Significance
The diet of fossil organisms can be inferred through isotopic analysis of skeletal tissues, largely assessed using isotopic and elemental systems such as carbon isotopes (δ13C) and strontium/calcium (Sr/Ca) and barium/calcium (Ba/Ca) concentration ratios. In the case of complex dietary habits such as omnivory, these systems must be used jointly together with new proxies. Based on the expectation that fractionation of bio-essential metals occurs during metabolism, we explore the isotopic variability of magnesium (δ26Mg) in tooth enamel sampled from an assemblage of modern mammals. We demonstrate that δ26Mg increases from herbivores to higher-level consumers, discriminating most of the identified trophic steps. This, combined with δ13C, Ba/Ca, and/or δ44Ca might prove useful in paleodietary studies.
Keywords: stable isotopes, magnesium, carbon, equatorial mammals, paleoecology
Abstract
Geochemical inferences on ancient diet using bone and enamel apatite rely mainly on carbon isotope ratios (δ13C) and to a lesser extent on strontium/calcium (Sr/Ca) and barium/calcium (Ba/Ca) elemental ratios. Recent developments in nontraditional stable isotopes provide an unprecedented opportunity to use additional paleodietary proxies to disentangle complex diets such as omnivory. Of particular relevance for paleodietary reconstruction are metals present in large quantity in bone and enamel apatite, providing that biologically mediated fractionation processes are constrained. Calcium isotope ratios (δ44Ca) meet these criteria but exhibit complex ecological patterning. Stable magnesium isotope ratios (δ26Mg) also meet these criteria but a comprehensive understanding of its variability awaits new isotopic data. Here, 11 extant mammal species of known ecology from a single locality in equatorial Africa were sampled for tooth enamel and, together with vegetation and feces, analyzed for δ26Mg, δ13C, Sr/Ca, and Ba/Ca ratios. The results demonstrate that δ26Mg incorporated in tooth enamel becomes heavier from strict herbivores to omnivores/faunivores. Using data from experimentally raised sheep, we suggest that this 26Mg enrichment up the trophic chain is due to a 26Mg enrichment in muscle relative to bone. Notably, it is possible to distinguish omnivores from herbivores, using δ26Mg coupled to Ba/Ca ratios. The potential effects of metabolic and dietary changes on the enamel δ26Mg composition remain to be explored but, in the future, multiproxy approaches would permit a substantial refinement of dietary behaviors or enable accurate trophic reconstruction despite specimen-limited sampling, as is often the case for fossil assemblages.
Reconstructing the diet of extinct vertebrates is a challenging task, especially when direct evidence such as stomach contents or feeding traces is lacking. Geochemical studies using the 13C/12C ratio, the strontium/calcium (Sr/Ca) ratio, and the barium/calcium (Ba/Ca) ratio of the mineral fraction of the skeleton, bone, and enamel apatite have proved as useful as other methodologies, such as morphofunctional and microwear analyses (e.g., refs. 1–7). Nevertheless, the geochemical tools used in paleodietary studies provide only limited ecological scope when used on their own. For instance, although stable carbon isotopes provide valuable dietary information, the constraints are restricted to the type of vegetation ingested or rely on any intermediate consumer’s tissue imprinting a distinctive carbon isotopic signature (debate in refs. 8 and 9). Thus, there is a need to explore novel isotopic systems and to implement a multiproxy approach, to tackle the complex mechanisms behind biological fractionation and ultimately reconstruct diets of our extinct relatives. One potential approach is to consider the isotope composition of bio-essential metals present in large proportions in osseous tissues, i.e., calcium (δ44Ca) and magnesium (δ26Mg). This offers two additional advantages. First, diagenetic modification of the original isotope compositions is likely to be limited because it would require unrealistic isotope compositions or concentrations in diagenetic fluids. Second, only a few micrograms of dental enamel and bone are necessary for the measurement of the isotopic ratios, allowing the analysis of precious fossil materials.
Previous studies highlighted calcium isotope compositions as a promising source for dietary inferences (e.g., refs. 10 and 11) but evidence for a clear trophic level effect in terrestrial vertebrates is questionable (12) and requires further investigation, notably in the light of underlying biological processes (13).
Magnesium has been suspected of biological fractionation during the synthesis of chlorophyll (14), it is the second most abundant metal after calcium in biological apatite, and the measurement of its variability in mammal bone from various extant ecosystems permits the further assessment of its reliability as a potential new isotopic tracer of diet (15). Moreover, the concentration of Mg in teeth can be high (in the range of 1,000–10,000 ppm).
Here, tooth enamel from the terrestrial vertebrate assemblage of the equatorial forest at La Lopé National Park, Gabon, was sampled and measured for its δ26Mg, δ13C, Sr/Ca, Ba/Ca, and Mg/Ca ratios. Although our dataset is from extant species, and therefore not subject to diagenesis, we chose to sample tooth enamel to allow direct comparison with fossil tooth enamel data.
Except for the ecosystems of Ituri and Kahuzi-Biega, Democratic Republic of the Congo (16, 17), no other equatorial mammalian assemblage has been analyzed for tooth or bone δ13C variability. Among the 45 species of large mammals (>2 kg) recorded, La Lopé National Park contains several species of primates of differing feeding ecologies, including our closest relatives the gorilla and the chimpanzee (18). These mammals live in a complex environment dominated by deep canopy forests, interspersed with savannas and forested galleries (18). This mosaic environment has existed for at least 9 ky (19) and offers a complex and intermediate setting between a closed C3 equatorial forest and an open C4 savanna, available to animals otherwise living uniquely in C3 forests (18). It also offers a setting where the two types of photosynthetic pathways are available, C3 and C4, and therefore allows a direct comparison of the new δ26Mg results across the expected C3–C4 dichotomy highlighted by δ13C measurements.
Samples consisted of tooth enamel belonging to 49 individuals from 10 species of small, medium, and large mammals (Dataset S1), including two species of large primates (Gorilla g. gorilla and Mandrillus sphinx) with known feeding ecologies in La Lopé National Park (20, 21). The carcasses of these animals were collected during biological surveys within a 10-km radius from the research station over a period of 20 y, thus representing a single localized origin. Feces representing the diversity of mammals in this study were also collected during a 2011 field survey. Plant material consisting of leaves belonging to 11 genera was also analyzed for δ26Mg and δ13C. Finally, the bone and muscle of four sheep raised in captivity were analyzed to better characterize the mechanisms of Mg fractionation in the body.
Results
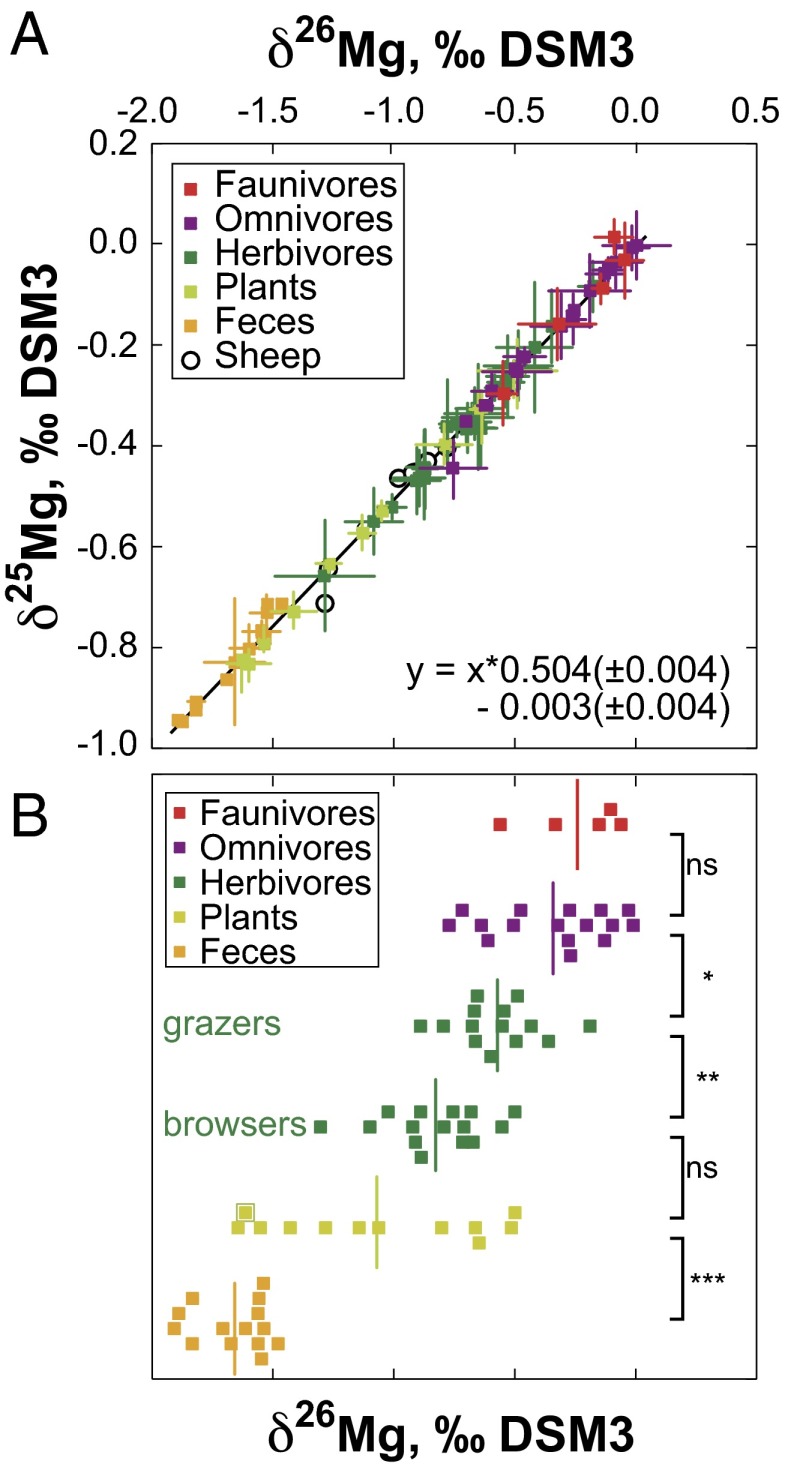
All of the samples fall on the Mg mass fractionation line (Fig. 1A and Dataset S1). An overall characteristic of this dataset is that mammal tooth enamel is 26Mg enriched relative to plants, whereas feces are depleted in 26Mg. Differences in δ26Mg, Sr/Ca, Ba/Ca, and Mg/Ca values between faunivores, omnivores, herbivores (grazers and browsers), and plants are summarized in Dataset S2. The total range of measured values for δ26Mg in tooth enamel is 1.34‰, from –1.29‰ to 0.05‰ (Dataset S1 and Fig. 1B). The highest values were measured in a group containing small faunivorous mammals, M. sphinx and Potamochoerus porcus (–0.76‰ to –0.05‰; n = 20), contrasting with the most negative values exhibited by Loxodonta cyclotis, G. g. gorilla, and duikers (range = –1.29‰ to –0.48‰; n = 18). Syncerus caffer nanus show intermediate Mg isotopic values overlapping with those of the other two groups (range = –0.87‰ to –0.17‰; n = 11). The total range of measured values for δ26Mg in plants is 1.12‰, from –1.6‰ to –0.48‰.
Fig. 1.
(A) δ26Mg and δ25Mg in all of the samples measured in the study. As expected from mass-dependent isotope fractionation, the slopes of the least-squares straight line fit to the data are close to 0.5. Error bars are 2 SD of the mean. (B) δ26Mg values grouped according to ecological category. Statistical differences between the δ26Mg values of the two groups were calculated using Kruskal–Wallis tests (Dataset S2). NS, nonsignificant P value; *P value = 0.01–0.05; **P value = 0.001–0.01; and ***P value <0.001. The C4 graminae plant sample is highlighted. Note the general tendency for Mg enrichment in heavier isotopes from feces to plants, herbivores, and omnivores/faunivores.
The total range of measured values for δ26Mg in feces is 0.33‰, from –1.49‰ to –1.82‰ (Dataset S1 and Fig. 1B). So far, feces from faunivores, omnivores, and herbivores have no distinct values and all represent the most 26Mg-depleted biological samples of the present dataset. The total range of measured values for δ26Mg in sheep is 0.53‰, with bone averaging –1.07‰ (n = 4) and muscle averaging –0.96‰ (n = 4).
Here, the environment of La Lopé displays a C3 influence comparable to that of Ituri forest (16, 17), but with a distinct C4 component highlighted by the analyzed Graminae possessing a δ13C value of –11.82‰, compared with the clustering of all other plants around –30‰. The total range of δ13C values for tooth enamel is 18.4‰, from –21.4‰ to –3‰. Two groups are distinct. S. caffer nanus (the forest buffalo) has the least negative values, ranging from –9.1‰ to –3‰. The rest of the mammals are all in a range from –21.9‰ to –12.5‰, with the exception of one outlier (G. g. gorilla) that clusters with the Syncerus dataset.
Elemental ratios for Sr/Ca, Ba/Ca, and Mg/Ca are provided in Dataset S1 and in Fig. 2 B–D. The log/log distribution of Sr/Ca and Ba/Ca ratios clusters along a straight line with a negative slope as in a previous study involving enamel analysis (3) (Fig. 2B).
Fig. 2.
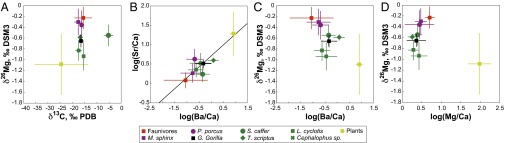
(A) δ26Mg vs. δ13C values showing that Mg and C isotopes bear independent ecological information. (B) log(Sr/Ca) vs. log(Ba/Ca), illustrating that the Ca biopurification process, which is responsible for the decrease of the Sr/Ca and Ba/Ca ratios up trophic chains, is at work at La Lopé. The regression line gives log(Sr/Ca) = 0.54 log(Ba/Ca) + 0.69, which is in accordance with that calculated at the Kruger National Park, using enamel [log(Sr/Ca) = 0.57 log(Ba/Ca) + 0.40] (3), but different from that calculated using bone [log(Sr/Ca) = 1.69 log(Ba/Ca) + 1.66] (15, 22). (C) δ26Mg vs. log(Ba/Ca), showing that omnivores lie between carnivores and herbivores. (D) δ26Mg vs. log(Mg/Ca), suggesting that Mg isotopic compositions and concentrations are not correlated. For all panels, error bars are 1 SD of the species means.
Discussion
Magnesium Isotopes in Plants As Related to Mammal Diet.
Mg has a central role in plant physiology and possibly fractionates during photosynthesis (14). A recent study showed enrichment in light Mg isotopes from the roots to the stems and to the leaves with a maximum ∆leaf-root of 0.65‰ (23). Thus, at the scale of a single plant, potentially different dietary sources with distinct δ26Mg values exist. In La Lopé, the analyzed plants fall in the δ26Mg range seen in other ecosystems (15, 23, 24) and cover the spectrum of values for mammal tooth enamel δ26Mg (below). Fruits, seeds, and roots are major constituents of the diet of many mammals (below). Although the Mg isotope composition of fruits and seeds is unknown, reported δ26Mg values for roots are high relative to those for leaves (23), and it could be hypothesized that herbivores focusing on such plant components would be indistinguishable from consumers at higher trophic positions. This could explain the relatively high δ26Mg values of P. porcus and M. sphinx but does not explain the high δ26Mg values of S. caffer nanus, not known for feeding on fruits, seeds, or roots. In our previous study it was uncertain whether a C4 photosynthetic pathway might be responsible for the relatively high δ26Mg values measured in osseous tissues of herbivores ingesting those plants, for example S. caffer (15). The present results show that this is unlikely, given the similar δ26Mg value of the graminae (a C4 plant) analyzed here to that of other C3 plants (Fig. 1B). The single sample of graminae measured in the mixed C3–C4 environment of La Lopé presents one of the lowest δ26Mg values of the plant dataset (–1.63‰). This value is inconsistent with the expectation that buffaloes source their Mg in a diet enriched in heavy Mg isotopes. However, it is currently premature to link the observed δ26Mg range in herbivorous mammals with the type (e.g., leaves, roots, fruits) or species of vegetation ingested and its potential effect on fractionation up the trophic chain.
The Ecological Significance of Magnesium Isotopes in Mammals.
Despite a comparatively larger variability in diet source for δ13C than for δ26Mg, there is much more variability in the measured enamel samples for δ26Mg than for δ13C. Here, all vertebrates, including faunivores, omnivores, and herbivores, with the exception of the buffaloes, indiscriminately overlap in δ13C, between –22‰ and –13‰ (Fig. 2A). The present dataset (Fig. 1B and Dataset S1) represents to our knowledge the first assessment of δ26Mg variability of mammalian tooth enamel in an equatorial ecosystem and adds to one previous study on two modern mammalian communities from South Africa (15). Here, the δ26Mg values allow the distinction of teeth from individuals representing taxa classified as omnivores and herbivores according to their known dietary behaviors as reported in the literature. Magnesium isotope values of herbivores are distributed in two groups (Fig. 1B and Dataset S2).
The first herbivore group has the most 26Mg-rich average values of all herbivores and is represented by the only grazer of the dataset: the forest buffaloes S. caffer nanus. Most individuals in this group present intermediate δ26Mg values, overlapping with those of the rest of the measured herbivores (elephants, bushbucks, duikers, and Gorilla) and also overlapping with the most negative δ26Mg values of the omnivore group. Only three buffaloes could be considered true outliers in having distinctly higher δ26Mg values than any other buffaloes. According to field observations at La Lopé (18), buffaloes eat graminae, which is consistent with the relatively high δ13C values of buffaloes seen in the present study (Fig. 2A). An intermediate δ26Mg value for buffaloes was also reported from a savanna ecosystem of South Africa, suggesting that either physiology or the type of plant ingested (namely C4) could be responsible for this value (15). Analysis of the Mg isotopic composition of buffaloes from a strict C3 context, such as buffaloes reported from the Ituri forest (16), would test whether these animals still stand out in δ26Mg value from other herbivores despite relying on different vegetation.
The second herbivore group possesses lower δ26Mg values and includes browsers: the forest elephant L. cyclotis, the bushbuck Tragelaphus scriptus, small duikers, and G. g. gorilla. The core (70%) of the diet of L. cyclotis consists of foliage and bark (25) and the diet of forest duikers mostly includes foliage and, when available, fruit fallen from the canopy (18). In La Lopé, G. g. gorilla relies primarily on fruit (40.9%) and leaves (33.8%) (13). Gorillas eat high-cellulose content food, which they can digest through a long gut passage (26). It is noteworthy that G. g. gorilla in La Lopé deliberately incorporates an average of 30% of ants in its diet (21). Whether this has any influence on δ26Mg values is unknown but should be explored because insects also make up a substantial part of the diet of hogs and mandrills, some of which display higher δ26Mg values. The analysis of the isotopic composition of consumer’s tissues, and comparison with the known ingested food, is needed.
The Mg isotope values of all herbivores (grazers and browsers) in the dataset differ significantly from those of the omnivores and faunivores (Dataset S2), which show the highest δ26Mg values of the present dataset (Dataset S1). Small faunivores are represented by Genetta sp. and are the heaviest of the dataset, overlapping with omnivores with nonsignificant differences. The omnivores analyzed here include P. porcus (i.e., hogs) and M. sphinx. The mandrill’s diet includes a large amount of fruit (46.7%) and seeds (34.4%) but comparatively very few leaves (5.7%) (18, 20). Moreover, its capacity to travel large distances makes it prone to incorporate a great variety of other foods, both vegetal and animal, and including insects (20). The diet of P. porcus is considered omnivore and also includes seeds (16, 18). Clearly, beyond the work of Bolou-Bi et al. (23), the Mg isotope systematics of plant organs (root, shoot, stem, fruit, and seed) need to be investigated to better understand whether the preferential consumption of a given plant component would lead to an omnivore, or even a herbivore, being indistinguishable from a faunivore.
Here, based on average values, omnivores can be distinguished from herbivores, using δ26Mg or Ba/Ca values (Fig. 2B) but not Sr/Ca or Mg/Ca values (Dataset S2). Choosing enamel over bone for paleodietary investigations reduces the risk of diagenetic alteration due to the higher crystallinity and hence lower solubility of apatite crystals. That Sr/Ca ratios measured in enamel are less sensitive to trophic level effect than Ba/Ca ratios has previously been demonstrated (2) and, unlike in bone, Sr/Ca ratios from enamel do not distinguish browsers from carnivores. Our results show that it is possible to discriminate browsers from omnivores, using δ26Mg in enamel (Dataset S2); thus δ26Mg is complementary to Ba/Ca ratios, and where Sr/Ca becomes uninformative, δ26Mg can be used instead (Fig. 2C). In addition, the Mg/Ca ratio does not reveal any relevant ecological patterning, with or without consideration of the Mg isotopic ratio (Fig. 2D), confirming early observations (27). Finally, omnivores cannot be distinguished from faunivores due to the small number of specimens analyzed for the latter group (n = 5; Dataset S2). Despite an obvious 26Mg enrichment up the trophic chain (Fig. 2B), the enamel δ26Mg values do overlap between ecological groups. This can be explained in several ways. First, as already mentioned above, dietary preference among herbivores and omnivores can enhance Mg isotopic variability, because plants exhibit Δ26Mgleaf-organ ∼ 0.6‰. Second, the bulk Mg isotope composition of an individual animal body most probably varies according to metabolic and dietary changes. For instance, aging is a metabolic change leading to a bulk isotopic drift when inward (diet) and outward (feces, next section) fluxes are not isotopically balanced. Such a mechanism has been proposed for zinc and copper isotopes (28, 29) in mammals. Dietary changes in Mg isotopes could occur in mammals during, for instance, weaning, because milk may have a Mg isotopic “signature” similar to that of Ca isotopes (30). Third, we tried as far as possible to collect a specific tooth type, but this was not always possible (Dataset S1). If the so-called isotopic heterogeneities discussed above are recorded in enamel, the disparate sampling of teeth could be an extra source of Mg isotopic variation among ecological groups.
Biological Fractionation of Magnesium Isotopes in Mammals.
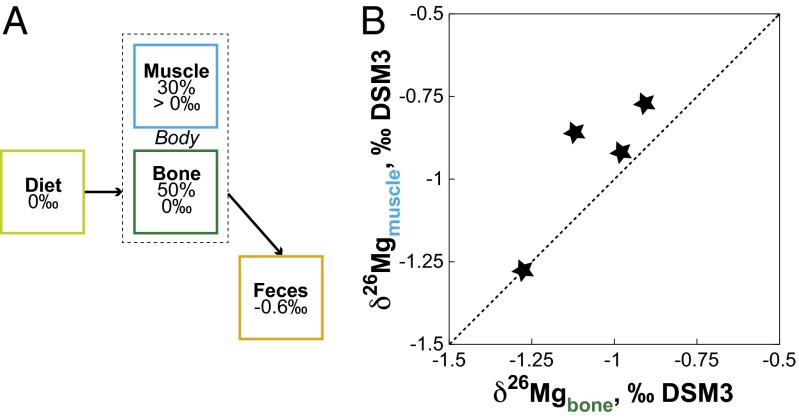
The present observations demonstrate that δ26Mg increases up the trophic chain, building on a previous study (15). This calls for an attempt at explaining the biological mechanisms at work at the scale of the mammalian body that result in trophic-level effects. To gain insights into the Mg isotopic balance in the body, we have collected feces because they represent the main Mg outward flux, plants being the inward Mg flux for herbivores. Feces are the most 26Mg-depleted biological material (–1.65‰), even more than most plants from the same ecosystem (–1.05‰), implying that during digestion light Mg is preferentially excreted whereas heavy Mg is preferentially assimilated. Using this basic observation, we can construct a very simple box model of Mg isotopes for mammals (Fig. 3A). The observed difference between diet and feces in δ26Mg values, and the seemingly equal δ26Mg value of bone with diet, implies that the mammalian body is isotopically unbalanced and requires an additional reservoir where δ26Mg values have to be higher than for bone (Fig. 3A). An inventory of Mg in mammals reveals that ∼50% of dietary Mg ends up in bone, ∼30% in muscle, and ∼20% in other soft tissues (31). Thus, muscles represent a possible candidate for a 26Mg-enriched reservoir in the mammalian body. To test this hypothesis, we measured the δ26Mg value of muscle and bone in four sheep specimens. The results show that muscles are slightly more 26Mg enriched than bone (Fig. 3B). Because mammals excrete isotopically light Mg material at every step of the trophic chain, all that remains in the body is Mg that is 26Mg enriched relative to the diet, and this includes edible parts readily accessible to higher-level consumers.
Fig. 3.
(A) Schematic box model of Mg isotopes in the body. For a given diet δ26Mg value, enamel is fractionated by +0.3‰, but the bone δ26Mg value remains unchanged because the fractionation between enamel and bone equals approximately –0.3‰ (15). The feces δ26Mg value is fractionated by –0.6‰ relative to diet, leaving the body isotopically unbalanced. Muscle (30% of body Mg) is a likely candidate to be the complementary 26Mg-enriched reservoir. (B) According to the box model, the δ26Mg value of muscle must be higher than that of bone. Measurements of δ26Mg in muscle and bone in four sheep confirm this hypothesis.
The physiology of Mg isotope fractionation needs to be elucidated, but an active mechanism could be transport through enterocytes, as already suspected for other metals (32). Potentially, this can have important implications for the difference in fractionation observed between browsers and grazers, the latter presenting more 26Mg-enriched values. Linking digestive physiology to Mg isotope values remains tentative until experimental studies exploring the processes of biological fractionation of Mg in mammals are conducted. Physiology appears to be a major determinant of Mg isotope fractionation in mammal tissues. As seen above, dietary Mg is equally partitioned between bone and soft tissues, which implies that several fractionation processes may explain the natural variation of magnesium isotopes. This contrasts with Ca, which ends up almost exclusively in bone (>99%). In addition, Ca is enriched in light isotopes up trophic chains whereas Mg, as shown here and in ref. 15, is enriched in heavy isotopes. Finally, the type of food ingested, in addition to potential intraseasonal variations, might still be responsible for the overlap between inferred trophic levels. For sure, Mg isotopes may not be used on their own but a multiproxy approach is warranted, using not only traditional tracers of diet such as δ13C and Ba/Ca, but also new systems (e.g., Ca) for which large reference datasets are still needed.
Conclusion and Perspectives
In this study, we provide to our knowledge the first dataset for Mg isotopic composition of tooth enamel from an extant equatorial mammalian assemblage and compare it with other geochemical proxies of known utility in ecological studies (Sr/Ca vs. Ba/Ca and δ13C). The main result is a general trend of increasing δ26Mg values from herbivores to omnivores. Our first results seem to indicate that soft tissues of the mammalian body become enriched in heavy Mg isotopes whereas the light isotopes are preferentially excreted in feces. Therefore, physiology appears to be a major determinant of Mg isotope fractionation in mammal tissues, but the type of food ingested might still be also responsible for the overlap between inferred trophic levels. Additional care must be taken to select as many specimens as possible of a single species. This will allow an assessment of species or consumer group δ26Mg variability and the placement of key taxa, for which available specimens are limited, in their ecological context. Moreover, δ26Mg adds substantial information to the already known and widely applied δ13C, allowing the discrimination of omnivores from some herbivores. Application of δ26Mg in paleoanthropology might add relevant data, especially given that interpretations gathered from the only other isotopic trophic tracer to be preserved in tooth enamel and to resist diagenesis (δ13C) still generate intense debates on the nature of our ancestor’s diet (e.g., refs. 8 and 9).
Methods
Sample Collection.
Vertebrate samples consisted exclusively of tooth enamel from extant mammals originating from La Lopé National Park, Gabon, and were collected and curated during a period of 20 y in a radius of 10 km around the Station d’Étude des Gorilles et des Chimpanzés (SEGC) (Dataset S1). The enamel surface was sampled with a diamond drill bit. Feces were collected in a 2-km radius around the SEGC during August 2011, following visual sightings (mandrills, elephants, and buffaloes) or during field surveys (duikers and hogs). Plant leaves were selected in a 2-km radius around the SEGC at the level of human reach to represent potential food material available to herbivorous mammals present in the area.
Carbon and Oxygen Analytical Technique.
Powdered enamel was sent to Iso-Analytical for sample preparation and analysis of δ13C and δ18O on tooth carbonate. Samples and reference carbonates were weighed into clean Exetainer tubes and the tubes placed in a drying oven for 24 h to remove moisture. Once the samples were dry, septum caps were fitted to the tubes. The tubes were then flushed with 99.995% helium. Phosphoric acid was then added to the samples for overnight digestion (33), allowing complete conversion of carbonate to CO2.
The CO2 gas liberated from samples was then analyzed by continuous flow–isotope ratio mass spectrometry (CF-IRMS). Carbon dioxide was sampled from the Exetainer tubes into a continuously flowing He stream, using a double-holed needle. The CO2 was resolved on a packed column gas chromatograph and the resultant chromatographic peak carried forward into the ion source of a Europa Scientific 20-20 IRMS, where it was ionized and accelerated. Gas species of different mass were separated in a magnetic field and then simultaneously measured using a Faraday cup collector array to measure the isotopomers of CO2 at m/z 44, 45, and 46.
The reference materials used during analysis of the samples were IA-R022 (Iso-Analytical working standard calcium carbonate, δ13CV-PDB = –28.63‰ and δ18OV-PDB = –22.69‰), NBS-18 (carbonatite, δ13CV-PDB = –5.01‰ and δ18OV-PDB = –23.2‰), and NBS-19 (limestone, δ13CV-PDB = +1.95‰ and δ18OV-PDB = –2.2‰). By using three carbonate reference materials treated in the same way as the samples, the need to apply corrections for temperature-dependent isotopic fractionation is removed. NBS-18 and NBS-19 are interlaboratory comparison standards distributed by the International Atomic Energy Agency. IA-R022 has been calibrated against and is traceable to NBS-18 and NBS-19.
Magnesium Analytical Technique.
Powdered samples of tooth enamel apatite were dissolved in 1 mL double-distilled concentrated HNO3 overnight, then evaporated, and redissolved in 350 μL 2.0 M HNO3. Feces were attacked in double-distilled concentrated HNO3 for 3 d on a hotplate, evaporated, redissolved in H202, evaporated, and redissolved in double-distilled concentrated HNO3, using a microwave digestion at École Normale Supérieure (ENS) de Lyon. Plant leaves were dried in an oven at a temperature of 60 °C for 48 h and then milled into powder, using liquid nitrogen and an agate mortar. Powdered plant was weighed and dissolved in 1 mL double-distilled concentrated HNO3 overnight. To break down the organic matter, this aliquot experienced an extra dissolving step, using an autoclave asher at the University of Bristol (3 h at a temperature of 300 °C and a pressure of 90 bar).
An aliquot of all solutions was taken for concentration analysis of Mg and other elements. This was done on an Element 2 inductively coupled plasma mass spectrometer (ICP-MS) by peak-height comparison with an in-house standard. A secondary standard with a similar matrix to the samples was not available for this study, but Mg, Ca, and Sr concentrations on the river standard SLRS-5 reproduce to 7–8% (2 SD) and are accurate relative to certified values to 3%.
Magnesium was separated from the remaining solution, using cation-exchange resin (AG-50WX-12, 200–400 mesh) with ultrapure 2.0 M HNO3 as the elution agent (details in ref. 34). To avoid isotopic fractionation during column separation, over 99% of the Mg was collected. This was verified by collecting and measuring the Mg content of a separate fraction of the eluant before and after the Mg elution peak.
The purified Mg fraction was measured for Mg isotopes on a Thermo Neptune Multicollector ICP-MS at the University of Bristol, using an Apex-Q for sample introduction (35). Purified apatite and plant samples were diluted in 2% HNO3 to a concentration of 100 ppb. Measurements were conducted at low resolution and each analysis consisted of 20 4-s integrations of the 24Mg, 25Mg, and 26Mg signals in static mode. Each analysis was preceded by a measurement of blank 2% HNO3, and the signals for this background were subtracted from the analyte signal before calculation of isotope ratios. Measurements of purified feces samples were conducted on a Nu instrument ICP-MS at the Laboratoire de Géologie de Lyon: Terre, Planètes, et Environnement, ENS de Lyon. Samples were diluted in 2% HNO3 to a concentration of 100 ppb. Delta values were obtained via bracketing analyses of DSM-3 (36), with CAM-1 used as a secondary standard. In each analytical session, samples (and occasional CAM-1) bracketed by DSM-3 were measured in turn. This sequence was then repeated three more times so that four separate analyses of the same sample solution were obtained. The uncertainties reported in Dataset S1 represent 2 SD of these four analyses.
δ26Mg and δ25Mg values presented for samples in Dataset S1 are defined as
For δ26Mg uncertainties mostly range between 0.01‰ and 0.16‰ and for δ25Mg between 0.01‰ and 0.18‰. The CAM-1 standard (n = 74 over a period of around 18 mo) yielded a mean δ26Mg of –2.57‰ ± 0.16‰ (2 SD) and a mean δ25Mg of –1.33‰ ± 0.10‰. These are close to published values, e.g., δ26Mg = –2.63‰ ± 0.05‰ (29). The δ26Mg and δ25Mg values obtained for all samples and standards measured in this study lie on a line (R2 = 0.997) with a slope of 0.5155, close to the theoretical value of 0.5.
Supplementary Material
Acknowledgments
This work would not have been possible without the support of E. Sarroca and G. Moussavou at Station d’Étude des Gorilles et Chimpanzés (SEGC), La Lopé, Gabon, as well as numerous persons at Centre International de Recherches Médicales de Franceville, Gabon, including J-P. Gonzales, M. Bourgarel, and R. Onanga. We thank the people in Park National de la Lopé, Gabon. The osteological collection at SEGC was built through the collecting efforts of C. Tuttin, K. Abernethy, L. White, and K. Jeffery. P. Pogge von Strandmann is thanked for setting up Mg isotope extraction in teeth in the initial part of the project. C. Coath provided much helpful assistance in the specimen laboratory. The sampling was conducted under the following Gabonese authorizations (to J.E.M.): Autorisation de recherche du Centre National de la Recherche Scientifique et Technologique no. AR0026/11/MENESSIC/CENAREST/CG/CST/CSAR and Autorisation d’entrée de l’Agence Nationale des Parcs Nationaux no. 000013/PR/ANPN/SE/CS/AEPN. J.E.M. was funded by a Marie-Curie Fellowship, FP7 framework (Project 273121: the Significance of Stable Isotopes as Dietary Indicators in Ancient Terrestrial Ecosystems). V.B. thanks the Fondation Mérieux and Fondation Bullukian for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417792112/-/DCSupplemental.
References
- 1.Ungar PS, Sponheimer M. The diets of early hominins. Science. 2011;334(6053):190–193. doi: 10.1126/science.1207701. [DOI] [PubMed] [Google Scholar]
- 2.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sponheimer M, Lee-Thorp JA. Enamel diagenesis at South African Australopith sites: Implications for paleoecological reconstruction with trace elements. Geochim Cosmochim Acta. 2006;70:1644–1654. [Google Scholar]
- 4.Balter V, et al. Were Neandertalians essentially carnivores? Sr and Ba preliminary results of the mammalian palaeobiocoenosis of Saint-Césaire. C R Acad Sci IIA. 2001;332(1):59–65. [Google Scholar]
- 5.Balter V, Braga J, Télouk P, Thackeray JF. Evidence for dietary change but not landscape use in South African early hominins. Nature. 2012;489(7417):558–560. doi: 10.1038/nature11349. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey LT, Dean MC, Jeffries TE, Penn M. Unlocking evidence of early diet from tooth enamel. Proc Natl Acad Sci USA. 2008;105(19):6834–6839. doi: 10.1073/pnas.0711513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin C, et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498(7453):216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontes-Villalba M, Carrera-Bastos P, Cordain L. African hominin stable isotopic data do not necessarily indicate grass consumption. Proc Natl Acad Sci USA. 2013;110(43):E4055. doi: 10.1073/pnas.1311461110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sponheimer M, et al. Reply to Fontes-Villalba et al.: On a reluctance to conjecture about animal food consumption. Proc Natl Acad Sci USA. 2013;110(43):E4056. doi: 10.1073/pnas.1314368110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skulan J, DePaolo DJ, Owens TL. Biological control of calcium isotopic abundances in the global calcium cycle. Geochim Cosmochim Acta. 1997;61:2505–2510. [Google Scholar]
- 11.Skulan J, DePaolo DJ. Calcium isotope fractionation between soft and mineralized tissues as a monitor of calcium use in vertebrates. Proc Natl Acad Sci USA. 1999;96(24):13709–13713. doi: 10.1073/pnas.96.24.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melin AD, et al. Calcium and carbon stable isotope ratios as paleodietary indicators. Am J Phys Anth. 2014;154(4):633–643. doi: 10.1002/ajpa.22530. [DOI] [PubMed] [Google Scholar]
- 13.Tacail T, Albalat E, Télouk P, Balter V. A simplified protocol for measurement of Ca isotopes in biological samples. J Anal At Spectrom. 2013;29(3):529–535. [Google Scholar]
- 14.Black JR, Yin Q, Casey WH. An experimental study of magnesium-isotope fractionation in chlorophyll-a photosynthesis. Geochim Cosmochim Acta. 2006;70:4072–4079. [Google Scholar]
- 15.Martin JE, Vance D, Balter V. Natural variation of magnesium isotopes in mammal bones and teeth from two South African trophic chains. Geochim Cosmochim Acta. 2014;130:12–20. [Google Scholar]
- 16.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004;138(1):5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 17.Levin NE, Simpson SW, Quade J, Cerling T, Frost S. The Geology of Early Humans in the Horn of Africa, Geological Society of America Special Papers. Geol Soc Am; 2008. pp. 215–234. [Google Scholar]
- 18.Tutin CEG, White LJT, Mackanga-Missandzou A. The use by rain forest mammals of natural forest fragments in an equatorial African savanna. Conserv Biol. 1997;11:1190–1203. [Google Scholar]
- 19.Peyrot B, et al. Les paléoenvironnements de la fin du Pléistocène et de l’Holocène dans la réserve de la Lopé (Gabon): Approche par les indicateurs géomorphologiques, sédimentologiques, phytologiques, géochimiques et anthropogènes des milieux enregistreurs de la dépression de la Lopé [Late Pleistocene and Holocene paleoenvironments in the reserve of La Lopé (Gabon): an approach using geomorphological, sedimentological, phytological, geochemical and anthropogenic indicators of the recording environments in the depression of La Lopé] Anthropologie. 2003;107:291–307. [Google Scholar]
- 20.Lahm SA. Diet and habitat preference of Mandrillus sphinx in Gabon: Implications of foraging strategy. Am J Primatol. 1986;11(1):9–26. doi: 10.1002/ajp.1350110103. [DOI] [PubMed] [Google Scholar]
- 21.Tutin CEG, Fernandez M. Composition of the diet of chimpanzees and comparisons with that of sympatric lowland gorillas in the Lopé reserve, Gabon. Am J Primatol. 1993;30(3):195–211. doi: 10.1002/ajp.1350300305. [DOI] [PubMed] [Google Scholar]
- 22.Balter V. Allometric constraints on Sr/Ca and Ba/Ca partitioning in terrestrial mammalian trophic chains. Oecologia. 2004;139(1):83–88. doi: 10.1007/s00442-003-1476-0. [DOI] [PubMed] [Google Scholar]
- 23.Bolou-Bi EB, Poszwa A, Leyval C, Vigier N. Experimental determination of magnesium isotope fractionation during higher plant growth. Geochim Cosmochim Acta. 2010;74:2523–2537. [Google Scholar]
- 24.Bolou-Bi EB, Vigier N, Poszwa A, Boudot J-P, Dambrine E. Effects of biogeochemical processes on magnesium isotope variations in a forested catchment in the Vosges Mountains (France) Geochim Cosmochim Acta. 2012;87:341–355. [Google Scholar]
- 25.White LJT, Tutin CEG, Fernandez M. Group composition and diet of forest elephants, Loxodonta africana cyclotis Matschie 1900, in the Lopé Reserve, Gabon. Afr J Ecol. 1993;31(3):181–199. [Google Scholar]
- 26.Remis MJ, Dierenfeld ES. Digesta passage, digestibility and behavior in captive gorillas under two dietary regimens. Int J Primatol. 2004;25:825–845. [Google Scholar]
- 27.Klepinger LL. Magnesium ingestion and bone magnesium concentration in paleodietary reconstruction: Cautionary evidence from an animal model. J Archaeol Sci. 1990;17:513–517. [Google Scholar]
- 28.Balter V, et al. Bodily variability of zinc natural isotope abundances in sheep. Rapid Commun Mass Spectrom. 2010;24(5):605–612. doi: 10.1002/rcm.4425. [DOI] [PubMed] [Google Scholar]
- 29.Jaouen K, et al. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics. 2013;5(8):1016–1024. doi: 10.1039/c3mt00085k. [DOI] [PubMed] [Google Scholar]
- 30.Chu NC, Henderson GM, Belshaw NS, Hedges REM. Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl Geochem. 2006;21:1656–1667. [Google Scholar]
- 31.Elin RJ. Assessment of magnesium status. Clin Chem. 1987;33(11):1965–1970. [PubMed] [Google Scholar]
- 32.Jaouen K, Pons ML, Balter V. Iron, copper and zinc isotopic fractionation up mammal trophic chains. Earth Planet Sci Lett. 2013;374:164–172. [Google Scholar]
- 33.Coplen TB, Kendall C, Hopple J. Comparison of stable isotope reference samples. Nature. 1983;302:236–238. [Google Scholar]
- 34.Pogge von Strandmann PAE. Precise magnesium isotope measurements in core top planktic and benthic foraminifera. Geochem Geophys Geosystems. 2008;9(12):Q12015. [Google Scholar]
- 35.Foster GL, Pogge von Strandmann PAE, Rae JWB. Boron and magnesium isotopic composition of seawater. Geochem Geophys Geosyst. 2010;11(8):Q08015. [Google Scholar]
- 36.Galy A, et al. Magnesium isotope heterogeneity of the isotopic standard SRM980 and new reference materials for magnesium-isotope-ratio measurements. J Anal At Spectrom. 2003;18:1352–1356. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.