Significance
The chemical dialog through which a host promotes long-term symbioses with particular microbial partners remains largely unexplored, especially within complex consortia like the human microbiota. Natural, monospecific associations, including that between bobtail squid and Vibrio fischeri, have proved useful for discovering shared strategies, such as rhythmic microbial signaling and symbiosis-induced development, subsequently found in mammalian associations. Here, we demonstrate that symbiont metabolism is driven by a diel provision of a squid-derived glycan, resulting in tissue acidification. This event alters bacterial physiology, favoring the cyclic production of bioluminescence, the functional basis of the symbiosis. More generally, studies of this association can help reveal mechanisms by which other hosts modulate the chemistry of symbiosis to regulate microbial community function.
Keywords: symbiosis, squid–vibrio, metabolism, chitin
Abstract
Glycans have emerged as critical determinants of immune maturation, microbial nutrition, and host health in diverse symbioses. In this study, we asked how cyclic delivery of a single host-derived glycan contributes to the dynamic stability of the mutualism between the squid Euprymna scolopes and its specific, bioluminescent symbiont, Vibrio fischeri. V. fischeri colonizes the crypts of a host organ that is used for behavioral light production. E. scolopes synthesizes the polymeric glycan chitin in macrophage-like immune cells called hemocytes. We show here that, just before dusk, hemocytes migrate from the vasculature into the symbiotic crypts, where they lyse and release particulate chitin, a behavior that is established only in the mature symbiosis. Diel transcriptional rhythms in both partners further indicate that the chitin is provided and metabolized only at night. A V. fischeri mutant defective in chitin catabolism was able to maintain a normal symbiont population level, but only until the symbiotic organ reached maturity (∼4 wk after colonization); this result provided a direct link between chitin utilization and symbiont persistence. Finally, catabolism of chitin by the symbionts was also specifically required for a periodic acidification of the adult crypts each night. This acidification, which increases the level of oxygen available to the symbionts, enhances their capacity to produce bioluminescence at night. We propose that other animal hosts may similarly regulate the activities of epithelium-associated microbial communities through the strategic provision of specific nutrients, whose catabolism modulates conditions like pH or anoxia in their symbionts’ habitat.
Animals exist in a microbial world and are reliant on beneficial associations with certain microbes for nutrition, defense, development, or other fitness factors (1). In the case of horizontally acquired symbioses, such as that in the gut, the success of the association hinges on the ability of microbial symbionts to colonize, be nourished by, and deliver a fitness advantage to the host, while maintaining a détente with its immune system (2–4). The negotiations underlying such mutually beneficial relationships must initiate upon first contact and continue throughout the period of association (5).
Three hallmarks of host–microbe interaction emerge from studies of the complex microbial consortia of animals. First, the provision of nutrients such as host-derived glycans contributes to the microbial community structure and is a source of microbe-derived metabolites such as short-chained fatty acids (SCFA) that promote the maturation of local and systemic immune functions (6–9). Second, the nutritional and environmental changes that mark the developmental trajectory of an organism from its juvenile to adult form are accompanied by distinct shifts in both the composition and functions of the maturing host’s microbiota (10, 11). Finally, circadian rhythms coordinate much of the communication between a host and its microbiota, leading to the maintenance of physiological homeostasis (12–14). Taken together, these themes indicate that the terms of a protracted symbiotic negotiation are subject to a dynamic equilibrium that encompasses nutritional, immune, developmental, and circadian inputs.
Given this complexity, the specific costs incurred and benefits derived from a long-term cooperation, as well as their underlying mechanisms, are often difficult to establish, particularly in symbioses where the microbial member provides nutrients to the host. Natural invertebrate model systems that maintain one or a few symbiotic microbial species, such as nematodes, medicinal leeches, and squid (15), provide a window through which we can discover themes conserved across the diversity of interactions of animals with their coevolved microbiota, whether simple or complex. In particular, the symbiosis of the bobtail squid Euprymna scolopes and the luminous bacterium Vibrio fischeri, in which the microbial symbiont can be manipulated without compromising the health of the host, presents a rare opportunity to study the chemical and immune dialogues of symbiotic partners at a cellular and molecular level (16).
The squid–vibrio symbiosis occurs within the light-emitting organ of E. scolopes and is based on the bacterium’s production of bioluminescence (17), which the host uses in its nocturnal behaviors, such as foraging and camouflage. The symbionts are obtained through horizontal transmission from the ambient seawater by each generation of juvenile squid (18) and are cultured in the epithelium-lined crypts of the light organ (Fig. 1A) throughout the animal’s ∼9-mo life. The symbiont induces postembryonic light-organ development (19), and the organ morphology reaches maturity in 4 wk (20). Host-derived chitin, a polymeric glycan of N-acetylglucosamine, is known to promote the species-specific colonization of the squid by V. fischeri (21). Chitin is synthesized by several types of squid tissue, including macrophage-like immune cells called hemocytes (22) (Fig. 1A), and has also been implicated, along with amino acids (23), as a nutrient provided to the symbiont population. Specifically, transcription of V. fischeri genes associated with the fermentation of chitin oligosaccharides (COS) is elevated during the nocturnal, bioluminescent phase of the symbiosis (24) (Fig. 1A). The importance of chitin in the chemical dialogue between squid and vibrio is reminiscent of the contribution of host glycans and structural polysaccharides to other host–microbe interactions (9). For example, (i) the catabolism of exoskeleton-derived chitin by Vibrio cholerae enhances transmission from an invertebrate vector to a susceptible host (25), (ii) pectin catabolism by the plant pathogen Xylella fastidiosa promotes transmission to leafhopper vectors (26), and (iii) foraging of mammalian mucin-derived glycans, such as fucose and sialic acid, forms a nutritional scaffold for the gut microbiota (27–29).
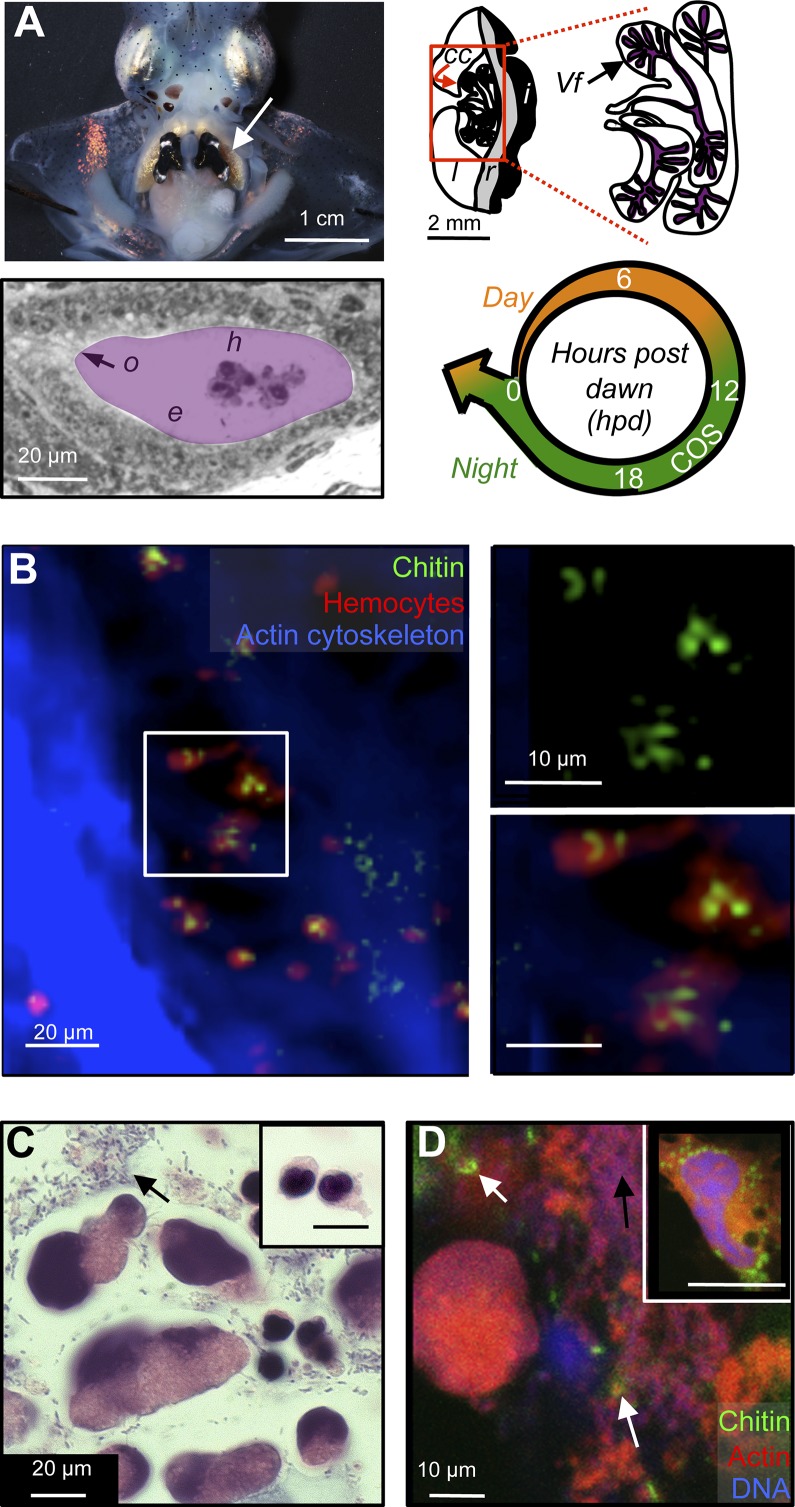
Fig. 1.
Light-organ crypts contain hemocyte-derived chitin. (A) Anatomy of the mature light organ. (Top Left) The mature, bilobed light organ is located ventrally, in the center of the mantle cavity (arrow). (Top Right) Schematic of one lobe, indicating the light-organ lens (l), reflector (r), and ink sac (i), as well as the bacteria-containing central-core tissue (cc) in the red box. Polarized epithelial cells form branched crypt spaces, in which the symbionts (Vf) reside. (Bottom Left) Symbiotic V. fischeri cells occupy extracellular crypts (purple), where they contact hemocytes (h) and the bordering epithelial cells (e); the outlet (o) to the mantle cavity allows the host to expel most of the crypt contents and symbionts every morning at dawn. (Bottom Right) The diel cycle of the mature squid–vibrio symbiosis. Throughout the animal’s life, about 95% of the symbiont population is expelled at dawn (arrow), and the remaining cells repopulate the crypts during the day (orange). At night (green), transcriptional evidence in the mature symbiosis suggests that symbionts metabolize host-derived chitin oligosaccharides (COS) (24) and produce luminescence. Numbers indicate hours post dawn (hpd). (B) Confocal micrograph, showing colocalization of chitin (fluorescent chitin-binding protein; green) and hemocytes (fluorescent DNase-I globular actin-binding protein; red) in central-core tissue (fluorescent phalloidin, a filamentous actin-binding protein; blue). Image is a 3D reconstruction of 40 1-µm confocal sections. Tissue was sampled just before nightfall (10 hpd). (Top Right and Bottom Right) Close-up of hemocytes in white box, highlighting particulate-chitin staining. (C) Differential interference contrast micrograph of crypt contents, sampled at the end of the night (22 hpd) and stained with hematoxylin (chromatin; dark blue), and eosin (proteins; pink). Two different morphologies of nucleated cells are seen in the crypt contents. Inset (same magnification) shows the single type of hemocyte morphology present in the hemolymph. Black arrow indicates extracellular material, which includes bacterial cells. (D) Detection of free particulate chitin (white arrows) and extracellular material (black arrows, here and in C) in the contents of the light-organ crypt, sampled 22 hpd. Fluorescent staining is as in B. Inset shows image of cytoplasmic particulate chitin within a hemocyte extracted from the hemolymph.
The squid–vibrio association is also characterized by daily rhythms of symbiont growth and bioluminescence (30, 31). Each morning at dawn, the host expels the contents of the light-organ crypts, including 95% of the symbiont population, into the surrounding seawater (Fig. 1A). The remaining symbiont cells repopulate the light organ within hours, by growing on substrates including amino acids and glycerophospholipids (23, 24), eventually providing the squid’s nocturnal bioluminescence. Light emission, which requires oxygen (32), is highest during the night (33). In the fully developed light organ, where the symbionts are oxygen limited (24, 33), the diel bioluminescent rhythm is potentiated by an acidic crypt environment, which creates a Bohr effect that releases oxygen from the carrier protein, hemocyanin (34). Here, we demonstrate that, in this mature state of light-organ development, the cyclic provision of COS to symbionts, combined with their fermentation of this glycan, leads to the nightly acidification of the symbiont-containing extracellular crypts. The combined nocturnal activities of host and symbiont thereby promote the diel cycle of bioluminescence: a rhythm critical to the long-term stability of this association.
Results and Discussion
Hemocytes Deliver Chitin to the Light Organ.
The diel transcription of chitin-utilization genes by symbiotic V. fischeri (24), together with the presence of chitin within the host’s macrophage-like hemocytes (22), suggests that the hemocytes convey this nutrient to the symbionts. To test this hypothesis, we first characterized the distribution of chitin in the light organ’s central core. Two major host cell types compose this tissue: the polarized microvillar epithelium that defines the symbiont-containing crypt spaces and the hemocytes that migrate into them (Fig. 1A). We collected samples of central core tissue at dusk [10 h postdawn (hpd), Fig. 1A] and just before dawn (22 hpd, Fig. S1A) and probed for the presence of particulate chitin. Fluorescently labeled chitin-binding protein (CBP) colocalized almost exclusively with the hemocyte-specific, cytoplasmic marker, DNase-I (Fig. 1B). Importantly, although soluble COS molecules (which do not bind CBP) may exist elsewhere, our observations support the idea that hemocytes are the main cell type within the light organ that contains particulate chitin.
Our previous observations of the contents of the light-organ crypts suggested that both live and dead hemocytes are present among the densely packed symbiotic bacteria (35). Thus, we hypothesized that chitin particles are delivered to the symbiont population by the death and lysis of chitin-containing hemocytes that have migrated into the crypts. A more extensive examination of crypt contents released at night (22 hpd) revealed two kinds of host cells: (i) compact 10-µm diameter cells and (ii) ∼20-µm variable-diameter cells with diffuse DNA staining, ruptured acidic vacuoles, and irregular membrane morphology (Fig. 1C and Fig. S1B), consistent with living and moribund hemocytes, respectively. The presence of the latter morphology in the nocturnal crypts raised the question of whether exposure to the conditions present in this environment (34) was sufficient to cause hemocyte damage.
When we suspended healthy hemocytes derived from hemolymph (pH ∼7) for 1 h in crypt contents buffered at either pH 7.5 or the nocturnal crypt pH of 5.5 (34), the initially intact hemocytes swelled, produced membrane blebs, and showed a loss of integrity of their acidic vacuoles (Fig. S1C). If the crypt contents were heat treated, they elicited the same effect. Thus, a heat-resistant, neutral-pH active, component of the crypt contents can trigger a change in hemocyte appearance that is consistent with hemocyte damage. In addition, when crypt contents were collected 2 h before dawn (22 hpd) and probed with CBP, we observed healthy-looking cells that contained particles of chitin, free chitin in the extracellular crypt matrix, and moribund cells morphologically similar to those observed in the in vitro studies described above (Fig. 1D). Collectively, the data are consistent with a model wherein chitin particles within host hemocytes are released into the crypts and become accessible to extracellular hydrolysis by both host and symbiont chitinases (21, 24, 36).
Symbiont-Dependent Hemocyte Trafficking into the Light Organ Is on a Diel Cycle.
For hemocytes to deliver COS to symbionts only during the nocturnal phase of the diel cycle, some aspect of their behavior must be rhythmic. We have previously reported a symbiosis-induced increase in the number of hemocytes associated with the light organ in immature, 2-d-old squid (37). We extended this observation by asking whether the increase in hemocytes was dependent on the time of day and, thus, a symbiosis-dependent diel migratory rhythm. Consistent with previous results (37), approximately four times more hemocytes were found in symbiotic than in aposymbiotic light organs of 2-d-old squid at dusk (10 hpd, Fig. 2A). However, we noted that this increase was transient: The number of hemocytes in symbiotic light organs returned to aposymbiotic levels by the end of the night (22 hpd), and no accumulation of hemocytes was noted in the crypts of the immature light organs. This pattern of hemocyte migration recurred the following day (Fig. S2A), demonstrating that the migration into symbiotic light organs at dusk is likely a diel pattern, rather than a single event in the trajectory of an immunological and/or developmentally triggered response to colonization. Thus, a symbiosis-dependent diel rhythm of hemocyte migration is established within the first few days following light-organ colonization.
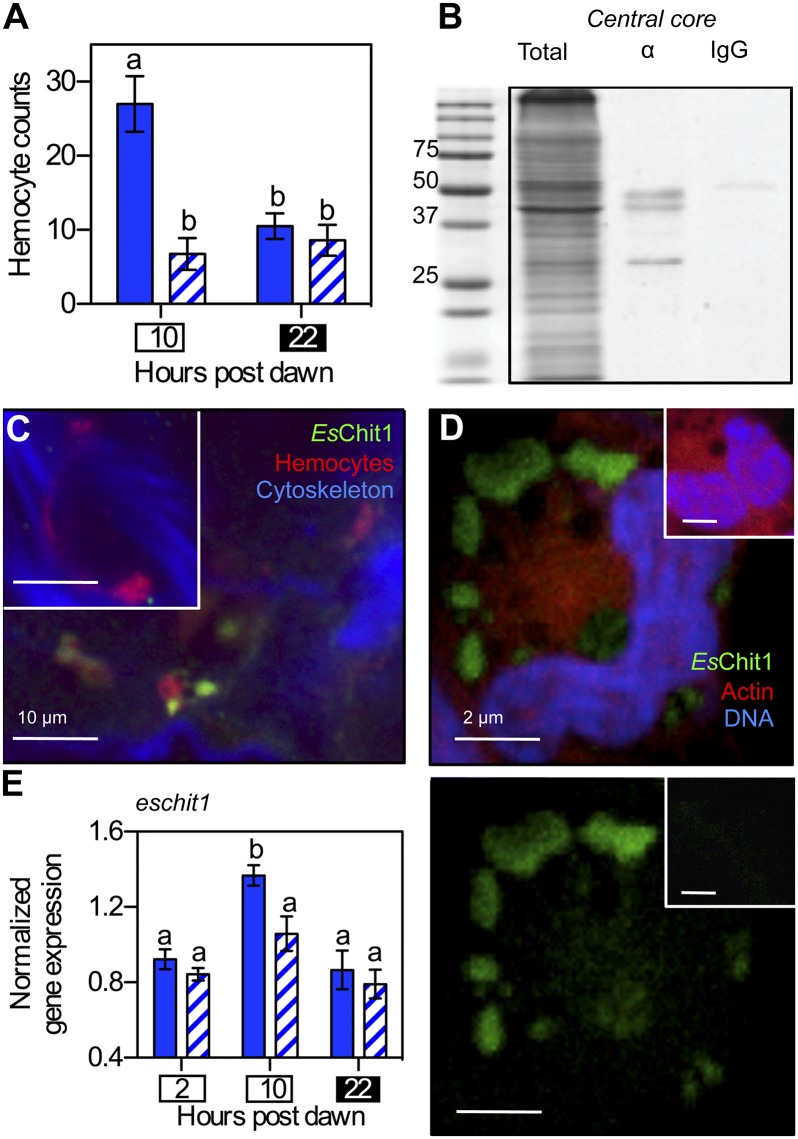
Fig. 2.
The diel migration of hemocytes to the light organ is symbiont dependent. (A) Enumeration of hemocytes present during the day (10 hpd; white box) and night (22 hpd; black box) in light-organ tissues of 2-d-old, immature symbiotic (solid bars) or aposymbiotic (hatched bars) animals. n = 15 light organs, error bars indicate SEM, and data are representative of three independent experiments. “a” and “b” indicate groups of statistically similar means, determined with two-way ANOVA with post hoc Bonferroni T-tests. (B) Western blot showing presence of EsChit1 in 25 µg of total soluble protein isolated from light-organ central cores. Total, total soluble protein; α, anti-EsChit1 antibody; IgG, Ig control. (C) Confocal micrograph localizing EsChit1 in whole adult (>4-wk-old) central cores at dusk (10 hpd). Inset shows preimmune control. (D) Localization of EsChit1 in hemocytes extracted from adult symbiotic squid. (Bottom) Anti-EsChit1 signal alone; (Top) anti-EsChit1 signal merged with rhodamine phalloidin (filamentous actin specific) and TOTO-3 (DNA specific). Insets show preimmune control. (E) Diel pattern of the transcription of host chitotriosidase (eschit1) in adult symbiotic (solid bars) and aposymbiotic (hatched bars) light organs; error bars indicate SEM, n = 5; statistical tests are as described in A.
We next investigated whether a similar rhythm of hemocyte migration occurred in mature light organs. At this stage of development, transcription of the predicted E. scolopes chitotriosidase gene eschit1 exhibits a diel periodicity, with expression highest at night (24). This gene is also transcribed by hemocytes (22). Western-blot analysis demonstrated that the chitotriosidase enzyme itself was present in central core tissue; i.e., the EsChit1 antibody (α-EsChit1) hybridized to a 50-kDa band (the molecular mass predicted for EsChit1), as well as two smaller bands in the soluble fraction of central-core proteins (Fig. 2B). This multiband pattern is consistent with the posttranslational processing that occurs during the activation of invertebrate chitinases (38). In addition, fluorescence immunocytochemistry and confocal microscopy of the central core localized the EsChit1 protein exclusively to the hemocytes found there (Fig. 2C). We confirmed these observations by isolating hemocytes and probing them directly for EsChit1: Cells from both mature and immature animals exhibited α-EsChit1-positive puncta (Fig. 2D and Fig. S2B), irrespective of symbiotic state. Thus, hemocytes express the majority of EsChit1 in light organ tissue, and eschit1 transcript can be associated with the presence of hemocytes.
To determine whether the diel rhythm of eschit1 transcription originally observed in mature central-core tissues occurred only in the symbiotic state, we profiled the transcript levels of this gene at three times of day, in both symbiotic and aposymbiotic mature light organs, by quantitative real-time PCR. Consistent with previous results (24), hemocyte-associated eschit1 transcript levels increased significantly in mature symbiotic light organs just before nightfall (10 hpd, Fig. 2E). This increase was not observed in aposymbiotic light organs, although transcript levels were comparable between the two groups, both in the morning and at the end of the night (2 hpd and 22 hpd, respectively) (Fig. 2E). Transcription of another, functionally distinct, hemocyte-associated E. scolopes gene, peptidoglycan-recognition protein 5 (espgrp5) (39), displayed the same symbiont-dependent peak at dusk as eschit1 (Fig. S2C). This pattern in hemocyte-associated transcripts mirrors the transient, symbiosis-dependent increase in hemocyte numbers observed in immature light organs and is consistent with a nightly hemocyte migration into the crypts of mature light organs, although it may also indicate a small symbiosis-dependent induction of these two genes as well. Although we do not know whether this rhythmic migration has circadian underpinnings, in vertebrates, macrophages migrate into tissues as part of a circadian cycle that is entrained by microbial cues (13).
Delivery of COS to the Symbionts Begins in the Mature Light Organ.
We next asked whether the symbionts metabolize COS and, if so, what consequences it might have for the association. To investigate COS metabolism within the crypts, we constructed a mutant strain of V. fischeri that would act as a biosensor. Catabolism of chitin (Fig. 3A) is a general characteristic of the Vibrionaceae, and this glycan is an important environmental nutrient and cue for both beneficial and pathogenic species in this family (25). To construct the COS biosensor, we deleted the V. fischeri nagB gene, which encodes glucosamine deaminase: the final enzymatic step by which COS and other amino sugars enter the central carbon metabolism of V. fischeri (36). Such a mutant cannot grow on this group of sugars (Fig. 3A and Fig. S3A). As with the homologous Escherichia coli mutant (40), in a complex growth medium, V. fischeri ∆nagB was sensitive to 100 µM of the COS monomer N-acetyl glucosamine (GlcNAc), a level even below that required for significant growth (Fig. S3B). As expected, this sensitivity was eliminated by genetically complementing the ∆nagB strain in cis at the Tn7 site on the V. fischeri chromosome, a neutral site for integration of DNA elements (41) (Fig. 3B). The effect of COS on ∆nagB growth was also relieved by the presence of a nongluconeogenic sugar of the pentose-phosphate pathway (e.g., fructose, glucose, or ribose; Fig. S3C), confirming that the growth-arrest phenotype is attributable to substrate inhibition (40).
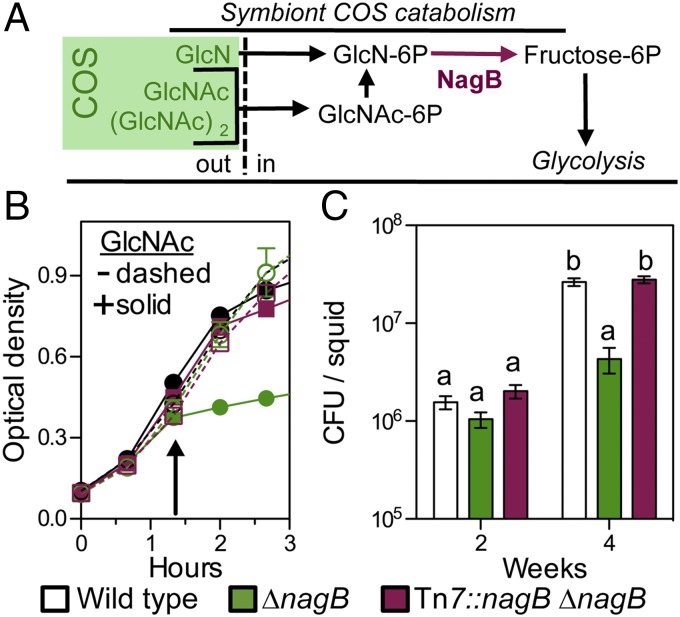
Fig. 3.
Symbionts sense chitin oligosaccharides (COS) only in the mature light organ. (A) Catabolism of COS in the genus Vibrio. COS are derivatives of amino di- and monosaccharides that represent enzymatic products of chitin hydrolysis (green box). After the COS are transported into the cell as the phosphorylated form, the acetyl and amino groups are removed from the hexose core before it enters glycolysis. The last common step in COS catabolism is the deamination of glucosamine-P by the enzyme NagB. To define amino sugar and COS catabolism in V. fischeri, we tested the ability of the ∆nagB mutant to grow on several sugars as a sole source of carbon (data shown in Fig. S3A). GlcNAc, N-acetyl glucosamine; (GlcNAc)2, N-acetylchitobiose; GlcN, glucosamine; out, extracellular or periplasmic space; in, intracellular space. (B) Growth of a ∆nagB V. fischeri mutant in seawater–tryptone medium (SWTO), either without (−) or with (+) the addition of 20 mM GlcNAc at the arrow. Error bars indicate SEM, n = 4. (C) Extent of colonization of the squid light organ at immature and mature stages of host development by wild type, ∆nagB, and an in cis complemented (∆nagB Tn7::nagB) strain. cfu, colony-forming units; error bars indicate SEM, n = 12; statistical tests on log-transformed data are as described in Fig. 2.
From these observations, we reasoned that V. fischeri ∆nagB would have difficulty colonizing the squid light organ under two conditions: (i) where COS is present at a level sufficient to support wild-type growth or (ii) where there is even a small amount of COS (e.g., ∼100 µM), but no ameliorating pentose-phosphate pathway sugars. We compared colonization levels in light organs populated by either wild-type or ∆nagB strains of V. fischeri during the first 4 wk postcolonization to determine when symbionts might encounter such environments (Fig. 3C). For at least 2 wk, squid were colonized equally well by wild-type or ∆nagB cells; however, by 4 wk, light organs colonized by the ∆nagB strain contained <15% as many symbiont cells as those colonized by either wild type or the in cis complemented strain (Fig. 3C). The ∆nagB strain showed no decrease in fitness during cocolonization with wild type throughout a 4-wk colonization (Fig. S3D), probably due to an effective lowering of the ambient COS concentration by wild-type metabolism. Similarly, coculture of ∆nagB and wild-type V. fischeri allowed growth of the mutant, even in the presence of up to 2.5 mM GlcNAc (Fig. S3E), suggesting that the concentration of COS available to symbionts in the light organ never exceeds a few millimolar. Although we cannot rule out the possibility that COS are present in the immature light-organ crypt at a level below the sensitivity of our biosensor strain (i.e., 10–100 µM), our data do indicate that COS catabolism emerges as a major metabolic strategy for symbiotic V. fischeri between 2 wk and 4 wk posthatch. These data also indicate that the presence of COS in the light-organ crypts is not directly linked to the squid’s diet: The squid consumes a chitin-rich diet throughout the rearing process. The developmental, diet-independent, regulation of COS availability echoes the life stage-specific presentation of surface sugars to glycan-foraging microbes during mammalian gut development (27).
Catabolism of COS by the Symbionts Drives a Diel Cycle of Crypt Acidification.
To determine whether the delivery of E. scolopes chitin, like that of mammalian glycans (27), shapes the ecology of the mature state of symbiosis, we asked whether the appearance of COS in the light-organ crypts induced any changes in symbiont physiology and/or the crypt environment. Catabolism of COS produces acetic acid in culture; thus, we performed a comparative study of COS catabolism, and associated acetate production, under aerobic culture conditions in four strains (Table S1): V. fischeri ES114 (the wild-type squid symbiont used in this study), V. fischeri MJ11 (a fish light-organ symbiont), Vibrio harveyi (a nonsymbiotic seawater isolate), and E. coli K-12 (an enteric strain found in the mammalian gut). When grown on either N-acetylglucosamine or glucose, the three Vibrio strains produced two to three times more acetate per unit growth, and more acetate per mole substrate, than did E. coli, suggesting that the aerobic catabolism of glycolytic substrates by Vibrio spp. relies more on substrate-level phosphorylation for energy generation than E. coli does. This excretion of acetate during aerobic growth is consistent with a metabolic imbalance called the Crabtree effect (42). In the context of the light-organ environment, the Crabtree effect would lead to the excretion of SCFA like acetate during growth on COS, thereby acidifying the crypt lumen and enhancing the allocation of ambient oxygen toward bioluminescence (34).
We reasoned that if SCFA accumulate as an excreted product of symbiont COS catabolism, they might cause acidification of the light-organ crypts. In a number of bacteria, including V. fischeri (Fig. S4A), exposure to low pH induces an acid-tolerance response (ATR) that mitigates a potentially lethal collapse of the cell’s proton motive force by protonated weak acids like SCFA (43). As expected (Table S1), during COS catabolism in unbuffered media, wild-type V. fischeri excreted sufficient acetate to acidify the culture supernatant to pH 5.5, inducing an ATR; however, when the COS medium was strongly buffered at pH 7.5, there was no ATR (Fig. 4A). The ∆nagB mutant, which is impaired in COS metabolism, did not acidify even an unbuffered COS medium and, thus, failed to induce an ATR. In contrast, when grown on glucose, a NagB-independent, acid-generating source of carbon, this mutant did reduce the pH and induce a robust ATR (Fig. S4B). Thus, we reasoned that the failure of the ∆nagB mutant to induce an ATR when COS is present was due to its inability to catabolize COS to acetate (Fig. 3A), rather than to causing a defect in the ATR mechanism itself.
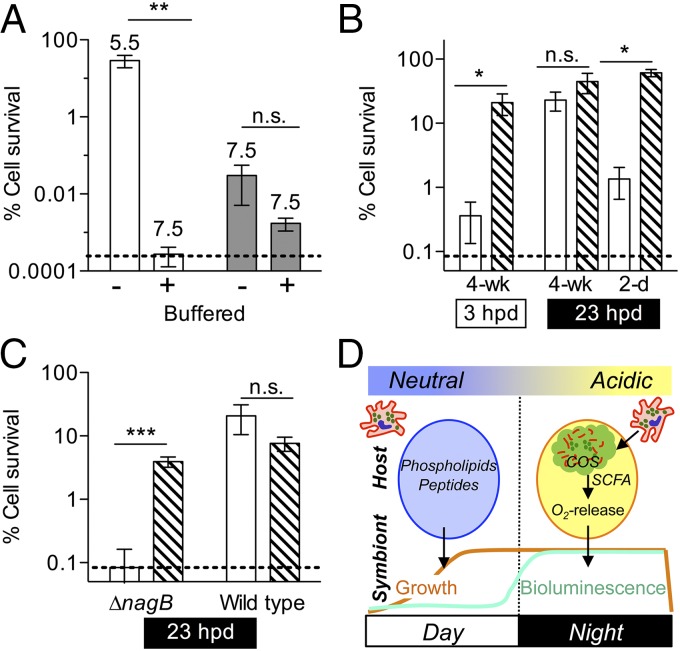
Fig. 4.
Acidification due to COS catabolism is sufficient to induce the V. fischeri acid tolerance response in symbiosis. V. fischeri was exposed to different conditions before challenge with 40 mM short-chained fatty acid (SCFA) medium at pH 4.5. Survival (induction of an acid tolerance response, ATR) was evaluated after 20 min. ANOVAs with post hoc Bonferroni T-tests were performed on log-transformed data; error bars, SEM; dashed line, limit of detection; n.s., no significance, *P < 0.05, **P < 0.01, ***P < 0.01. (A) Wild-type and ∆nagB V. fischeri were grown with 40 mM GlcNAc in either unbuffered or buffered medium (final culture pHs are indicated above bars) (n = 6). (B) Symbiotic wild-type V. fischeri was released from mature light organs during either the day (3 hpd) or the night (23 hpd). Alternately, symbionts were released from six individual pools of 20 2-d-old light organs at night (23 hpd). Released symbionts were assayed immediately for ATR (open bars) or after a period of preexposure to 30 mM SCFA medium at pH 5.5 (hatched bars) as a positive control (n = 5). (C) ∆nagB or wild-type V. fischeri were released from mature light organs at night (23 hpd). Symbionts were assayed for ATR either immediately following release (open bars), or after preexposure for 1 h (hatched bars) as in B, before acid killing (n = 6). (D) Model of diel metabolic cross-talk between the adult host and its symbionts. During the day, growth of symbionts on host-derived nutrients (peptides and phospholipids) in the crypt spaces (circles) is pH neutral (24). At dusk, hemocytes (red) migrate into the light-organ crypt lumen, lyse, and release chitin oligosaccharides (COS, green). The COS are catabolized by symbionts to produce SCFA, which acidify the crypt lumen and promote oxygen release (34). Oxygen fuels the enzymatic production of light by symbiont luciferase, the functional basis of the squid–vibrio mutualism.
We next used the ATR as a reporter to indicate whether symbiont catabolism of COS acidifies the light-organ crypts; specifically, we released V. fischeri cells from the light organ and immediately determined whether they had recently induced an ATR. Wild-type symbionts exhibited an ATR when released from the light organs of mature squid at night (18 hpd and 23 hpd, Fig. 4B and Fig. S4C), consistent with the acidic pH measured in crypt contents at night (34). In contrast, symbionts released in the morning (3 hpd) showed no evidence of ATR induction, even though they were physiologically capable of inducing this response if they were exposed to acidic conditions before the assay (Fig. 4B). In contrast, the ∆nagB strain of V. fischeri failed to exhibit an ATR when released from the mature light organ at night, indicating it had not experienced a low pH in the crypts (Fig. 4C and Fig. S4C). Wild-type symbionts released from immature (2-d-old) light organs, even at night, also showed no evidence of an ATR (Fig. 4B and Fig. S4D), consistent with the notion that the immature host does not provide its symbionts sufficient COS (or other fermentable carbon sources) to promote acidification.
To estimate the amount of symbiont acid production that would be needed to reduce the pH of the crypts to an ATR-inducing 5.5, we measured the buffering capacity of freshly isolated squid hemolymph. Hemolymph, which is the major constituent of the crypt environment (34), required the addition of ∼7.5 mM acetic acid to reach a pH of 5.5 (Fig. S4E). The incomplete oxidation of a molecule of N-acetyl glucosamine produces at least one molecule of acetate (Table S1) and could stoichiometrically produce as many as three. Thus, a nightly catabolism by the symbiont population of a few millimolar COS would be sufficient to create the level of acidification measured in the adult light-organ crypts (SI Calculations).
Conclusions
Collectively, the data presented here support the hypothesis that (i) a nocturnal release of chitin from hemocytes in the light-organ crypts characterizes the mature diel cycle of the squid–vibrio symbiosis, (ii) symbiont catabolism of COS acidifies the crypt environment and induces the V. fischeri ATR at night, and (iii) this cycle is not active in the immature association, but develops only as the host matures (Fig. 4D). Previous work has shown that an acidic crypt pH potentiates the release of oxygen from hemocyanin (34) at the time when the symbiont’s nocturnal light production increases (33). Thus, the host’s cyclic provision of chitin, coupled with the symbionts’ physiological responses to this rhythm, regulates the output of bioluminescence, the central product of the association (17). To acidify the light-organ crypts to their nighttime pH of ∼5.5 (34) would require an amount of acetate released by catabolism of 11 µg of COS; we estimate that this amount of substrate could be transported into the crypts by ∼2,000 hemocytes or 5% of the total circulating population (SI Calculations) (35, 44). The nightly sacrifice of this number of immune cells may appear to be a costly strategy for the host, and we cannot be certain that other, complementary mechanisms of COS delivery do not exist. However, 5% is less than the reported percentage of circulating hemocytes normally replaced by mollusks every few days (44), suggesting that the accumulation of a fraction of hemocytes in the crypt may be one strategy by which senescent cells are efficiently repurposed. Whether or not this strategy of delivering a specific glycan is costly to the host, if immune-cell derived chitin is the key that unlocks a nocturnal cycle of symbiont bioluminescence, then the sacrifice of a small fraction of its hemocytes may be a viable strategy for maintaining a stable, cooperative association.
pH homeostasis is emerging as a factor that regulates not only the phylogenetic composition of complex microbial communities in the oral cavity and gut, but also immune maturation and host health (45–47). In the squid–vibrio symbiosis, the diel acidification of the host tissue environment by bacterial metabolism is likely to affect activities beyond the bioluminescence phenotype of this association. The squid encodes homologs of zebrafish enzymes that inactivate immunogenic microbe-associated molecular pattern (MAMP) molecules. These enzymes lose activity at a low pH (48–51), suggesting that diel tissue acidification might lead to a local or systemic increase in MAMP levels at night. MAMPs promote the establishment of symbiosis by signaling pattern recognition receptors (PRRs) (8); thus, it is possible that acidification underlies a daily activation of PRR signal cascades. In addition, induction of bacterial acid tolerance has been associated with cross-protection and attenuation of immune-related antimicrobial compounds (52, 53). Consequently, an understanding of the mechanisms by which bacteria, whether beneficial or pathogenic, modulate the pH of colonized tissues is of critical importance to deciphering the molecular basis of these interactions.
Materials and Methods
See SI Materials and Methods for additional experimental details, including strains (Table S2) and primers (Table S3) used in this study, and the full sequence of EsChit1 (Fig. S5).
ATR Assays.
The induction of the V. fischeri ATR was monitored by assaying resistance to killing by exposure to a pH 4.5 mixture of SCFA for 20 min (54). To obtain symbiotic V. fischeri for the ATR assay, we collected a homogenate of either central-core tissues from individual adult (>4-wk-old) squid light organs or 50 whole juvenile (2-d-old) squid; both preparations were suspended at ∼107 cfu/mL.
Detection of Chitin, and Immunocytochemistry.
Chitin was detected in hemocytes and light-organ tissues, using commercially available FITC- or TRITC-conjugated CBP (New England Biolabs) (22). Light-organ tissues and hemocytes from the hemolymph of >4-wk-old squid, or whole tissue from 2-d-old light organs, were collected and prepared for immunocytochemistry (ICC) and confocal microscopy, using standard procedures (22, 48).
Supplementary Material
Acknowledgments
We thank S. Nyholm, K. Vetsigian, R. Welch, H. Blackwell, and J. Reed for contributive discussions and Nell Bekiares and the Kewalo Marine Laboratory, University of Hawaii-Manoa, for support during animal experiments. This work was funded by NIH Grants RR12294/OD11024 (to E.G.R. and M.J.M.-N.) and AI050661 (to M.J.M.-N.). J.A.S. was funded by a National Science Foundation Graduate Research Fellowship, by the Chemical Biology Training Program [University of Wisconsin (UW)–Madison, NIH–National Institute of General Medical Sciences Grant T32 GM008505], and by the Microbes in Health and Disease Training Program (UW–Madison NIH–National Institute of Allergy and Infectious Diseases Grant T32 AI055397).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KM592978).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418580112/-/DCSupplemental.
References
- 1.McFall-Ngai M, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110(9):3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schluter J, Foster KR. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 2012;10(11):e1001424. doi: 10.1371/journal.pbio.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kereszt A, Mergaert P, Maróti G, Kondorosi E. Innate immunity effectors and virulence factors in symbiosis. Curr Opin Microbiol. 2011;14(1):76–81. doi: 10.1016/j.mib.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod R. The Evolution of Cooperation. Basic Books; New York: 1984. [Google Scholar]
- 6.Lee W-J, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10(6):416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 7.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert SF. A holobiont birth narrative: The epigenetic transmission of the human microbiome. Front Genet. 2014;5:282. doi: 10.3389/fgene.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaston J, Goodrich-Blair H. Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev. 2010;34(1):41–58. doi: 10.1111/j.1574-6976.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 11.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 12.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106(50):21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Mandel MJ. Models and approaches to dissect host-symbiont specificity. Trends Microbiol. 2010;18(11):504–511. doi: 10.1016/j.tim.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 16.McFall-Ngai MJ. The importance of microbes in animal development: Lessons from the squid-vibrio symbiosis. Annu Rev Microbiol. 2014;68(1):177–194. doi: 10.1146/annurev-micro-091313-103654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabb EV, Visick KL. Vibrio Fisheri: Squid Symbiosis. The Prokaryotes. Springer; New York: 2013. pp. 497–532. [Google Scholar]
- 18.McFall-Ngai M, Heath-Heckman EA, Gillette AA, Peyer SM, Harvie EA. The secret languages of coevolved symbioses: Insights from the Euprymna scolopes-Vibrio fischeri symbiosis. Semin Immunol. 2012;24(1):3–8. doi: 10.1016/j.smim.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doino JA, McFall-Ngai M. A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol Bull. 1995;189:347–355. doi: 10.2307/1542152. [DOI] [PubMed] [Google Scholar]
- 20.Koch EJ, Miyashiro T, McFall-Ngai MJ, Ruby EG. Features governing symbiont persistence in the squid-vibrio association. Mol Ecol. 2014;23(6):1624–1634. doi: 10.1111/mec.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kremer N, et al. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe. 2013;14(2):183–194. doi: 10.1016/j.chom.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heath-Heckman EA, McFall-Ngai MJ. The occurrence of chitin in the hemocytes of invertebrates. Zoology. 2011;114(4):191–198. doi: 10.1016/j.zool.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graf J, Ruby EG. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc Natl Acad Sci USA. 1998;95(4):1818–1822. doi: 10.1073/pnas.95.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wier AM, et al. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci USA. 2010;107(5):2259–2264. doi: 10.1073/pnas.0909712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meibom KL, et al. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci USA. 2004;101(8):2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killiny N, Almeida RP. Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc Natl Acad Sci USA. 2009;106(52):22416–22420. doi: 10.1073/pnas.0908562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng KM, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashyap PC, et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA. 2013;110(42):17059–17064. doi: 10.1073/pnas.1306070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath-Heckman EA, et al. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis. MBio. 2013;4(2):e00167-13. doi: 10.1128/mBio.00167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KH, Ruby EG. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol. 1994;60(5):1565–1571. doi: 10.1128/aem.60.5.1565-1571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn AK. Vibrio fischeri metabolism: Symbiosis and beyond. Adv Microb Physiol. 2012;61:37–68. doi: 10.1016/B978-0-12-394423-8.00002-0. [DOI] [PubMed] [Google Scholar]
- 33.Boettcher K, Ruby E, McFall-Ngai M. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179(1):65–73. [Google Scholar]
- 34.Kremer N, et al. The dual nature of haemocyanin in the establishment and persistence of the squid-vibrio symbiosis. Proc Biol Sci. 2014;281(1785):20140504. doi: 10.1098/rspb.2014.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyholm SV, McFall-Ngai MJ. Sampling the light-organ microenvironment of Euprymna scolopes: Description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol Bull. 1998;195(2):89–97. doi: 10.2307/1542815. [DOI] [PubMed] [Google Scholar]
- 36.Miyashiro T, et al. The N-acetyl-D-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol Microbiol. 2011;82(4):894–903. doi: 10.1111/j.1365-2958.2011.07858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koropatnick TA, Kimbell JR, McFall-Ngai MJ. Responses of host hemocytes during the initiation of the squid-Vibrio symbiosis. Biol Bull. 2007;212(1):29–39. doi: 10.2307/25066578. [DOI] [PubMed] [Google Scholar]
- 38.Renkema GH, et al. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur J Biochem. 1997;244(2):279–285. doi: 10.1111/j.1432-1033.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 39.Collins AJ, Schleicher TR, Rader BA, Nyholm SV. Understanding the role of host hemocytes in a squid/vibrio symbiosis using transcriptomics and proteomics. Front Immunol. 2012;3:91. doi: 10.3389/fimmu.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernheim NJ, Dobrogosz WJ. Amino sugar sensitivity in Escherichia coli mutants unable to grow on N-acetylglucosamine. J Bacteriol. 1970;101(2):384–391. doi: 10.1128/jb.101.2.384-391.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCann J, Stabb EV, Millikan DS, Ruby EG. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl Environ Microbiol. 2003;69(10):5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69(1):12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krulwich TA, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol. 2011;9(5):330–343. doi: 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol. 2009;11(2):483–493. doi: 10.1111/j.1462-2920.2008.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 46.Guo L, He X, Shi W. 2014. Intercellular communications in multispecies oral microbial communities. Front Microbiol 5:328.
- 47.Wolf KJ, et al. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem. 2014;62(4):237–250. doi: 10.1369/0022155413519650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Troll JV, et al. Peptidoglycan induces loss of a nuclear peptidoglycan recognition protein during host tissue development in a beneficial animal-bacterial symbiosis. Cell Microbiol. 2009;11(7):1114–1127. doi: 10.1111/j.1462-5822.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rader BA, Kremer N, Apicella MA, Goldman WE, McFall-Ngai MJ. Modulation of symbiont lipid A signaling by host alkaline phosphatases in the squid-vibrio symbiosis. MBio. 2012;3(3):e00093-12. doi: 10.1128/mBio.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Royet J, Gupta D, Dziarski R. Peptidoglycan recognition proteins: Modulators of the microbiome and inflammation. Nat Rev Immunol. 2011;11(12):837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- 51.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2(6):371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 53.Foster JW. When protons attack: Microbial strategies of acid adaptation. Curr Opin Microbiol. 1999;2(2):170–174. doi: 10.1016/S1369-5274(99)80030-7. [DOI] [PubMed] [Google Scholar]
- 54.Merrell DS, Camilli A. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol Microbiol. 1999;34(4):836–849. doi: 10.1046/j.1365-2958.1999.01650.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.