suggesting that BATF plays a critical function in the earliest hours of effector differentiation. Second, BATF associates with its binding sites within 24 h of T-cell activation, which suggests that it could play a regulatory role as early as hours after antigen encounter (11). Finally, during Th17 differentiation, loss of BATF results in decreased chromatin accessibility at some regions normally bound by BATF, which suggests that BATF may play a role as a pioneer TF (21). Our findings of a transient role for BATF during the initiation of effector CD8+ T-cell development are consistent with its function as pioneer TF.
Our study also helps to explain why effector CD8+ T-cell differentiation can be initiated by brief, transient TCR activation. Studies of temporally limited antigen exposure have shown that a few hours of TCR stimulation can initiate cell division and induce cytolytic function in naive CD8+ T cells (5, 7, 8). These studies indicate that CD8+ T cells encounter an irreversible decision point within hours of antigen encounter (5). Our findings suggest that transcriptional regulation by BATF may be one component of that decision point. BATF may launch differentiation by irreversibly engaging the effector transcriptional program within the first 24 h of stimulation.
Although we have used this experimental approach to investigate the role of BATF in the early commitment events in effector CD8+ T-cell differentiation, this strategy also could be used to discover genes that regulate differentiation and longevity of memory CD8+ T cells and the mechanisms leading to CD8+ T-cell exhaustion. We also anticipate that pooled in vivo screens of gene function will be possible using this approach. Moreover, the use of a bone marrow chimeric system results in transduction of all hematopoietic-derived lineages with the inducible shRNA vector. This system, therefore, provides a feasible strategy for analyzing gene function in other cell types that are refractory to viral transduction, such as naive CD4+ T cells or B cells.
Methods
Mice.
Wild-type C57BL/6J, Ly5.1 (CD45.1), and Thy1.1 mice were purchased from The Jackson Laboratory. The P14 TCR Tg mice were previously described (22). All mice were used according to the Harvard Medical School Standing Committee on Animals and National Institutes of Animal Healthcare Guidelines.
Generation of Bone Marrow Chimeras.
LSK cells from bone marrow were enriched using anti-CD117 microbeads (Miltenyi Biotech) and then sorted using a BD FACSAria cytometer. Sorted cells were plated overnight in StemSpan SFEM (Stemcell Technologies) with 100 μg/mL recombinant stem cell factor, thrombopoietin, IL-7, and Flt3-ligand (PeproTech). Cells were then spin infected with lentiviral supernatants at 650 × g for 90 min at 37 °C on 100 μg/mL RetroNectin (Takara Bio)-coated plates. Fresh medium was added after 1 h. The following day, the cells were washed in PBS (Gibco) and 50,000 cells were injected i.v. into recipient mice that had been irradiated with two doses of 600 cGy, 3 h apart.
Lentivirus Production.
The 293T cells were seeded in DMEM with 10% (vol/vol) FBS. The following day, the cells were transfected with shRNA construct pLKO.1 or 1xLacO (now available from Sigma-Aldrich under the name “MISSION 1X LacO Inducible”) and the packaging plasmids Pax2 (gag, pol) and VSV-G using TransIT-LT1 (Mirus Bio) or ExGen 500 transfection reagents (Thermo Scientific Fermentas). Viral supernatants were collected 48–72 h later.
In Vitro Knockdown of GFP.
A stable GFP-expressing Jurkat cell line was constructed using PGK-eGFP lentivirus. GFP-Jurkat cells were lentivirus-transduced with shRNA targeting GFP under constitutive (pLKO.1, with puromycin resistance) or inducible (1xLacO, with Thy1.1 reporter) promoters. Varying doses of dioxane-free IPTG (Promega) were added at the indicated concentrations and durations. GFP expression was assessed with Accuri C6 flow cytometer (BD Biosciences).
T-Cell Transfers and Infections.
CD8+ T cells were magnetically separated using the CD8a+ T Cell Isolation Kit II (Miltenyi Biotech) and then GFP+ CD8+ congenic cells were sorted using a BD FACSAria cytometer. P14 CD8+ T cells (104 to 106 cells per animal) were injected into recipient mice i.v. Mice were subsequently infected intraperitoneally with 2 × 105 p.f.u. LCMV Armstrong or with influenza intranasally. For influenza virus infection, the mice were anesthetized with 2.5% avertin and infected with 0.5 LD50 H1N1 influenza virus (PR8), engineered to express GP33–41 peptide of LCMV (PR8-GP33) (23). Both viruses were a generous gift from E. John Wherry (University of Pennsylvania School of Medicine, Philadelphia).
Flow Cytometry and Cell Sorting.
Spleen or bone marrow tissue was homogenized into single cell suspension and resuspended in staining buffer (2 mM EDTA and 1% FBS in PBS; Gibco) together with combinations of the indicated antibodies. Data were acquired using LSR II or Accuri C6 (BD Biosciences) cytometers and analyzed with FlowJo software (v9.7.2; TreeStar).
shRNA Construct Generation.
Target sequences of the shRNA used are: shBatf 1 (CCGCAAAGAGATCAAACAGCT), shBatf 2 (CTGGACAAGTATTGAACACAA), shBatf 3 (GAGCTCAAGTACTTCACATCA), shLacZ (CCGTCATAGCGATAACGAGTT), shRFP (GCTTCAAGTGGGAGCGCGTGA), and shGFP (ACAACAGCCACAACGTCTATA). Cloning methods can be found at www.broadinstitute.org/rnai/public/.
Additional Methods.
Additional descriptions for all methods are available in SI Methods, including antibodies used, infections, immunoblotting, and RT-QPCR.
Supplementary Material
Acknowledgments
We thank John G. Doench, E. John Wherry, and the members of the W.N.H. and A.H.S. laboratories for meaningful discussions. This work was supported by the National Institutes of Health Grants AI091493, AI057266, AI082630 (to W.N.H.), AI38310 (to A.H.S.), and Cancer Research Institute Predoctoral Emphasis Pathway in Tumor Immunology (J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413291112/-/DCSupplemental.
References
- 1.Doering TA, et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37(6):1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 4.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165(12):6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 7.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2(5):423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 8.van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4(4):361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 9.Wong P, Pamer EG. Cutting edge: Antigen-independent CD8 T cell proliferation. J Immunol. 2001;166(10):5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 10.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 11.Kurachi M, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol. 2014;15(4):373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amit I, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326(5950):257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209(9):1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506(7486):52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, et al. In vivo RNA interference screens identify regulators of antiviral CD4(+) and CD8(+) T cell differentiation. Immunity. 2014;41(2):325–338. doi: 10.1016/j.immuni.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson AL, et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12(7):1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevrier N, et al. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147(4):853–867. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciofani M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 23.Mueller SN, et al. Qualitatively different memory CD8+ T cells are generated after lymphocytic choriomeningitis virus and influenza virus infections. J Immunol. 2010;185(4):2182–2190. doi: 10.4049/jimmunol.1001142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.