Significance
Maintenance of a pool of natural killer (NK) cells with optimal immune function is crucial for host defense against pathogens or cancerous tumor formation. Here we identify intracellular osteopontin (OPN-i) as an essential molecular component responsible for maintenance of functional NK cell expansion. Absence of OPN-i results in failure to maintain normal NK cellularity and increased cell death following stimulation by cytokine interleukin-15. OPN-deficient NK cells fail to successfully navigate the contraction phase of the immune response, resulting in impaired expansion of long-lived NK cells and defective responses to viral infection and tumor cells. Insight into the contribution of OPN-i to NK cell responses may provide the basis for improved approaches to immunotherapy for infectious disease and cancer.
Keywords: cellular maintenance, contraction, long-lived cell, memory cell, T-box transcription factor
Abstract
Natural killer (NK) cells play an essential role in the immune response to infection and cancer. After infection or during homeostatic expansion NK cells express a developmental program that includes a contraction phase followed by the formation of long-lived mature memory-like cells. Although this NK cell response pattern is well established, the underlying mechanisms that ensure efficient transition to long-lived NK cells remain largely undefined. Here we report that deficient expression of intracellular osteopontin (OPN-i) by NK cells results in defective responses to IL-15 associated with a substantial increase in the NK cell contraction phase of homeostatic expansion, defective expression of the Eomes transcription factor, and diminished responses to metastatic tumors. The OPN-i–deficient phenotype is accompanied by increased NK cell apoptosis, impaired transition from immature to mature NK cells, and diminished ability to develop memory-like NK cells that respond to mouse cytomegalovirus. Gene pathway analysis of OPN-i–deficient NK cells suggests that the mechanistic target of rapamycin pathway may connect OPN-i to Eomes and T-bet expression by mature NK cells following up-regulation of OPN-i after IL-15 stimulation. Identification of OPN-i as an essential molecular component for maintenance of functional NK cell expansion provides insight into the NK cell response and may provide the basis for improved approaches to immunotherapy for infectious disease and cancer.
Homeostatic proliferation of lymphocytes is a central mechanism used by the immune system to maintain sufficient numbers of immune cells to mount rapid and effective responses against potential pathogens (1–4). Periods of lymphopenia also induce a homeostatic developmental program characterized by early proliferation that precedes a contraction phase followed by expansion and persistence of long-lived memory-like cells (1, 3, 5). Increased understanding of mechanisms that guide homeostatic expansion of immune cells is essential for developing improved strategies for immunotherapy of infectious disease or cancer.
Natural killer (NK) cells exhibit the highest levels of cytotoxic activity within the immune system and altered NK cell numbers or functionality may have a profound impact on overall immune status, including protective immunity against viruses and tumors (6, 7). NK cells that undergo homeostatic expansion under lymphopenic conditions can give rise to progeny capable of robust proliferation and effector activity against diverse pathogen challenges (5). The factors that promote and regulate progression through the distinct stages of homeostatic NK cell expansion are not well understood.
Homeostasis, survival, and development of NK cells from common lymphoid progenitor (CLP) cells in the bone marrow (BM) are critically dependent on IL-15, and mice unable to produce or respond to IL-15 (Il15−/−, Il15ra−/−, and Rag2−/−γC−/− mice) lack mature NK cells and display developmental arrest at an immature NK cell stage (8, 9). Developmental and survival signals downstream of the IL-15 receptor (IL-15R) in NK cells are thought to be mediated, in part, by the basic leucine zipper transcription factor (TF) Nfil3 (nuclear factor interleukin-3 regulated; also known as E4BP4) (10, 11). However, whereas expression of Nfil3 is essential for early development of NK cells, survival and homeostasis of mature NK cells is Nfil3 independent (12). The serine-threonine kinase mechanistic target of rapamycin (mTOR) downstream of IL-15R is also critical for development and peripheral activation of NK cells, in part through induction of several critical TFs, including Eomes and T-bet (13). The mechanisms that integrate these multiple signaling pathways that contribute to NK cell development are poorly understood.
The phosphoprotein osteopontin (OPN) is expressed as a secreted (OPN-s) or intracellular (OPN-i) isoform that arises from different OPN translational initiation sites (14). OPN-s promotes survival and maintenance of activated CD4+ T cells and excessive OPN-s can result in relapse and disease exacerbation of murine experimental autoimmune encephalomyelitis (15). OPN-s also contributes to activation of NKT cells and development of ConA-induced hepatitis (16). Although OPN is also expressed by NK cells (16), the potential contribution of OPN isoforms to NK cell homeostasis, survival, and function has not been determined.
We report here that OPN expression by NK cells is up-regulated after ligation of IL-15R. Analysis of mice containing targeted expression of OPN isoforms indicated that expression of OPN-i, but not OPN-s, is essential for NK cell expansion and differentiation. These findings implicate OPN-i as a previously unidentified intermediate within the IL-15 signaling pathway that ensures passage of NK cells through the contraction phase of homeostatic expansion and full differentiation into long-lived NK cells.
Results
OPN-i–Deficient Mice Harbor Reduction of Mature NK Cells.
To define the potential contribution of OPN to regulation of NK cells at steady state, we initially measured OPN at the mRNA and protein levels at different stages of NK maturation, according to surface expression of CD11b and CD27: CD11b–CD27– (R0), CD11b–CD27+ (R1), CD11b+CD27+ (R2), and CD11b+CD27– (R3) (Fig. 1A) (17, 18). Peak levels of Spp1 mRNA and OPN protein were expressed by CD11b+ R2 mature NK cells, during the transition into terminally differentiated R3 cells (Fig. 1A), suggesting a potential contribution of OPN to the development of mature NK cells.
Fig. 1.
Reduced mature NK cells from OPN-i–deficient mice. (A) Quantitative RT-PCR analysis of Spp1 mRNA (Middle) and OPN and actin protein levels (Right) expressed by the indicated splenic NK cell subsets (Left, defined by CD11b and CD27 expression) sorted from B6 mice. Spp1 expression was normalized to that of the Rps18 control and results are presented relative to that of R0, set as 1. Ratios of OPN to actin protein levels are indicated as follows: R0, CD11b–CD27–; R1, CD11b–CD27+; R2, CD11b+CD27+; and R3, CD11b+CD27–. (B) Representative FACS plots show splenic NK1.1+TCRβ− NK cells from the indicated mouse stains. Histogram overlays of CD11b, CD43, and B220 expression are shown (Right). (C) Quantitation of absolute numbers of total NK cells, immature CD11b–, and mature CD11b+ NK cells from spleen and BM of the indicated mouse strains (n = 6 mice per group) is shown. **P < 0.01 (error bars, mean ± SEM). (D) Sublethally irradiated Rag2−/−γC−/− recipients injected with mixtures of lymphocyte-depleted OPN-i–KI or OPN-KO BM cells (CD45.1−CD45.2+) and CD45.1+CD45.2+ WT BM cells were analyzed 12 wk later. Representative FACS plots show the percent of NK cells (NK1.1+CD122+CD3−) from each donor along with the indicated CD45.1−CD45.2+ donor NK subsets in E.
To define the potential contribution of OPN isoforms to regulation of NK cell homeostasis and function, we analyzed NK cells from mice that express only OPN-i [EIIa-Cre+Spp1flstop, hereafter “OPN-i–knock-in” (KI)] (19), and wild-type (WT) mice that express both isoforms or from OPN-deficient (EIIa-Cre–Spp1flstop, hereafter “OPN-KO”) mice that express neither OPN isoform. Immunoblot analysis showed that NK cells from OPN-i–KI mice expressed similar levels of OPN protein as NK cells from WT littermates (Fig. S1A). Moreover, secreted OPN was not detectable in supernatants of freshly isolated or activated T cells and NK cells from either OPN-i–KI or OPN-KO mice (Fig. S1B). We then evaluated NK cell numbers, subsets, and phenotype in various tissues of OPN-i–KI and OPN-KO mice compared with WT littermates. The number of NK cells in spleens of mice lacking OPN was reduced to roughly 50% of the NK numbers of WT mice, and expression of OPN-i fully remedied this defect (Fig. 1 B and C). We noted that immature NK cells, characterized as CD11b–CD27+ and expressing CD51 (integrin αν), CD61 (integrin β3), and B220 receptors, were modestly elevated in OPN-KO mice compared with WT and OPN-i–KI mice (Fig. 1B and Fig. S2 A and B). A corresponding decrease in the most mature NK cell subset (CD11b+CD27–, expressing Ly49D, Ly49H, and CD43 receptors), was evident in spleen and BM of OPN-KO but not OPN-i–KI mice (Fig. 1B and Fig. S2 A and C). Separate enumeration of mature CD11b+ and immature CD11b– NK cells revealed that OPN-KO NK cells contained ∼2.5-fold fewer mature NK cells compared with WT and OPN-i-KI counterparts, whereas numbers of immature NK cells did not differ significantly (Fig. 1C), suggesting that OPN-i deficiency was associated with defective NK cell maturation.
The OPN-i–Deficient Developmental Phenotype Is NK Cell Intrinsic.
OPN-i expression in plasmacytoid dendritic cells (pDC) is essential for efficient production of IFNα and full expression of NK effector cell activity (20). To investigate whether the impaired NK response noted above reflected a cell-intrinsic role of OPN-i in NK cells, mixed BM chimeric mice were generated by reconstituting sublethally irradiated Rag2−/−γC−/− hosts with an equal number of BM cells from OPN-KO or OPN-i–KI (CD45.2+) and WT (CD45.1+CD45.2+) mice. The frequency of OPN-KO NK cells was twofold less than OPN-i–KI cells 12 wk postreconstitution, which reflected a twofold reduction of mature CD11b+CD43+ NK cells (Fig. 1 D and E), suggesting that defective NK cell maturation noted in intact animals reflected an intrinsic NK cell defect.
We next investigated whether reduced numbers of mature NK cells in OPN-KO mice reflected diminished survival, decreased proliferation, or both. Proliferation of OPN-KO splenic NK cells was similar to that of OPN-i–KI counterparts, according to expression of Ki67, a marker that identifies actively dividing cells. In contrast, OPN-KO NK cells displayed increased levels of annexin V expression (Fig. 1 D and E), indicating that, whereas NK cells lacking OPN-i are able to undergo unimpaired homeostatic proliferation, they are less able to survive this process.
OPN-i–Deficiency Impairs NK Cell Function.
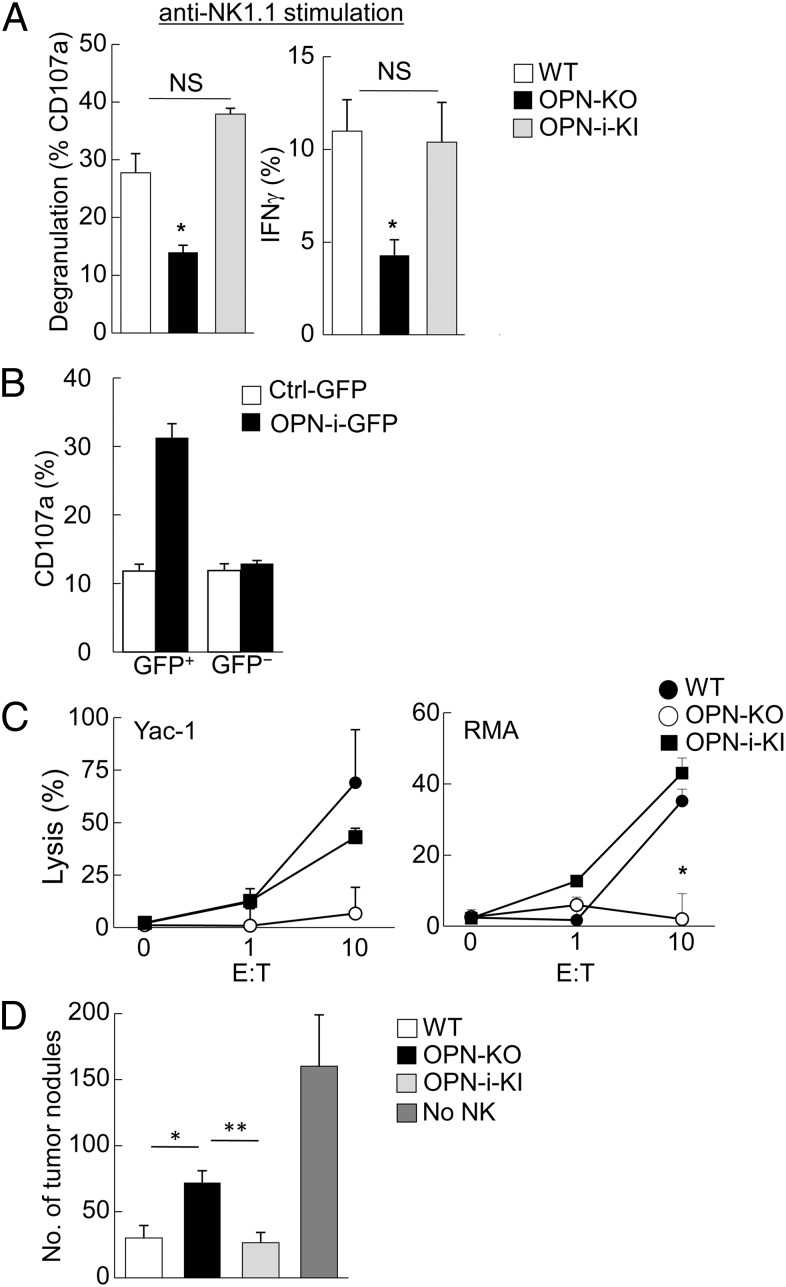
Defective differentiation of OPN-KO NK cells from immature precursors to mature progeny is accompanied by increased apoptosis and potential functional impairment. We examined the response of splenic NK cells to NK1.1 ligation in vitro. Compared with WT and OPN-i–KI NK cells, OPN-KO NK cells showed significantly reduced degranulation (measured by CD107a expression) and diminished IFNγ production after NK1.1 engagement (Fig. 2A). OPN deficiency did not affect the response to phorbol 12-myristate 13-acetate (PMA) plus ionomycin (Fig. S2D), suggesting that OPN-KO NK cells were responsive to stimuli that bypassed receptor-based physiological pathways. Impaired NK1.1 responses could be reconstituted by OPN-i expression, because OPN-KO NK cells transduced with a lentivirus that expressed OPN-i (GFP+) displayed significantly increased NK1.1-induced degranulation compared with cells transduced with control virus (Fig. 2B). OPN-KO NK cells also failed to mediate significant lysis of Yac-1 and RMA targets, in contrast to WT and OPN-i–KI NK cells, which displayed vigorous target cell killing (Fig. 2C). We further investigated the cytotoxic potential of OPN-KO NK cells in vivo using a well-established B16 melanoma model (7) that allows enumeration of metastatic lung nodules after i.v. injection of NK cells and B16F10 cells into Rag2−/−γC−/− mice (Fig. 2D and Fig. S3A). Mice reconstituted with OPN-KO NK cells displayed fewer NK cells and failed to reduce tumor metastasis compared with recipients of WT and OPN-i–KI NK cells (Fig. 2D and Fig. S3 B and C). Taken together, these results indicated that OPN-i deficiency increased NK cell apoptosis, resulted in diminished NK cell lytic activity and cytokine secretion, as well as impaired antitumor responses in vivo.
Fig. 2.
OPN-i–deficiency impairs NK cell function. (A) Splenic NK cells from the indicated strains were stimulated for 5 h with plate-bound anti-NK1.1 Ab before flow cytometric analysis of CD107a (degranulation) and IFNγ expression by incubation with anti-CD107a and anti-IFNγ. (B) OPN-KO HSCs were transduced with lentivirus expressing OPN-i (OPN-i–GFP) or a control GFP vector before transfer as sorted GFP+ cells into sublethally irradiated Rag2−/−γC−/− mice. CD107a expression was analyzed in splenic NK cells 8 wk after reconstitution and stimulation as in A. (C) IL-2–cultured NK cells from the indicated mice were incubated with Yac-1 or RMA target cells at the indicated ratios before specific lysis was determined 4 h later. (D) B16F10 cells were injected i.v. into Rag2−/−γC−/− hosts 10 d after transfer of sorted NK cells from the indicated mice (as shown in Fig. S3). Lung metastasis nodules were enumerated at d 25. n = 5 mice per group. *P < 0.05, **P < 0.01. Values are shown as mean ± SEM. Data are representative of at least three independent experiments.
Impaired Response of OPN-KO NK Cells to IL-15.
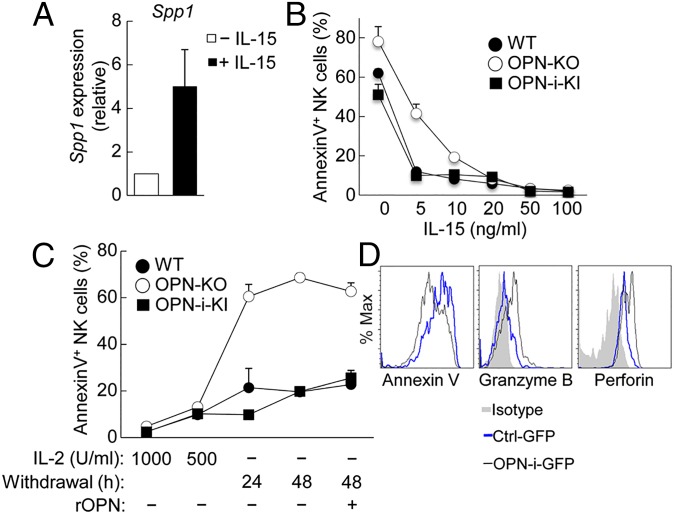
The phenotypic impairment displayed by OPN-KO NK cells was reminiscent of NK cells from mice carrying genetic disruptions of IL-15 signaling, resulting in greatly reduced numbers of mature NK cells and a residual NK population consisting mainly of immature CD11b– cells (8, 9). We therefore analyzed the potential impact of IL-15 on OPN expression by NK cells. IL-15 treatment induced a fivefold increase in Spp1 mRNA expression in cultured NK cells (Fig. 3A), consistent with earlier gene profiling analysis of NK cells 24 h after activation in vitro with IL-15 (Gene Expression Omnibus: GSE7764) (21). We then compared the response of OPN-KO NK cells with WT and OPN-i–KI NK cells to increasing concentrations of IL-15. Although higher concentrations of IL-15 protected NK cells from apoptosis (measured by annexin V expression), limiting concentrations of IL-15 revealed defective protection of OPN-KO NK cells compared with WT and OPN-i–KI cells (Fig. 3B). Moreover, withdrawal of IL-2, which shares a similar signaling pathway with IL-15, greatly increased apoptosis of OPN-KO NK cells compared with their OPN-i–expressing counterparts (Fig. 3C). The defective survival response to IL-2/IL-15 was independent of OPN-s, because supplementation by recombinant OPN did not diminish apoptosis levels (Fig. 3C), suggesting that regulation of NK cell survival by IL-15 signals may require expression of OPN-i.
Fig. 3.
Impaired IL-15 responses of OPN-KO NK cells in vitro. (A) Quantitative RT-PCR analysis of Spp1 mRNA expressed by B6 splenic NK cells treated with (+) or without (–) IL-15 (100 ng/mL) for 24 h. Results (normalized as in Fig. 1A) are presented relative to that of (–), set as 1. (B and C) Percent of annexin V+ NK cells (from the indicated mice) incubated with increasing concentrations of IL-15 for 24 h (B) and with or without IL-2 at the indicated time points (C), as well as with (+) or without (–) addition of recombinant OPN (C), assessed by flow cytometry. Values represent the average of three independent experiments with error bars of mean ± SEM. (D) Lentivirally transduced OPN-KO HSCs were transferred into Rag2−/−γC−/− hosts as in Fig. 2B. Expression of annexin V, intracellular granzyme B, and perforin was analyzed by flow cytometry in splenic NK cells 8 wk after reconstitution followed by incubation with IL-15 (100 ng/mL) for 24 h.
To further define the potential contribution of OPN-i to the NK-cell IL-15 response, we transduced OPN-KO hematopoietic stem cells (HSCs) with lentivirus expressing OPN-i (or a control GFP vector) and transferred sorted GFP+ cells into sublethally irradiated Rag2−/−γC−/− mice. Analysis of splenic NK cells 8 wk postreconstitution after in vitro stimulation with IL-15 showed that expression of OPN-i in OPN-KO NK cells reduced the apoptotic fraction of NK cells and greatly increased the proportion of NK cells expressing granzyme B and perforin (Fig. 3D), indicating that OPN-i expression promotes IL-15–dependent NK cell survival and cytotoxic potential long after development from BM precursors in lymphopenic hosts.
Excessive Contraction During Homeostatic Expansion of OPN-KO NK Cells.
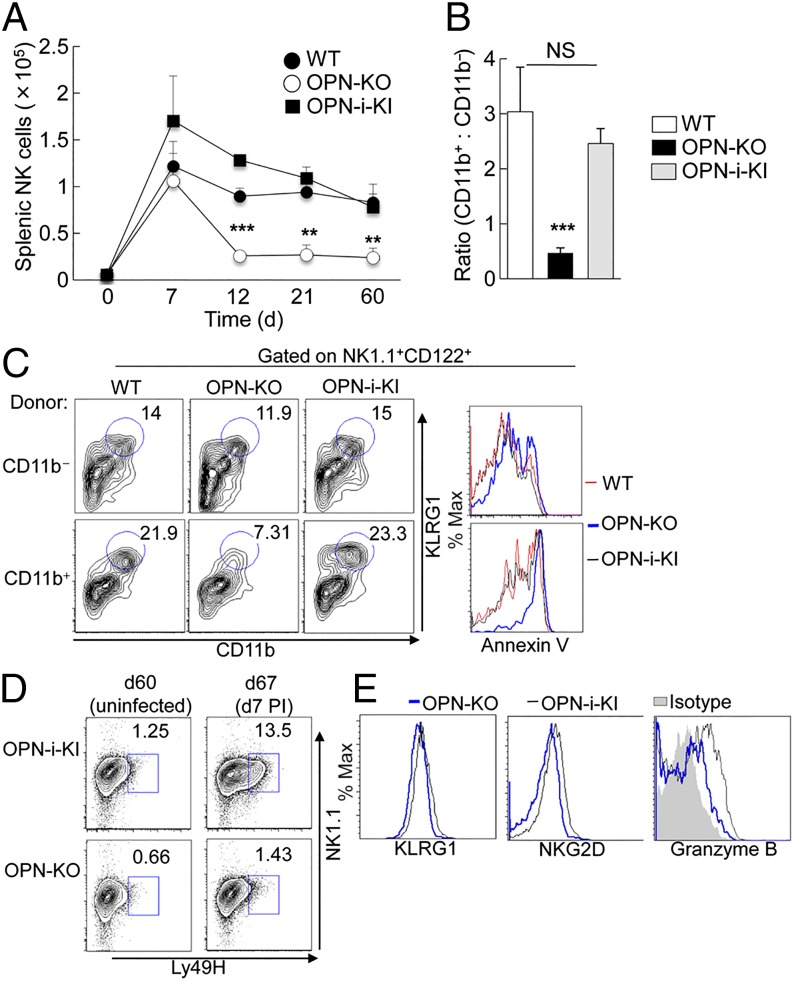
Maintenance of mature NK cell survival in vivo also depends on IL-15 (1, 3, 5), which may require OPN-i expression. We directly analyzed the contribution of OPN-i to homeostatic expansion in a lymphopenic setting after transfer of sort-purified NK cells from WT, OPN-KO, and OPN-i–KI mice into Rag2−/−γC−/− hosts (Fig. 4A). All groups of NK cells expanded robustly after transfer, peaking at similar numbers at day 7. However, by day 12, when WT and OPN-i–KI NK cells maintained substantial numbers despite the “contraction phase,” the numbers of adoptively transferred OPN-KO NK cells were approximately one-third of their OPN-i–expressing counterparts at 12, 21, and 60 d posttransfer (Fig. 4A). Moreover, WT and OPN-i–KI NK cells displayed ratios of mature:immature (CD11b+:CD11b−) NK cells of ∼2.5:1, in contrast to the CD11b+:CD11b− 0.5:1 ratio of OPN-KO NK cells (Fig. 4B), consistent with a maturation defect of NK cells lacking OPN-i (Fig. 1D).
Fig. 4.
Impaired homeostatic expansion of OPN-KO NK cells in lymphopenic hosts. (A and B) The 5 × 105 sorted splenic NK cells from WT, OPN-KO, and OPN-i–KI mice were transferred into Rag2−/−γC−/− hosts. (A) Graph shows the absolute number of splenic NK cells at various time points after transfer. (B) Ratios of CD11b+ mature NK cells to CD11b− immature NK cells from the indicated strain at d 21 after transfer from A are shown. n = 3–4 mice per time point per group. **P < 0.01, ***P < 0.001 (error bars, mean ± SEM). (C) Sorted CD11b− immature and CD11b+ mature NK cells from WT, OPN-KO, and OPN-i–KI mice were transferred into Rag2−/−γC−/− hosts, separately. FACS plots show percent of CD11b+KLRG1+ splenic NK cells (CD122+NK1.1+) at d 16 after transfer. Histogram overlays (Right) show expression of annexin V on transferred NK cells from WT (red line), OPN-KO (blue line), and OPN-i-KI (black line) mice. (D) Rag2−/−γC−/− hosts reconstituted with sorted NK cells from OPN-KO and OPN-i-KI mice as in A were infected with MCMV at day 60. Splenic NK cells were analyzed 7 d postinfection (PI) (compared with uninfected mice) for expression of Ly49H+NK1.1+ NK cells (gated on CD122+ cells). (E) Splenic NK cells of day-7 postinfection mice from OPN-i–KI (black line) and OPN-KO (blue line) were analyzed for surface expression of KLRG1 and NKG2D and intracellular staining of granzyme B.
To determine whether fewer OPN-KO NK cells were recovered at later time points simply because they failed to proliferate efficiently, we performed adoptive transfer experiments in which carboxyfluorescein succinimidyl ester (CFSE)-labeled splenic NK cells from WT, OPN-KO, or OPN-i–KI mice were injected i.v. into Rag2−/−γC−/− hosts. The proportion of NK cells displaying decayed CFSE fluorescence represents dividing NK cells. At day 3 after injection, despite an impaired response to NKp46 stimulation by OPN-KO NK cells (Fig. S4A), no significant difference was noted in proliferation among WT, OPN-KO, or OPN-i–KI NK cells, consistent with similar Ki67 expression by these cells in BM chimeras (Fig. 1D and Fig. S4B).
To test whether reduction of mature OPN-KO NK cells reflected a survival defect of mature NK cells or a differentiative block that inhibits development of mature NK cells, CD11b−CD27+ immature and CD11b+ mature NK subsets were sorted from WT, OPN-KO, or OPN-i–KI mice before adoptive transfer into Rag2−/−γC−/− hosts. Eight days after transfer, WT and OPN-KO CD11b− immature donor NK cells proliferated (Ki67 expression) and ∼65% became CD11b+ mature NK cells (Fig. S5 A and B). However, a lower proportion of OPN-KO CD11b− donor NK cells acquired expression of the terminal differentiation marker CD43 compared with WT and OPN-i–KI NK cells (Fig. S5C) and OPN-KO CD11b+ donor NK cells showed higher levels of staining for annexin V (Fig. S5B), suggesting a possible early phenotypic difference between OPN-i–deficient and OPN-i–expressing NK cells at the outset of the contraction phase. These differences became more apparent at day 16 posttransfer, at which point OPN-KO KLRG1+ CD11b+ donor NK cells were reduced approximately three-fold and displayed higher levels of apoptosis compared with WT and OPN-i–KI NK cells (Fig. 4C). Taken together, these findings suggest that differences in NK cell responses mainly reflect impaired survival of mature OPN-KO NK cells during homeostatic responses.
Generation of Long-Lived NK Cells Requires OPN-i.
NK cells that survive the contraction phase of homeostatic proliferation can develop into long-lived NK cells that are poised to respond to viral infection with characteristics of memory-like NK cells (5). We determined whether OPN-i expression was required for generation of long-lived Ly49H+ NK cells, which can display robust antigen-driven expansion in response to mouse cytomegalovirus (MCMV) infection. At day 60 after transfer of OPN-KO or OPN-i–KI NK cells into Rag2−/−γC−/− hosts, similar levels of Ly49H+ NK cells were present in uninfected mice (Fig. 4D). Seven days after challenge with MCMV, the proportion of splenic Ly49H+ OPN-i–KI NK cells increased by ∼10-fold, compared with a 2-fold increase in OPN-KO NK cells. The defective response of Ly49H+ OPN-KO NK cells to MCMV was associated with impaired up-regulation of Ly49H, KLRG1, NKG2D, and granzyme B (Fig. 4 D and E). Thus, OPN-i expression is essential not only for the survival of mature NK cells but also for generation of long-lived Ly49H+ NK cells that respond to viral infection.
Molecular Mechanisms for OPN-i–Dependent Regulation of NK Cell Homeostasis.
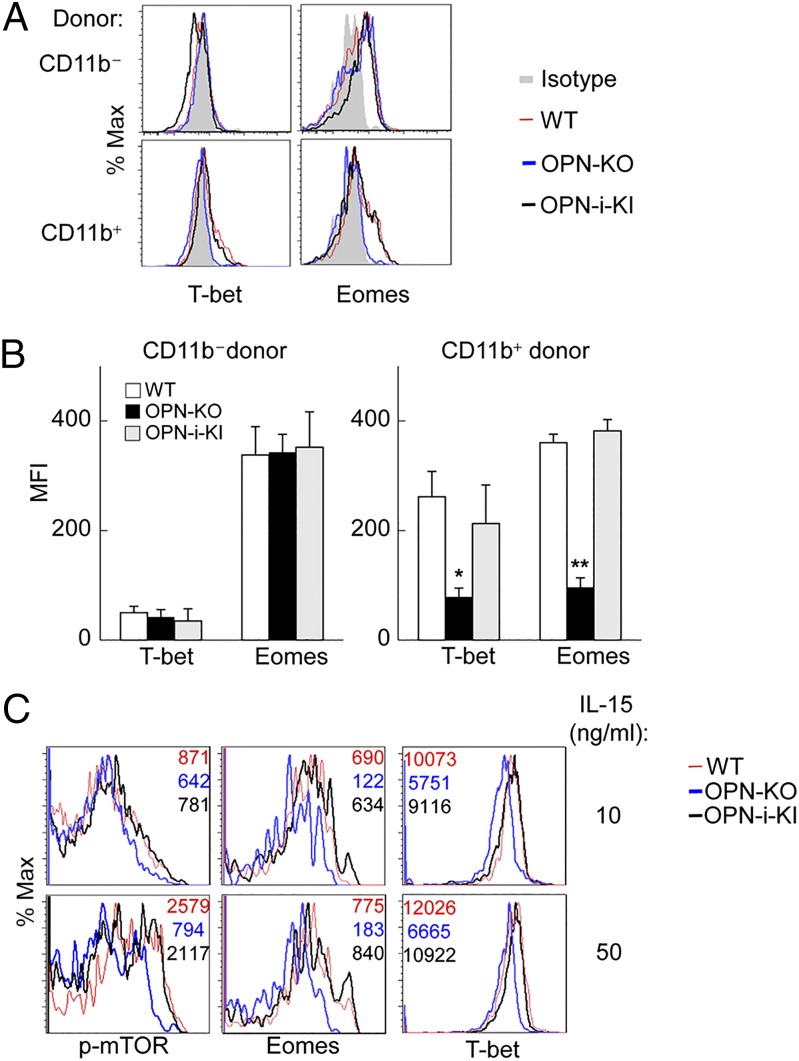
The above findings suggested that OPN-i is required for successful differentiation of NK cells into a mature subset and successful navigation through the contraction phase of homeostatic expansion. Moreover, OPN-i–dependent expansion was essential for NK cell inhibition of tumor metastasis in vivo and the development of long-term memory-like phenotype and responses to MCMV infection. Because Eomes and T-bet are essential regulators of NK cell homeostasis, maturation and function (7, 22, 23) and phenotypic impairment of OPN-KO NK cells may reflect defective expression of these TFs, we analyzed expression of Eomes and T-bet at the protein level 16 d after NK cell transfer into Rag2−/−γC−/− hosts (Fig. 5A). Immature CD11b– NK cells differentiated into T-betlo Eomes+ cells independent of OPN status. However, transition to mature (CD11b+) NK cells was accompanied by up-regulation of T-bet and maintenance of Eomes expression by mature OPN-i–expressing but not OPN-KO NK cells (Fig. 5 A and B). These findings support the view that the developmental defect of OPN-KO NK cells noted above is associated with failure of mature NK cells to maintain Eomes and T-bet expression (23).
Fig. 5.
Molecular mechanism of OPN-i–dependent regulation of NK cell homeostasis. (A) Splenic NK cells from Rag2−/−γC−/− hosts reconstituted with either CD11b− immature or CD11b+ mature NK cells (from WT, OPN-KO, and OPN-i–KI mice) (as in Fig. 4C) were analyzed for intracellular expression of T-bet and Eomes at d 16 posttransfer. (B) Quantitation of Eomes and T-bet mean fluorescence intensity (MFI) in NK cells from A is shown. n = 3–5 mice per group. *P < 0.05, **P < 0.01 (error bars, mean ± SEM). (C) Expression of p-mTOR, Eomes, and T-bet in splenocytes from indicated mice strains (n = 3 mice per group) cultured in IL-15 (10 or 50 ng/mL) for 24 h. Representative histogram overlays are shown. Numbers indicate MFI of indicated proteins.
To gain insight into the potential contribution of OPN-i to up-regulation of Eomes and T-bet expression and its impact on NK cell homeostasis, maturation, survival, and function, global gene expression analysis was performed using sorted OPN-i–KI and OPN-KO NK cells from Rag2−/−γC−/− recipients at day 10 after adoptive transfer (Fig. S6A), when OPN-KO NK cells display striking survival defects during contraction (Fig. 4A). Consistent with up-regulated Eomes and T-bet protein levels by OPN-i–KI NK cells compared with OPN-KO NK cells (Fig. 5 A and B), expression of Eomes and Tbx21 mRNA increased in OPN-i–KI cells (Fig. S6B). Many transcripts associated with Eomes and T-bet activity were also up-regulated, including those encoding molecules associated with NK cell maturation (e.g., Klrg1 and Tox) (23–26), survival (e.g., Bcl2, Il2rβ, and Il2rγ) (27, 28), effector function [e.g., Klrk1 (encoding NKG2D), Prf1 (encoding perforin), Gzmk (encoding granzyme k)] and memory formation (e.g., Cxcr6 and Thy1) (29, 30) (Fig. S6B). These results are consistent with our observations that OPN-i–KI NK cells expressed increased protein levels of KLRG1, NKG2D, and cytotoxic molecules compared with their OPN-KO counterparts (Figs. 3D and 4 C and E). Consistent with the essential role of the mTOR pathway to up-regulation of Eomes and T-bet expression (13, 31), we noted a highly significant enrichment of mTOR pathway gene expression by OPN-i–KI cells (P < 0.0001) compared with OPN-KO NK cells according to gene set enrichment analysis (GSEA) or the National Institute of Allergy and Infectious Diseases DAVID Bioinformatics Resource (http://david.abcc.ncifcrf.gov) that identify pathways in the KEGG database (Fig. S6C and D). NK cell mTOR activity is controlled mainly by IL-15 signaling (13). Analysis of phosphorylated mTOR (p-mTOR) expressed by NK cells after incubation with IL-15 showed a dose-dependent increase of p-mTOR and up-regulation of the Eomes and T-bet TFs. Moreover, OPN-i–deficient NK cells displayed impaired up-regulation of p-mTOR as well as diminished expression of Eomes and T-bet (Fig. 5C). These results suggested that the contribution of OPN-i to elevated Eomes and T-bet expression during transition from the immature to the mature NK phenotype may be associated with up-regulation of an mTOR intermediary (13).
Discussion
Maintenance of a pool of NK cells with optimal effector function is essential for host defense against pathogens or cancerous tumor formation. Although mechanisms responsible for homeostatic proliferation of T lymphocytes have been well characterized, the signals guiding NK cell homeostasis are only dimly understood. Here we provide evidence that mice carrying a deletion of an intracellular isoform OPN (OPN-i) fail to maintain normal NK cellularity and harbor mature NK cells that undergo increased apoptosis in response to IL-15. This phenotype is associated with failure of OPN-KO NK cells to successfully navigate the contraction phase during homeostatic expansion, resulting in impaired expansion of long-lived NK cells equipped to respond to MCMV infection.
These data suggest that IL-15–dependent NK cell responses and homeostasis require expression of OPN-i for survival in vitro and in vivo. Previous studies have shown that IL-15 signaling in NK cells results in increased expression and antiapoptotic activity of Bcl-2 and its family members (8, 32), whereas IL-15 deprivation promotes accumulation of the proapoptotic factors Bim and Noxa (33). The signals downstream of IL-15 that may control the balance of antiapoptotic and proapoptotic signals have been attributed to Erk, PI3K, and mTOR kinases (13, 33). Our gene profile analysis suggests an association between OPN-i expression and increased mTOR activity, consistent with the similar phenotypes of OPN-KO NK cells and mTOR-deficient NK cells, although the latter phenotype is more severe (13). Identification of OPN-i as an intermediate of the IL-15 antiapoptotic pathway suggests an unappreciated complexity of IL-15 signals that may control the life span of NK cells through interactions with the mTOR system and warrants further investigation.
The failure of OPN-KO NK cells to successfully navigate the contraction phase in lymphopenic hosts may reflect impaired expression of both Eomes and T-bet by mature NK cells. Impaired expression of Ly49 receptors and increased NK cell apoptosis may reflect diminished Eomes expression, because expression of Eomes is essential for maintenance of mature NK cells and induction of Ly49 receptor expression, including Ly49H, which mediates resistance against MCMV infection (34, 35). Although Eomes and T-bet play distinct roles in the regulation of NK cell maturation, they cooperate to promote expression of perforin and enhance IL-15 responsiveness and compound mutations of both TFs exert a synergistic effect on the NK phenotype (23, 27). Mechanisms that may underpin OPN-i–dependent regulation of these two TFs are under investigation.
Although NK cells have been considered relatively short-lived components of innate immunity, recent studies have revealed that NK cells can exhibit features of adaptive immune responses, including antigen-dependent clonal expansion and the ability to differentiate into long-lived memory-like cells (29, 35, 36). Homeostatic expansion also drives NK cells to become long-lived cells and acquire memory-like features after transfer into lymphopenic mice (5). Although generation of these two pools of long-lived NK cells follows a similar developmental program, several differences exist between antigen-activated and homeostasis-driven memory-like NK cells (including a less activated phenotype after homeostatic expansion) (5). Recent studies have begun to elucidate molecular elements regulating memory NK cell formation after viral infection, including regulation of expansion by the Zbtb32 TF and regulation of contraction by Bim (37, 38). The current study reveals for the first time to our knowledge that the intracellular isoform OPN-i makes an essential contribution to the formation of long-lived NK cells with a memory-like phenotype after homeostatic expansion. Whether OPN-i is important for the generation of antigen-driven memory NK cells is not directly addressed by these studies. Because IL-15, Eomes, and the mTOR pathway are essential for memory formation by subsets other than NK cells, including CD8+ T cells (39), the potential contribution of OPN-i to the memory response of these T-cell subsets warrants further study.
In summary, we have shown that expression of OPN-i by NK cells is essential for successful navigation through the contraction phase of expansion and generation of long-lived NK cells with increased functionality. Homeostatic expansion of NK cells is a critical response to chronic infection, chemotherapy, or as a consequence of hematopoietic stem cell transplantation. Identification of OPN-i as an essential molecular element in this process provides insight into NK cell biology and may provide the basis for improved approaches to immunotherapy for infectious diseases or cancer.
Materials and Methods
Mice.
C57BL/6J (B6) (Jackson Labs), Rag2−/−γC−/−, CD45.1+ C57BL/6 (Taconic Farms), and EIIa-Cre– Spp1flstop (OPN-KO), EIIa-Cre+Spp1flstop (OPN-i-KI) mice (19) were housed in pathogen-free conditions. All experiments were performed in compliance with federal laws and institutional guidelines as approved by Dana-Farber Cancer Institute’s Animal Care and Use Committee.
NK Cell Stimulation and Flow Cytometry.
Details of NK cell stimulation and flow cytometry can be found in SI Materials and Methods.
Adoptive Transfer, Lentiviral Transduction, and MCMV Infection.
Details of adoptive transfer, lentiviral transduction, and MCMV infection are provided in SI Materials and Methods.
In Vitro and in Vivo Cytotoxicity Assays.
Details of in vitro and in vivo cytotoxic assays are provided in SI Materials and Methods.
Analysis of Spp1 mRNA and OPN Protein Expression.
Detailed analysis of Spp1 mRNA and OPN protein expression are provided in SI Materials and Methods.
Microarray.
Microarray details can be found in SI Materials and Methods.
Statistical Analyses.
Statistical analyses were performed using Student’s t test with GraphPad Prism V6 as indicated. Error bars indicate mean ± SEM. A P value of <0.05 was considered to be statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplementary Material
Acknowledgments
We thank Yue Shao (Dana-Farber Cancer Institute Microarray Facility) for microarray analysis and A. Angel for manuscript and figure preparation. This work was supported in part by National Institutes of Health Research Grant AI48125, a gift from The LeRoy Schecter Research Foundation (to H.C.), the Benacerraf Fellowship and National Research Service Award Fellowship (T32 CA070083) (to J.W.L.), and a Belgian-American Educational Foundation Fellowship (to B.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423011112/-/DCSupplemental.
References
- 1.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2(8):547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 2.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 3.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208(2):357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, et al. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest. 2013;123(10):4410–4422. doi: 10.1172/JCI69369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008;180(12):8004–8010. doi: 10.4049/jimmunol.180.12.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper MA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206(13):2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gascoyne DM, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10(10):1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 12.Firth MA, et al. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med. 2013;210(13):2981–2990. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marçais A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15(8):749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105(20):7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8(1):74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 16.Diao H, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21(4):539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Chiossone L, et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 19.Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85α–osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7(5):498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26(6):798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Lazarevic V, Glimcher LH, Lord GM. T-bet: A bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13(11):777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon SM, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36(1):55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11(10):945–952. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntington ND, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178(8):4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 26.Luevano M, Madrigal A, Saudemont A. Transcription factors involved in the regulation of natural killer cell development and function: An update. Front Immunol. 2012;3:319. doi: 10.3389/fimmu.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillard GO, et al. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7(8):e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinthorne JF, Beli E, Duriancik DM, Gardner EM. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J Immunol. 2013;190(2):712–722. doi: 10.4049/jimmunol.1201837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranson T, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci USA. 2003;100(5):2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8(8):856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 35.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 37.Min-Oo G, Bezman NA, Madera S, Sun JC, Lanier LL. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med. 2014;211(7):1289–1296. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15(6):546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.