Significance
The emergence of human infection with a novel H7N9 avian influenza reassortant in China raises a pandemic concern. However, it is not fully understood how these H9N2 chicken viruses facilitated the genesis of the novel H7N9 viruses. Here we show that a “fittest” genotype (G57) emerged with changed antigenicity and improved adaptability in chickens. It became predominant in vaccinated farm chickens and caused widespread outbreaks before the H7N9 virus emergence, increasing reassortment between H9N2 and other subtype viruses and finally providing all of their internal genes to the novel H7N9 viruses. The prevalence and variation of H9N2 influenza virus in farmed poultry could provide an important early warning of the emergence of novel reassortants with pandemic potential.
Keywords: H9N2, H7N9, chicken influenza virus, genotype, infectivity
Abstract
The emergence of human infection with a novel H7N9 influenza virus in China raises a pandemic concern. Chicken H9N2 viruses provided all six of the novel reassortant’s internal genes. However, it is not fully understood how the prevalence and evolution of these H9N2 chicken viruses facilitated the genesis of the novel H7N9 viruses. Here we show that over more than 10 y of cocirculation of multiple H9N2 genotypes, a genotype (G57) emerged that had changed antigenicity and improved adaptability in chickens. It became predominant in vaccinated farm chickens in China, caused widespread outbreaks in 2010–2013 before the H7N9 viruses emerged in humans, and finally provided all of their internal genes to the novel H7N9 viruses. The prevalence and variation of H9N2 influenza virus in farmed poultry could provide an important early warning of the emergence of novel reassortants with pandemic potential.
Human infection with a novel avian-origin H7N9 influenza A virus causing severe respiratory symptoms and mortality was first reported in eastern China in March 2013 (1). To date, the novel virus has caused two outbreaks of human infection, including 375 known cases and 115 deaths as of 11 March 2014 (2). Phylogenetic analysis suggests that the virus is a triple reassortant of H7, N9, and H9N2 avian influenza viruses (3, 4). The H7 and N9 genes may have been transferred from migratory birds to domestic ducks and then to chickens in the live poultry markets (3–5), after which reassortment with enzootic H9N2 viruses formed the H7N9 viruses identified in humans (3–5).
H9N2 influenza virus has low pathogenicity for avians, replicating mainly in the upper respiratory tract and causing mild or no overt signs of illness in specific pathogen-free (SPF) chickens (6). In 1994, the H9N2 subtype was first identified in chicken farms in the Guangdong province of south China (7); it has since become widespread in chickens and has caused great economic loss from reduced egg production and highly lethal coinfections (8–11). To reduce the impact of H9N2 infection in chickens, the flocks have been vaccinated since 1998 with commercial inactivated vaccines, such as A/chicken/Guangdong/SS/1994 (Ck/GD/SS/94), A/chicken/Shandong/6/1996 (Ck/SD/6/96), and A/chicken/Shanghai/F/1998 (Ck/SH/F/98) (8, 12, 13). These H9N2 vaccines initially limited the outbreaks and virus spread. However, despite multiple doses, the H9N2 vaccines became less effective, especially after 2007, and H9N2 influenza virus continues to circulate in vaccinated chicken flocks and has caused sporadic disease outbreaks (8, 10, 12–20). However, the recent prevalence and molecular evolution of the H9N2 viruses in chickens especially in the flocks receiving large-scale vaccination, and their role in the emergence of human H7N9 virus, are not fully understood. In this study, we systematically investigated the prevalence and evolution of H9N2 viruses mainly focusing on farm chickens and their role in the genesis of the novel H7N9 viruses.
Results
Increased Isolation of H9N2 Viruses from Farm Chickens Preceded the Outbreak of H7N9 Infection in Humans.
We retrospectively investigated the infection of farm chickens with H9N2-subtype influenza viruses, mainly in northeast China, from January 2010 to December 2013 (Fig. 1 and Table S1). Tracheal swabs or lung samples were taken for virus isolation and identification from 1975 chicken farms reporting illness in 19 provinces during 2010–2013. All of these chickens had been vaccinated against H9N2 virus but showed respiratory disease signs and/or a 5–20% reduction in egg production. Of the 1975 farms, 637 were positive for H9N2 infection, with a virus isolation rate as high as 32.25% (Table S1). Mean H9N2 isolation rates increased annually, from 22.08% in 2010 to 47.08% in 2013. Further, these H9N2 infections occurred even during the warm season (July through September), when influenza infection is usually reported less often (Fig. 1). Despite vaccination, H9N2 virus infection in the investigated regions increased dramatically after 2010, indicating an antigenic variant caused an outbreak in farm chickens that was maximal before and during the 2013 emergence of the novel H7N9 virus in humans.
Fig. 1.
Isolation rate (%) of H9N2 influenza viruses during 2010–2013 on chicken farms reporting illness in our investigated regions. Histogram indicates monthly isolation rates; red horizontal line (connected with dots) indicates mean annual isolation rates; and gray horizontal dashed line indicates 95% CI.
Reduced Genetic Diversity of H9N2 Viruses in Chickens During 2010–2013 Indicates Widespread Outbreak.
Widespread influenza outbreaks caused by antigenic drift variants are usually thought to be characterized by reduced genetic diversity of the viruses, either genome-wide or in the hemagglutinin (HA) segment (21–23). Thus, a widespread outbreak can be reasonably inferred by high sequence similarity of viruses isolated during a specified time span across a broad region. We wondered whether other regions beyond our investigation were also affected. To address this question, we analyzed the temporal dynamics of genome-wide sequence similarities of H9N2 viruses by collecting all available H9N2 sequences from chicken viruses in China during 1994 through 2013. Pairwise identity comparison showed a decrease in diversity in all segments of these viruses, starting mainly in 2009–2010 and reaching a peak during 2010–2013 (Fig. 2 and Fig. S1). Somewhat less similarity was found in the HA genes of 2011 viruses and the neuraminidase (NA) genes of 2013 viruses. The decreased sequence diversity in almost all segments at approximately the same time point suggests that a virus with the greatest fitness was selected, gained dominance, and caused a widespread outbreak in chickens throughout China. A genetic “bottleneck” is proposed in influenza virus evolution (22). This is the first observation, to our knowledge, of such a bottleneck in the H9N2 viruses (Fig. 2 and Fig. S1), despite their prevalence in China for the last 20 y.
Fig. 2.
Reduced genetic diversity of the eight segments of H9N2 influenza viruses isolated from chickens during the widespread outbreaks in China (2010–2013). Pairwise comparison of nucleotide sequence of all H9N2 chicken viruses is plotted as a heatmap, if a given pair of sequences shares more than 50% of the full length. Viruses isolated from 1994 through 2013 are ordered by isolation time from left to right on the x axis and from top to bottom on the y axis. Ticks on the axes indicate the isolation years. Red dotted line indicates the year 2010.Color indicates identity levels from ≤90% (red) to 100% (blue) or identity levels unavailable due to the shared sequence length less than 50% (white).
Genotype G57 of H9N2 Influenza Virus Became Predominant in Chickens Since 2010.
To understand the phylogenetic evolution of the prevalent H9N2 viruses, we constructed phylogenetic trees for all of the eight segments. Phylogenetic trees were automatically partitioned into clusters (24) using a 20th percentile cutoff. Clades were then manually merged, if necessary, based on the branching posterior and reported classification (19, 20, 25). In each tree of eight segments, most of the 2010–2013 viruses belonged to the same clade (Fig. 3A and Fig. S2). Within each prevalent clade, the majority of 2010–2013 viruses further formed a major group with few early viruses (Fig. 3A and Fig. S2), indicating they shared the common ancestor surviving the bottleneck event. In the polymerase basic 2 (PB2), NP (nucleoprotein), HA, NA, and nonstructural (NS) genes, a minor population of the prevalent viruses formed a different clade (HA) or minor group (HA and other segments). On the basis of clade classification, 69 genotypes were found in H9N2 chicken viruses in China from 1994 through 2013 (Dataset S1). Most of the 2010–2013 viruses were from a single genotype classified as genotype 57 (G57). G57 virus was first detected in chickens in Jiangxi and Jiangsu provinces in 2007, and its prevalence increased sharply during 2009. Since 2010, G57 has been the predominant genotype in circulation throughout China (Dataset S1 and Fig. 3B).
Fig. 3.
Phylogenetic analysis of H9N2 influenza viruses isolated from chickens during 1994–2013 and H7N9 viruses isolated from humans in China during the first 2 wk of the 2013 outbreak. (A) Phylogenetic trees of surface genes with the H9N2 chicken viruses and those of internal genes with the chicken H9N2 and the novel H7N9 human viruses. Internal gene trees with only H9N2 viruses are not shown here due to the same topology. Clades with most of 2010–2013 viruses were fully shown, and others were collapsed (indicated by gray triangles). Color of line at right of each leaf node indicates year of isolation (see color bar). Timescale is in years. Vertical black lines mark major (long line) and minor (short line) groups of H9N2 viruses, mainly from 2010 through 2013. Black arrows mark the position of H7N9 viruses. Also see Fig. S2. (B) Diversity of genotypes of H9N2 viruses isolated from chickens in China, 1994–2013. Here are shown the recurrent genotypes that were persistent across at least 2 y. All of the genotypes can be found in Dataset S1. (C) Summary of the genesis of genotype G57 by reassortment events. Virus particles are represented by ovals. The eight gene segments are horizontal bars (from the top: PB2, PB1, PA, HA, NP, NA, M, and NS). Red segments are clades of G57; and blue segments are other clades. A broken arrow indicates an interspecies gene transmission. Ck/BJ/94, A/chicken/Beijing/1/1994; Qa/HK/97, A/quail/HongKong/G1/1997; Ck/SH/98, A/chicken/Shanghai/F/1998; Ck/JS/00, A/chicken/Jiangsu/1/2000; Ck/HuN/05, A/chicken/Hunan/5260/2005; Dk/ST/04, A/duck/Shantou/163/2004; Ck/ZJ/07, A/chicken/Zhejiang/HJ/2007 (G57 genotype). Another G57 virus (A/chicken/Jiangxi/12/2007) detected in 2007 is not shown.
This predominant genotype was generated by continued reassortment that can be traced to the year 1994 (Fig. 3C). The G57 NA gene was derived from the A/chicken/Beijing/1/1994-like lineage. In 1998, four new lineages of the polymerase basic (PB1), polymerase acidic (PA), NP, and NS genes were produced in a chicken virus, A/chicken/Shanghai/F/1998 (SH/98), which caused a severe outbreak in chickens in eastern China (26). After 1998, the SH/98-like virus reassorted with the A/quail/Hong Kong/G1/1997-like and A/chicken/Jiangsu/1/2000-like viruses, introducing matrix (M) and the HA genes to generate the A/chicken/Hunan/5260/2005-like virus in 2005. The virus had seven of the eight gene segments of the G57 genotype. Then the final segment, PB2, was transmitted from ducks (A/duck/Shantou/163/2004-like) to chickens, where it formed the predominant G57 genotype in 2007. Since 2007, G57 viruses have spread to domestic aquatic birds, wild birds, and swine (Dataset S1); in 2014, this genotype was isolated from a human patient in Hong Kong (Dataset S1), indicating a wide range of infected hosts.
Clearly, the widespread outbreak in 2010–2013 established the single dominant G57 genotype in chickens, ending more than 10 y of cocirculation of multiple genotypes and producing numerous H9N2 viruses carrying the same gene constellation.
Improved Infectivity of G57 H9N2 Viruses in Chickens.
Reassortment seems advantageous because it can rapidly create high fitness genotypes (27). To determine the effect of reassortment on the infectivity of G57, we selected nine representative H9N2 chicken viruses from G57 or other genotypes to test their replication in inoculated and contact chickens (Fig. 4, Top, A–C). Specifically, four 2010–2011 viruses were from G57, one virus was from G68 (only with different HA segment compared with G57) isolated in 2011, and four viruses were from other major genotypes circulating before 2010. After inoculation, although all of the tested viruses had a 100% isolation rate in tracheas (Fig. 4A), most of the G57 viruses replicated to significantly higher titers than did the earlier viruses of other genotypes (titers 10–100 times as high; P < 0.05; Fig. 4B). The G57 viruses also showed clearly enhanced replication in the cloaca, to titers 10–1,000 times as high as those of earlier isolates (Fig. 4B), explaining the 100% detection rate of all inoculated G57 viruses but only 20–60% of inoculated earlier viruses (Fig. 4A). In addition, most of the G57 viruses were shed from the cloaca for a longer period [until 7 days postinfection (dpi); Fig. 4C]. The replication ability of G68 virus was moderate between G57 viruses and other genotypes of earlier viruses (Fig. 4 A–C). Similar to its replication in inoculated chickens, G57 exhibited improved infection with higher viral titers also in contact chickens followed by the G68 virus, although there was a 100% isolation rate among different genotypes except for G02 in the contact chickens. (Fig. 4 A–C). These results suggest this improved adaptation in chickens may be one reason for the widespread outbreak of G57 H9N2 viruses during 2010–2013.
Fig. 4.
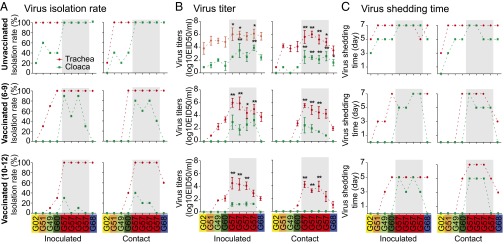
Infection of H9N2 chicken viruses in unvaccinated and vaccinated chickens. Nine representative H9N2 chicken viruses were selected from G57 or other genotypes to test their infection in inoculated and contact chickens. As shown in the figure, from left to right on each x axis, they are A/chicken/Beijing/3/1999 (G02); A/chicken/Hebei/0617/2007 (G51); A/chicken/Shandong/22/2008 (G49); A/chicken/Shandong/ZB/2007 (G60); A/chicken/Jiangsu/TS/2010 (G57); A/chicken/Hebei/YT/2010 (G57); A/chicken/Shandong/sd01/2010 (G57); A/chicken/Shandong/06/2011 (G57); and A/chicken/Guangdong/01/2011 (G68). Chickens (n = 10 per group) were inoculated intranasally with 1× 106 EID50 of each virus to test viral replication. After 24 h, five chickens were placed in physical contact with inoculated birds to test viral transmission. Tracheal (red dashed line) and cloacal (green dashed line) swabs were collected at 3, 5, and 7 dpi for virus detection and/or titration. All of the infection experiments were carried out on unvaccinated (Top) and vaccinated chickens (Middle, with antibody titers of 6–9; Bottom, with antibody titers of 10–12). Antibody titers were expressed as the log2 reciprocal of the end point in HI test. The dashed lines show the differences of infection among different genotype viruses but have no meanings for the real connection with these genotypes. (A) Isolation rates of H9N2 viruses in chickens. (B) Mean titers of H9N2 viruses at 3 dpi. Error bars are SD. The titers of the G57/G68 viruses were significantly higher than those of other genotypes of the earlier viruses (*P < 0.05, **P < 0.01, one-way ANOVA). (C) The viral shedding time of H9N2 viruses.
Antigenic Drift of G57 H9N2 Viruses Isolated Since 2010.
Antigenic drift, induced mainly in the HA glycoprotein, can decrease a vaccine’s efficacy and cause outbreaks of viral infection (21–23, 28). Phylogenetic analysis revealed that the 2010–2013 H9N2 viruses were mainly in clade 6 and clade 9, whereas the vaccine strains were in clade 2 (Fig. S2). To determine whether antigenic drift had been induced in the prevalent G57 H9N2 viruses, we investigated this possibility by hemagglutinin inhibition (HI) testing of 26 representative H9N2 viruses. As the outbreak of H9N2 influenza in chickens occurred mainly after 2010 and the H7N9 viruses are estimated to have emerged in 2011–2012 (4, 12), we selected viruses from 2010 to 2011 for HI testing, on the basis of their HA phylogenic topology. Some early representative viruses from different antigenic groups were included (20), as were two representative vaccine strains as controls (Ck/SD/6/96 and Ck/SH/F/98; Table S2). The G57 viruses prevalent in 2010–2011, as well as some earlier viruses (mainly 2009), had lower HI titers eight or more times the HI titers of the vaccine strains in reactions with vaccine antisera. These results demonstrated that the recent H9N2 viruses were antigenically distinct from the vaccine strains.
To determine whether antigenic drift had reduced the protective effect of vaccines in the field, we compared the infective efficiency of H9N2 viruses in vaccinated inoculated and contact chickens. The vaccinated chickens were divided into groups with low antibody levels (HI titer, 6–9) and high antibody levels (HI titer, 10–12). Nine viruses used in the above unvaccinated chicken experiment (Fig. 4 A–C, Upper) were also selected in the vaccinated chicken study because they are also the representatives based on the HA lineage (Fig. S2) and HI reaction (Table S2). In the inoculated vaccinated chickens, the G57 viruses from 2010/2011 consistently maintained their growth in trachea, with a 100% isolation rate regardless of antibody level (Fig. 4A). However, the replication of early viruses from other genotypes was clearly inhibited, as shown by a 0–70% isolation rate (except for A/chicken/Shandong/ZB/2007, ZB/07) in chickens with low antibody levels (Fig. 4, Middle) and a 0–30% rate in those with high antibody levels (Fig. 4A, Bottom). ZB/07 was an antigenic variant identified previously (17). After placing vaccinated chickens in contact with inoculated birds, all of the G57 viruses maintained 100% transmission in trachea regardless of antibody level (Fig. 4A), whereas the transmission rate of early viruses decreased markedly, and only one (ZB/07) was isolated, at a low rate (40%), in chickens with high antibody levels (Fig. 4A). Although all of the G57 viruses maintained 100% replication and transmission, their titers in the trachea and cloaca decreased but remained significantly higher (P < 0.05 or P < 0.01, ANOVA) than those of the early viruses (Fig. 4B). By comparison of their shedding time (Fig. 4C), G57 viruses had longer shedding time until 7 dpi in contact vaccinated chickens with high antibody titers (10–12). Although the shedding time of ZB/07 was similar with those of G57 viruses, the numbers of H9N2-positive chickens was less (Fig. 4A). Notably, G57 viruses showed improved replication and transmission in the cloaca of vaccinated chickens, whereas none of the other genotypes of early viruses was detected (Fig. 4, Middle and Bottom), similar to that observed in unvaccinated chickens (Fig. 4, Top). One G68 virus showed a moderate level in replication and transmission between G57 and other earlier genotype viruses (Fig. 4). These results suggest that recent antigenic variants of G57 viruses possessed the fitness to escape immunization pressure and cause the widespread outbreak of H9N2 infection in chickens in 2010–2013.
G57 H9N2 Chicken Virus Provided All Six of Their Internal Genes to the Novel H7N9 Viruses.
Previous studies demonstrate that all six of the internal genes of the novel H7N9 viruses are from H9N2 chicken viruses circulating in China (3–5). To determine whether these internal genes were all from the clades of G57 genotype viruses, we constructed phylogenetic trees of all internal genes of the available 1994–2013 H9N2 chicken viruses and of the H7N9 human viruses isolated during the first 2 wk of the 2013 H7N9 outbreak. Later H7N9 isolates were eliminated to better identify the cause of the initial outbreaks because these viruses underwent further gene exchange with local H9N2 viruses during viral transmission after that time point (29). The six internal genes of the H7N9 human isolates grouped closely with those of the recent 2010–2013 H9N2 chicken viruses as shown in previous studies (3–5), and all belonged to the clades of the G57 genotype (Fig. 3A and Fig. S2). Specifically, the internal genes of the initial H7N9 isolates were the most similar to those of the major groups of H9N2 G57 viruses, with the exception of the NS gene. The NS genes of H7N9 human isolates belonged to a minor group that included H9N2 G57 viruses isolated mainly in the Jiangsu and Zhejiang provinces nearby Shanghai, where the first outbreaks of human H7N9 infection occurred. Eleven novel reassortants of subtypes other than H7N9 (e.g., H5N2, H7N7, and H10N8 viruses) isolated in avian, swine, or humans, mainly after 2010, also possessed G57-like internal genes (Dataset S1).
Discussion
The segmented genome feature of influenza viruses allows reassortment of segments from different viruses, generating novel influenza viruses with pandemic potential (28, 30, 31). In the present study, we investigated the roles of the evolution of H9N2 viruses in the genesis of the reassortant H7N9 viruses. Our results revealed that H9N2 influenza viruses evolved by reassortment and mutation over their 20 y of prevalence in chickens, and the “fittest” genotype, G57, was selected recently. The G57 viruses with changed antigenicity and improved fitness resulted in viral escape from immunization and caused widespread outbreaks in 2010–2013 and finally became the single predominant H9N2 genotype in farm chicken population. This genotype contributed its six internal segments to the novel H7N9 viruses by reassortment.
The widespread use of vaccines against H9N2 viruses in chickens has exerted continuous mutation pressure on the viral HA gene, resulting in antigenic changes. Our study showed a weakened effect of vaccine antisera on the recent H9N2 chicken isolates and heightened virus replication and transmission in vaccinated chickens. These results, combined with the reduced diversity of the 2010–2013 viruses and the close phylogenetic grouping of their HA genes, suggest that the observed antigenic changes are common in the recent H9N2 chicken viruses. In addition to the antigenic characteristics of HA, the genomic context and compatibility of certain segment combinations may be an important contributor to viral fitness (22). As shown in the study on unvaccinated chickens, G57 viruses exhibited the highest replication ability in inoculated and contact chickens than other genotypes, indicative of an optimized segment combination of G57. In another recent genotype G68, which had the same segments with G57 viruses except for the HA segment, a representative virus (A/chicken/Guangdong/01/2011) still had higher viral titers in inoculated or contact chickens than early viruses of other genotypes, indicating other segments also contributed to the enhanced infection ability for the viruses prevailing after 2010. When testing infection in vaccinated chickens, G57 viruses showed superiority in replication and transmission ability. These results suggest that under immunization pressure and selection of the optimal gene constellation by passing through the immunological bottleneck the G57 virus eventually became the dominant genotype during the 2010–2013 outbreak. This outbreak was the first country-wide event of H9N2 viruses in chickens since 1998, when extensive agricultural vaccination began in China.
The formation of the H9N2 ecological environment, as described above, was the first step in the genesis of the H7N9 viruses, as it greatly increased the likelihood of reassortment between H9N2 and other subtypes of influenza viruses. The introduction of H7 and/or N9 viruses into chickens and subsequent reassortment with the H9N2 viruses was the final step. Jiangsu, Shanghai, and Zhejiang, the first provinces that experienced outbreaks of the novel H7N9 viruses on humans (1, 3), are ideally situated for the cocirculation of H9N2, H7, and N9 viruses. These provinces are among the largest chicken consumption markets and are located along the avian migratory route in the Yangtze river delta. Molecular clock dating of divergence times estimates that these H7N9 viruses emerged in ∼2011–2012 (4, 13), during the widespread H9N2 outbreaks in chickens. Also since the H9N2 outbreaks, the appearance of more than 10 non-H7N9 novel reassortants further highlights the significant contribution of the predominant G57 genotype viruses in reassortment with other subtype viruses and raises concerns for the generation of potential pandemic viruses.
In conclusion, our study provides a comprehensive picture of how H9N2 influenza viruses infecting chickens contributed to generation of the H7N9 viruses that have infected humans (Fig. S3). We demonstrated how selection and enrichment of the fittest G57 H9N2 genotype in farm chickens and explained the acquisition of this genotype’s internal genes by the novel H7N9 viruses. The prevalence and variation of H9N2 influenza virus in farmed poultry could provide an important early warning of the emergence of novel reassortants with pandemic potential.
Materials and Methods
Ethics Statement.
All animal research was approved by the Beijing Association for Science and Technology (approval ID SYXK, Beijing, 2007–0023) and performed in compliance with the Beijing Laboratory Animal Welfare and Ethics guidelines, as issued by the Beijing Administration Committee of Laboratory Animals, and in accordance with the China Agricultural University (CAU) Institutional Animal Care and Use Committee guidelines (ID: SKLAB-B-2010-003) approved by the Animal Welfare Committee of CAU.
Virus Isolation and Identification.
From January 2010 to December 2013, chicken farms in 19 provinces of China (Anhui, Beijing, Guangdong, Guangxi, Fujuan, Hebei, Heilongjiang, Henan, Hubei, Jiangsu, Jiangxi, Jilin, Liaoning, Neimenggu, Shaanxi, Shandong, Sichuan, Tianjin, and Xinjiang) sent chickens with disease signs or lung tissues from deceased chickens to our laboratory for diagnosis. The chicken flocks that were sampled were reported to exhibit overt respiratory illness and/or a 5–20% reduction in egg production, despite having received H9N2-subtype influenza vaccination. For virus isolation, tracheal swabs were taken and placed in 1.0 mL transmission medium [50% (vol/vol) glycerol in PBS] containing antibiotics, as previously described (12). Lungs were homogenized in PBS containing antibiotics. Samples were centrifuged at 1,000 × g for 10 min at 4 °C, and 0.2 mL of the supernatants was inoculated into the allantoic cavities of 10-d-old SPF embryonated chicken eggs and incubated for 48 h at 37 °C. Allantoic fluid was harvested and tested for HA activity. HA-positive samples were identified serologically with antisera to each known influenza virus subtype, as previously described (20). H9N2-containing allantoic fluid was harvested and stored as stock at−80 °C until use. Usually, several chickens or samples were sent from one farm to the laboratory at one time. If one or more than one H9N2 virus was isolated, the farm was noted as an H9N2-positive farm. The virus isolation rate was determined by dividing the number of H9N2-positive farms by the total number of farms surveyed.
Genomic Sequencing.
H9N2 viruses (n = 76) isolated from 2010 through 2013 were selected for sequencing on the basis of isolation time and location. At least one strain per province per month was sequenced; if there were multiple isolates in a single month, additional viruses were selected according to location. H9N2 viruses (n = 11) isolated during 2008–2009 from diseased, vaccinated farm chickens were also sequenced genomically. Viral RNA was extracted by using the QIAamp Viral RNA Minikit (Qiagen), and standard RT-PCR was performed with primers specific for H9N2 influenza virus, using the One Step RT-PCR Kit (Qiagen). Full genomic DNA was sequenced by either Sanger sequencing on an ABI 3730 genetic analyzer (Applied Biosystems) or high-throughput next-generation sequencing (Ion torrent).
Sequence Collection and Alignment.
All previously published sequences of Chinese H9N2 influenza A virus (1994–2013) and relative sequences were collated from Influenza Research Database (FluDB) (www.FluDB.org), Global Initiative on Sharing Avian Influenza Data (www.gisaid.org), and the Influenza Virus Resource at the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov/genomes/FLU). All replicate submissions were removed by identifying sets of isolates with identical sequences for any segment, with each isolate containing one to eight segments. All sequences of H7N9 influenza A viruses published before April 2014 were acquired from the above databases and combined with the sequences generated in this study (Table S3) for further analysis. All of the accession codes of public sequences can be found in Table S4 and Dataset S2. The resulting sequences of each gene segment were aligned using MAFFT v6 (32), manually adjusted to correct frame shift errors, and subsequently translated. Downstream phylogenetic analyses were performed on regions of the alignments containing few gaps across sequences. These regions consist of the following intervals: PB2, 1,168–2,199; PB1, 61–1,437; PA, 796–2,103; HA, 190–1,563; NP, 67–1,014; NA, 91–1,272; MP, 88–948; and NS, 79–807.
Phylogenetic Analysis and Clade Classification.
RAxML was used to construct maximum likelihood phylogenies for each segment (33). The general-time reversible model of nucleotide substitution was assumed, and the rates were assumed to vary according to the discrete Gamma distribution with four rate categories. Two sets of trees were constructed: one set including all H9N2 isolates from all hosts since 1994 isolated from China and Hong Kong (all segments) and one set additionally including all H7N9 isolates from all hosts from China and Hong Kong (all six internal segments). One hundred bootstrap maximum-likelihood (ML) trees were used to construct a majority rule consensus tree from each bootstrap sample using sumtrees (34). Bayesian phylogenetic trees were constructed for each segment using Bayesian Evolutionary Analysis Sampling Trees Software (BEAST) (35). The model of substitution and rate variation in each tree was the same as in the corresponding RAxML tree, with the additional assumption of a log-normally distributed molecular clock. BEAST trees were initiated with the RAxML majority rule consensus tree to reduce the computation time required for the MArcov Chain Monte Carlow (MCMC) sampler to reach the stationary distribution. Maximum clade credibility (MCC) trees were constructed using 2,000 equidistant samples from the final 30% of the posterior sample of trees (n = 25 × 106 total samples) using a python script and tree annotator (35). The resulting MCC trees were partitioned into clades using a median branch length distance threshold of 0.20 (24). Briefly, clades with median root-to-tip distances equal to or less than 20% of the median root-to-tip distances of all clades in the tree were selected as clusters. Clades were then manually merged, if necessary, based on the branching posterior and reported classification (19, 20, 25). Each clade was assigned a unique clade ID.
Genotypic Analysis.
Virus genotypes were analyzed in H9N2 viruses isolated from chickens in China during 1994–2013. Isolates were genotyped if sequences and clade assignments were available for all eight segments. The genotypes of the resulting isolates were determined by the combination of clade assignments of each of the eight segments. Genotype IDs were assigned according to the isolation time of the initial founder isolate.
HI Assay.
The HI assay was used to antigenically characterize the H9N2 viruses isolated in China in this and previous studies. The assay used chicken antisera to two representative H9N2 vaccine strains (A/chicken/Shandong/6/1996 and A/chicken/Shandong/F/1998). The HI assay was performed as previously described (36), with an initial serum dilution of 1:10. The HI titer was expressed as the reciprocal of the highest serum dilution in which hemagglutination was inhibited.
Virus Infection in Chickens.
These experiments were designed as previously described (19, 20) and performed in unvaccinated and vaccinated chickens. Three-week-old SPF white leghorn chickens were vaccinated s.c. once with A/chicken/Shandong/6/1996-based commercial vaccine (HA content, 10 log2). HI titers to homologous antigen were measured in twofold serum dilutions 3 wk after inoculation and expressed as the log2 reciprocal of the end point. The vaccinated chickens were divided into two groups for infection experiments: those with a low antibody level (HI titer, 6–9) and those with a high antibody level (HI titer, 10–12). Six-week-old SPF white Leghorn chickens were used as the unvaccinated group. Nine H9N2 viruses (Fig. 4) were selected to inoculate chickens. In inoculated groups, birds (n = 10 per group) were inoculated intranasally with 106 EID50 (egg infective dose at which 50% of inoculated eggs are infected) of each virus in a 0.2-mL volume. Tracheal and cloacal swabs were collected at 3, 5, and 7 dpi. The virus titers of positive samples were measured by determining the EID50; the lower limit of detection was 1.0 log10 EID50/mL In contact groups, at 24 h after infection, five chickens were placed in physical contact (i.e., in the same cage, sharing food and water) with inoculated birds. Tracheal and cloacal swabs were collected at 3, 5, and 7 dpi for viral detection and titration, as described above.
Statistical Analysis.
Experimental groups were statistically compared by ANOVA. P < 0.05 was considered to indicate a statistically significant difference.
Supplementary Material
Acknowledgments
We thank Furkat Anwar for assistance with animal experiments; Robert Huether and Amber Smith for helpful suggestions on data analysis; and Sharon Naron, Kin-chow Chang, and Lu Lu for great editorial assistance. This work was supported by National Natural Science Foundation of China Grants 31430086 and 31272535; National Basic Research Program (973 Program) Grant 2011CB504702; the China Scholarship Foundation; Key Technologies Research and Development Program of China Grant 2013BAD12B01; the China Agriculture Research System, Poultry-Related Science and Technology Innovation Team of Peking; National Institute of Allergy and Infectious Disease Contract HHSN266200700005C; and the American Lebanese Syrian Associated Charities.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences generated in this study have been deposited in the Genbank database (accession nos. are listed in Table S3).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422456112/-/DCSupplemental.
References
- 1.Gao R, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (2014) Human infections with avian influenza A(H7N9) virus. Available at www.who.int/influenza/human_animal_interface/influenza_h7n9/140225_H7N9RA_for_web_20140306FM.pdf?ua=1. Accessed December 9, 2014.
- 3.Liu D, et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: Phylogenetic, structural, and coalescent analyses. Lancet. 2013;381(9881):1926–1932. doi: 10.1016/S0140-6736(13)60938-1. [DOI] [PubMed] [Google Scholar]
- 4.Lam TT, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013;502(7470):241–244. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu A, et al. Sequential reassortments underlie diverse influenza H7N9 genotypes in China. Cell Host Microbe. 2013;14(4):446–452. doi: 10.1016/j.chom.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Guo YJ, et al. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267(2):279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Zhang ZJ, Chen WB. Isolation and identification of avian influenza virus. Chin J Vet Med. 1994;20(10):3–5. [Google Scholar]
- 8.Li C, et al. Evolution of H9N2 influenza viruses from domestic poultry in Mainland China. Virology. 2005;340(1):70–83. doi: 10.1016/j.virol.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Dong G, et al. Reassortant H9N2 influenza viruses containing H5N1-like PB1 genes isolated from black-billed magpies in Southern China. PLoS ONE. 2011;6(9):e25808. doi: 10.1371/journal.pone.0025808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi J, et al. Phylogenetic and molecular characterization of H9N2 influenza isolates from chickens in Northern China from 2007-2009. PLoS ONE. 2010;5(9):e13063. doi: 10.1371/journal.pone.0013063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YK, et al. Continuing evolution of H9N2 influenza viruses in Southeastern China. J Virol. 2004;78(16):8609–8614. doi: 10.1128/JVI.78.16.8609-8614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, et al. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998-2002) J Gen Virol. 2008;89(Pt 12):3102–3112. doi: 10.1099/vir.0.2008/005652-0. [DOI] [PubMed] [Google Scholar]
- 13.Wu ZQ, et al. Cloning and phylogenetic analysis of hemagglutinin gene of H9N2 subtype avian influenza virus from different isolates in China during 2002 to 2009. Poult Sci. 2010;89(6):1136–1143. doi: 10.3382/ps.2010-00695. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, et al. Phylogenetic analysis of hemagglutinin genes of 40 H9N2 subtype avian influenza viruses isolated from poultry in China from 2010 to 2011. Virus Genes. 2012;45(1):69–75. doi: 10.1007/s11262-012-0742-9. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, et al. Diversified reassortant H9N2 avian influenza viruses in chicken flocks in northern and eastern China. Virus Res. 2010;151(1):26–32. doi: 10.1016/j.virusres.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, et al. Phylogenetic analysis of the hemagglutinin genes of twenty-six avian influenza viruses of subtype H9N2 isolated from chickens in China during 1996-2001. Avian Dis. 2003;47(1):116–127. doi: 10.1637/0005-2086(2003)047[0116:PAOTHG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, et al. Evaluation of the protective efficacy of a commercial vaccine against different antigenic groups of H9N2 influenza viruses in chickens. Vet Microbiol. 2012;156(1-2):193–199. doi: 10.1016/j.vetmic.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. Molecular and antigenic characterization of H9N2 avian influenza virus isolates from chicken flocks between 1998 and 2007 in China. Vet Microbiol. 2012;156(3-4):285–293. doi: 10.1016/j.vetmic.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, et al. A novel genotype H9N2 influenza virus possessing human H5N1 internal genomes has been circulating in poultry in eastern China since 1998. J Virol. 2009;83(17):8428–8438. doi: 10.1128/JVI.00659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, et al. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008. Vet Microbiol. 2010;146(3-4):215–225. doi: 10.1016/j.vetmic.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Rambaut A, et al. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453(7195):615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHardy AC, Adams B. The role of genomics in tracking the evolution of influenza A virus. PLoS Pathog. 2009;5(10):e1000566. doi: 10.1371/journal.ppat.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boni MF. Vaccination and antigenic drift in influenza. Vaccine. 2008;26(Suppl 3):C8–C14. doi: 10.1016/j.vaccine.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosperi MC, et al. ARCA collaborative group A novel methodology for large-scale phylogeny partition. Nat Commun. 2011;2:321. doi: 10.1038/ncomms1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu KM, et al. The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000 to 2005. J Virol. 2007;81(19):10389–10401. doi: 10.1128/JVI.00979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P, et al. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998-2002) J Gen Virol. 2008;89(Pt 12):3102–3112. doi: 10.1099/vir.0.2008/005652-0. [DOI] [PubMed] [Google Scholar]
- 27.Moya A, Holmes EC, González-Candelas F. The population genetics and evolutionary epidemiology of RNA viruses. Nat Rev Microbiol. 2004;2(4):279–288. doi: 10.1038/nrmicro863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui L, et al. Dynamic reassortments and genetic heterogeneity of the human-infecting influenza A (H7N9) virus. Nat Commun. 2014;5:3142. doi: 10.1038/ncomms4142. [DOI] [PubMed] [Google Scholar]
- 30.Nelson MI, Holmes EC. The evolution of epidemic influenza. Nat Rev Genet. 2007;8(3):196–205. doi: 10.1038/nrg2053. [DOI] [PubMed] [Google Scholar]
- 31.Cox NJ, Subbarao K. Global epidemiology of influenza: Past and present. Annu Rev Med. 2000;51:407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 32.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukumaran J, Holder MT. DendroPy: A Python library for phylogenetic computing. Bioinformatics. 2010;26(12):1569–1571. doi: 10.1093/bioinformatics/btq228. [DOI] [PubMed] [Google Scholar]
- 35.Bouckaert R, et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2014;10(4):e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards S. OIE laboratory standards for avian influenza. Dev Biol (Basel) 2006;124:159–162. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





