Significance
Rapid eye movement (REM) sleep is a critical component of restful sleep, yet the mechanisms that control REM sleep are incompletely understood. Brainstem cholinergic neurons have been implicated in REM sleep regulation, but heterogeneous cell types in the area have made it difficult to determine the specific role of each population, leading to a debate about the importance of cholinergic neurons. Therefore, we selectively activated brainstem cholinergic neurons to determine their role in REM sleep regulation. We found that activation of cholinergic neurons during non-REM sleep increased the number of REM sleep episodes but not REM sleep duration. Our data demonstrate that brainstem cholinergic neurons are important modulators of REM sleep and clarify their role in REM sleep initiation.
Keywords: rapid eye movement sleep, acetylcholine, optogenetics, mesopontine tegmentum, mouse
Abstract
Rapid eye movement (REM) sleep is an important component of the natural sleep/wake cycle, yet the mechanisms that regulate REM sleep remain incompletely understood. Cholinergic neurons in the mesopontine tegmentum have been implicated in REM sleep regulation, but lesions of this area have had varying effects on REM sleep. Therefore, this study aimed to clarify the role of cholinergic neurons in the pedunculopontine tegmentum (PPT) and laterodorsal tegmentum (LDT) in REM sleep generation. Selective optogenetic activation of cholinergic neurons in the PPT or LDT during non-REM (NREM) sleep increased the number of REM sleep episodes and did not change REM sleep episode duration. Activation of cholinergic neurons in the PPT or LDT during NREM sleep was sufficient to induce REM sleep.
Rapid eye movement (REM) sleep is tightly regulated, yet the mechanisms that control REM sleep remain incompletely understood. Early pharmacological and unit recording studies suggested that ACh was important for REM sleep regulation (1, 2). For example, injection of cholinergic drugs into the dorsal mesopontine tegmentum reliably induced a state very similar to natural REM sleep in cats (3–6). Unit recordings from the cholinergic areas of the mesopontine tegmentum revealed cells that were active during wakefulness and REM sleep, as well as neurons active only during REM sleep (7–13). Electrical stimulation of the laterodorsal tegmentum (LDT) in cats increased the percentage of time spent in REM sleep (14), and activation of the pedunculopontine tegmentum (PPT) in rats induced wakefulness and REM sleep (15). If cholinergic PPT and LDT neurons are necessary for REM sleep to occur, as the early studies suggest, then lesioning the PPT or LDT should decrease REM sleep. In cats, lesions of the PPT and LDT do disrupt REM sleep (16, 17), but lesions in rodents have had little effect on REM sleep or increased REM sleep (18–22). Additionally, c-fos studies have found very few cholinergic cells activated under high-REM sleep conditions. When c-fos–positive cholinergic neurons in the PPT and LDT are found to correlate with the percentage of REM sleep, they still account for only a few of the total cholinergic cells in the area (23). Juxtacellular recordings of identified cholinergic neurons in the LDT found these cells had wake and REM active firing profiles, with the majority firing the highest during REM sleep (13). These discrepancies have led to alternative theories of REM sleep regulation, where cholinergic neurons do not play a key role (18, 19, 23, 24 and reviewed in 25, 26).
The PPT and LDT are made up of heterogeneous populations of cells, including distinct populations of cholinergic, GABAergic, and glutamatergic neurons (27–29). Many GABAergic neurons are active during REM sleep, as indicated by c-fos (23), and both GABAergic and glutamatergic neurons have been found with maximal firing rates during REM sleep in the LDT and medial PPT (13). To distinguish the differential roles of each cell type in REM sleep regulation, a method that can modulate specific cell types in the behaving animal is needed. Optogenetics now provides this ability to target specific subpopulations of neurons and control them with millisecond temporal resolution (30). Therefore, we aimed to determine the role of cholinergic neurons in the PPT and LDT in REM sleep regulation using optogenetics.
Results
Channelrhodopsin Expression Was Selective to Cholinergic Neurons in the PPT and LDT and Functional in Vitro.
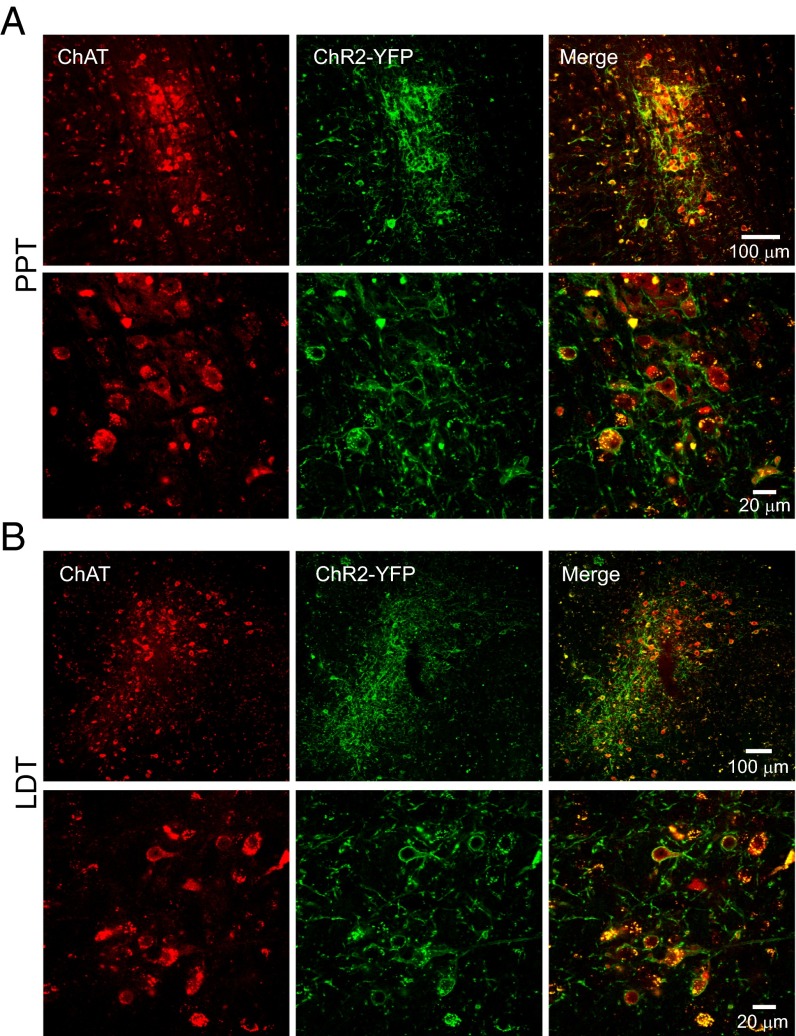
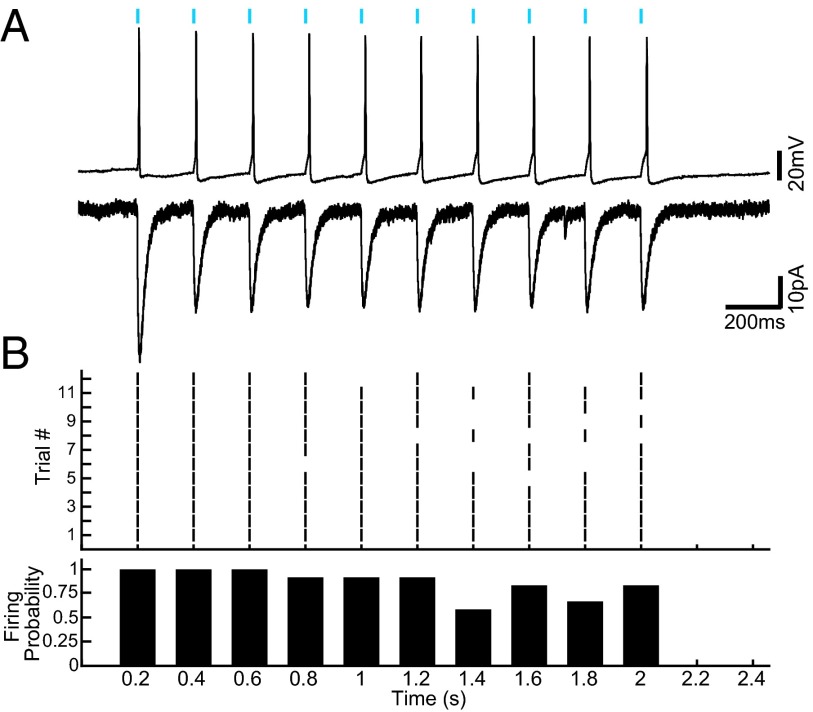
Mice expressing channelrhodopsin (ChR2) conjugated to YFP under the choline acetyltransferase (ChAT) promoter and WT littermates were used (six PPT ChAT-ChR2+, five PPT ChAT-ChR2−, five LDT ChAT-ChR2+, and six LDT ChAT-ChR2− mice per group, and one ChAT-ChR2+ patch-clamp mouse) (31). Immunohistochemistry for ChAT confirmed that ChR2 was expressed selectively in cholinergic neurons in the PPT and LDT. Quantification revealed that 96.1% (2,636 of 2,742) and 94.0% (1,992 of 2,119) of ChAT-positive neurons were also positive for ChR2-YFP in the PPT and LDT, respectively. Colocation of ChAT and ChR2-YFP in this range is consistent with previous reports demonstrating selective expression in the cortex (100%), striatum (100%), globus pallidus (100%), and medial habenula (98.2%) for the same mouse strain (31). No ChR2-YFP–only neurons were found (Fig. 1). Fig. 2A demonstrates that LDT neurons (one shown, n = 2 tested) had reliable rapid-onset action potentials following 5-ms light pulses. Fig. 2B shows the ability of a cell to follow the light pulses at 5 Hz over 2 s. The cell never missed the first light pulse, but the probability of a spike occurring decreased with progressive light pulses. The likelihood of a spike occurring varied as a function of the starting membrane potential. If the cell started near −55 mV, it was more likely to fire and follow the light pulses. If the cell started closer to −60 mV, it missed more light pulse-induced action potentials. The latency from start of the light pulse to the beginning of the action potential ranged from 4.6 ms for the first light pulse to 20 ms for the 10th light pulse in the series.
Fig. 1.
ChAT and ChR2-YFP were colocalized in the PPT (A; bregma, −4.84 mm) and LDT (B; bregma, −5.02 mm). Confocal images of coronal brain sections stained for ChAT and ChR2-YFP in the PPT (A, Top) and LDT (B, Top) are shown. (Magnfication: 25×.) Confocal images show robust colocalization of ChAT to the cell bodies and ChR2-YFP to the cell membrane in the PPT (A, Bottom) and LDT (B, Bottom). (Magnification: 40×.)
Fig. 2.
Blue light-induced reliable action potentials in cholinergic LDT neurons expressing ChR2-YFP. (A) Current-clamp (Top) and voltage-clamp (Bottom) recordings of a ChR2-YFP–expressing cholinergic LDT neuron during photostimulation (5-ms blue light pulse at 5 Hz for 2 s, blue bars). (B) Twelve 10-pulse trials for one neuron, showing the ability of the neuron to follow the light pulse (Top) and the probability of an action potential for each light pulse (Bottom).
Activation of Cholinergic Neurons in the PPT and LDT Increases the Probability of REM Sleep.
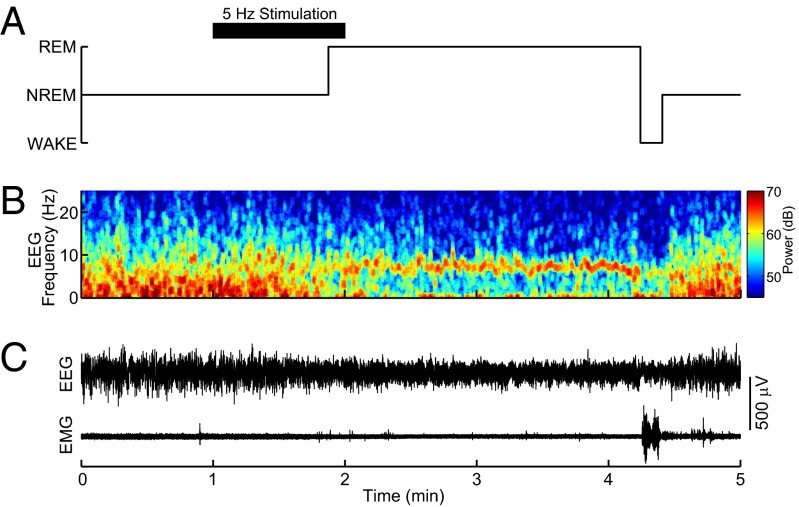
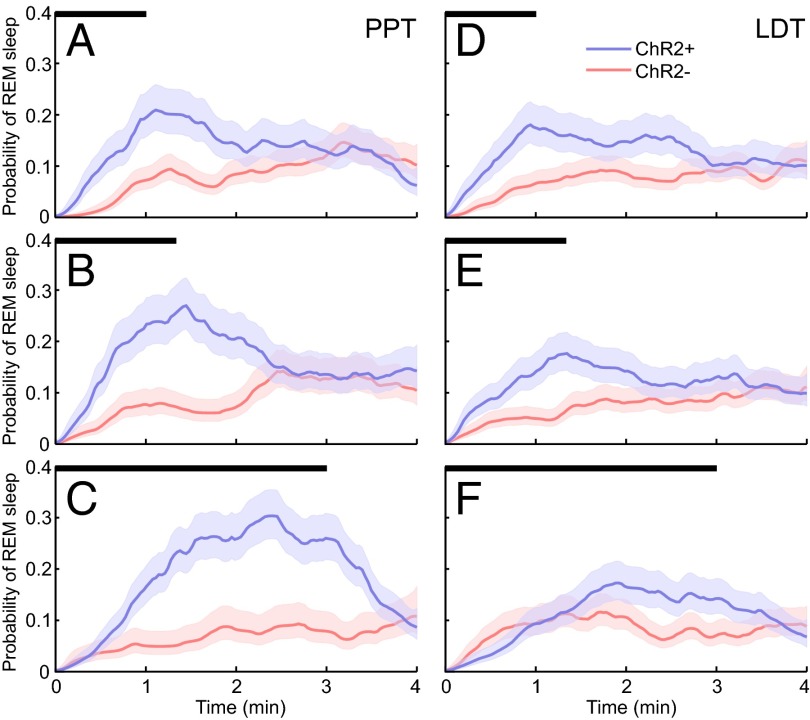
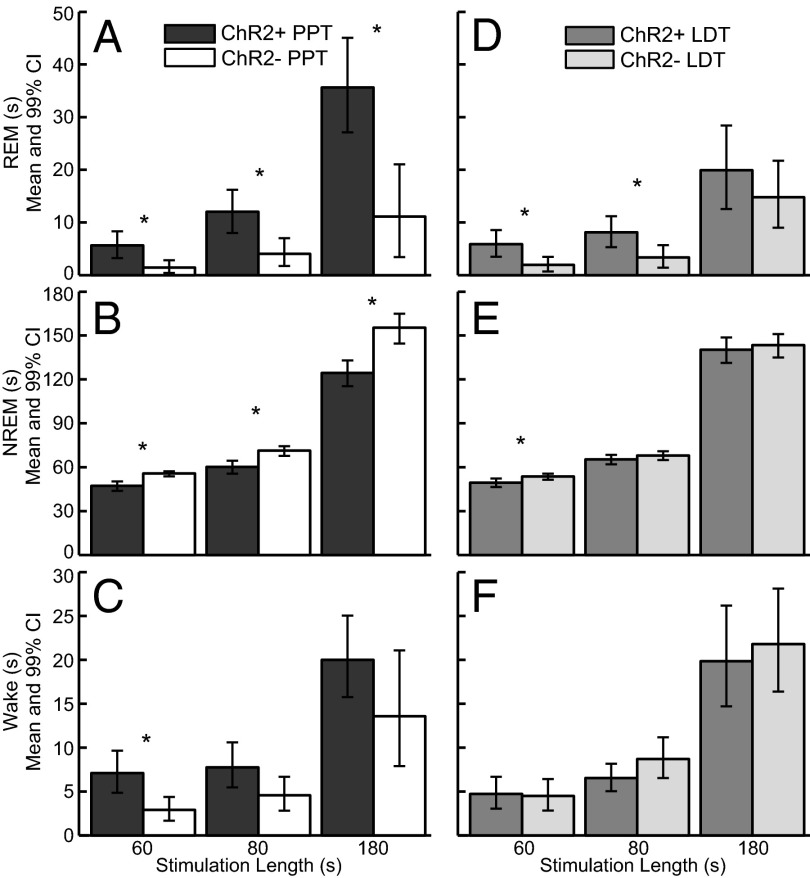
Mice expressing ChR2 in cholinergic neurons (ChR2+) and their WT littermates (ChR2−) were implanted with bilateral fiber optics for the PPT or LDT, as well as EEG and electromyogram (EMG) electrodes. REM sleep was classified by high levels of theta (5–9 Hz) in the EEG and no muscle tone. EEG and EMG traces demonstrate that optogenetic activation of cholinergic neurons in the PPT during non-REM (NREM) sleep induced REM sleep (Fig. 3). The probability of REM sleep over time increased between ChR2+ mice (n = 11) and ChR2− mice (n = 11) for all stimulations for both the PPT and LDT (Fig. 4). REM sleep probability was significantly higher [nonoverlapping confidence intervals (CIs)] for 30–60 s beyond the stimulation for ChR2+ PPT and LDT 60-s and 80-s stimulations compared with ChR2− 60-s and 80-s stimulations. REM sleep probability for the PPT 180-s stimulation reached its peak of 0.30 (95% CI: 0.26–0.35) at 2 min and 25 s, and then started to decline. REM sleep probability for the LDT 180-s stimulation significantly increased between 2 and 3 min (nonoverlapping CIs) compared with the ChR2− 180-s stimulation. Group data from 22 mice and an average of 173 stimulations per condition (range: 99–226 stimulations per condition; Table 1) show that optogenetic activation of the PPT or LDT increased REM sleep (Fig. 5 A and D). PPT 180-s stimulation increased REM sleep more than the LDT 180-s stimulation for ChR2+ mice (difference of means = 15.72 s, 99% CI: 3.90–27.51 s). NREM sleep decreased between ChR2+ mice and ChR2− mice for all PPT stimulations and the 60-s LDT stimulation (Fig. 5 B and E). Wakefulness did not change for either PPT or LDT stimulation, except for a small increase in wakefulness for the 60-s PPT stimulation (Fig. 5 C and F). Inferences of the differences between groups were calculated using 99% CIs (details are provided in Table 2 and SI Materials and Methods, Data Analysis).
Fig. 3.
Representative example of optogenetic induction of REM sleep in ChAT-ChR2+ mice showing the unprocessed EEG and EMG traces. (A) Hypnogram of wake, NREM sleep, and REM sleep shows the changing sleep states across the stimulation. The black bar indicates the timing of optical stimulation (5-ms pulse at 5-Hz for 60 s). Sleep-wake states were determined by analyzing the power spectra of the EEG (B) and the raw EEG and EMG (C).
Fig. 4.
Probability of REM sleep as a function of time (mean and 95% CI). Probability of REM sleep in ChR2+ (blue) and ChR2− (red) mice for PPT stimulation across 60-s (A), 80-s (B), and 180-s (C) stimulations (stimulation time marked by black bars). Probability of REM sleep for LDT stimulation across 60-s (D), 80-s (E), and 180-s (F) stimulations (stimulation time marked by black bars).
Table 1.
Number of stimulations per condition
| Stimulation length, s | ChR2+ | ChR2− | |
| PPT | 60 | 150 | 151 |
| 80 | 168 | 146 | |
| 180 | 171 | 99 | |
| LDT | 60 | 196 | 181 |
| 80 | 226 | 219 | |
| 180 | 163 | 183 |
Fig. 5.
Group data demonstrate that optogenetic activation of cholinergic neurons in the PPT and LDT increased REM sleep. Activation of the PPT (A) increased REM sleep during the stimulation window for 60-s, 80-s, and 180-s stimulations, whereas activation of the LDT (D) increased REM sleep for the 60-s and 80-s stimulations. PPT activation decreased NREM sleep for all stimulations (B), whereas LDT activation only decreased NREM sleep for the 60-s stimulation (E). (C and F) Wakefulness during the stimulation window did not change, except for a small increase for the 60-s PPT stimulation. *Significant differences between groups with 99% confidence (details of the numbers used to generate the inference are provided in Table 2).
Table 2.
Difference of ChR2+ and ChR2− means and 99% CIs
| Stimulation length, s | Difference of means | Lower CI | Upper CI | Significance | |
| PPT | |||||
| REM | 60 | 4.2 | 1.5 | 7.2 | Increase |
| 80 | 8.0 | 3.0 | 13.0 | Increase | |
| 180 | 24.5 | 11.6 | 37.0 | Increase | |
| NREM | 60 | −8.4 | −12.1 | −4.8 | Decrease |
| 80 | −11.2 | −16.5 | −5.7 | Decrease | |
| 180 | −30.9 | −43.8 | −16.2 | Decrease | |
| Wake | 60 | 4.2 | 1.6 | 7.2 | Increase |
| 80 | 3.2 | 0.0 | 6.3 | No change | |
| 180 | 6.4 | −2.2 | 13.9 | No change | |
| Stimulations that induced REM, % | 60 | 0.2 | 0.1 | 0.3 | Increase |
| 80 | 0.2 | 0.1 | 0.3 | Increase | |
| 180 | 0.4 | 0.2 | 0.5 | Increase | |
| REM episode duration | 60 | 8.2 | −49.2 | 54.2 | No change |
| 80 | 21.5 | −8.1 | 50.5 | No change | |
| 180 | −17.7 | −65.0 | 29.9 | No change | |
| LDT | |||||
| REM | 60 | 3.9 | 1.1 | 6.9 | Increase |
| 80 | 4.8 | 1.1 | 8.4 | Increase | |
| 180 | 5.1 | −4.7 | 15.6 | No change | |
| NREM | 60 | −4.1 | −7.6 | −0.6 | Decrease |
| 80 | −2.6 | −6.9 | 1.7 | No change | |
| 180 | −3.1 | −14.9 | 8.7 | No change | |
| Wake | 60 | 0.2 | −2.3 | 2.8 | No change |
| 80 | −2.2 | −5.1 | 0.5 | No change | |
| 180 | −1.9 | −10.3 | 6.4 | No change | |
| Stimulations that induced REM, % | 60 | 0.1 | 0.0 | 0.2 | Increase |
| 80 | 0.1 | 0.0 | 0.2 | Increase | |
| 180 | 0.1 | −0.1 | 0.2 | No change | |
| REM episode duration | 60 | 12.3 | −33.4 | 52.0 | No change |
| 80 | 4.1 | −32.3 | 39.2 | No change | |
| 180 | 1.2 | −26.3 | 28.8 | No change |
Increase in REM Sleep Is Due to More REM Sleep Episodes.
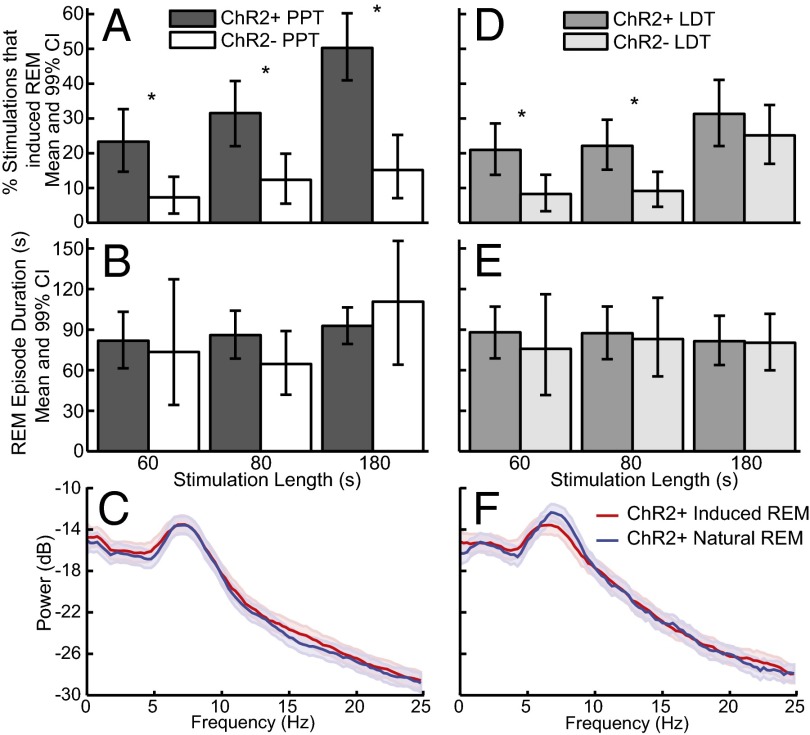
The increase in REM sleep occurred by increasing the number of REM sleep episodes (percentage of stimulations that induced REM sleep; Fig. 6 A and D) but not the duration of REM sleep episodes (Fig. 6 B and E). The induced REM sleep was electrophysiologically similar to natural REM sleep. Power spectral analysis of the EEG during induced REM sleep was not significantly different from the power spectra during natural REM sleep in the same ChR2+ mice (Fig. 6 C and F), as indicated by overlapping 95% CIs.
Fig. 6.
Optogenetic activation of cholinergic neurons in the PPT and LDT increased the number of REM sleep episodes but not REM sleep episode duration. The percentage of stimulations that induced REM sleep per number of REM sleep episodes increased for the PPT (A) and LDT (D). The duration of REM sleep episodes that started within the stimulation window did not change for the PPT (B) or LDT (E). The power spectra were not different for PPT-induced (C) or LDT-induced (F) REM sleep compared with noninduced REM sleep in the same ChR2+ mice. *Significant differences between groups with 99% confidence (details of the numbers used to generate the inference are provided in Table 2).
Fiber Optics Were Localized to the PPT and LDT.
Fig. 7 summarizes the results of the histological analyses demonstrating that the tips of the fiber optics were localized just above the PPT or LDT bilaterally. Placing the tips of the fibers at the top of the nuclei ensured as complete as possible activation of the entire nuclei based on Yizhar et al.’s (32) calculations of light dissipation in the brain. Stained sections were compared with the mouse brain atlas (33) to identify their final location (Fig. 7A). The average PPT stereotaxic coordinates were 4.80 mm posterior to bregma, 1.14 mm lateral to the midline, and −3.15 mm ventral. The average LDT stereotaxic coordinates were 5.03 mm posterior to bregma, 0.59 mm lateral to the midline, and −3.03 mm ventral.
Fig. 7.
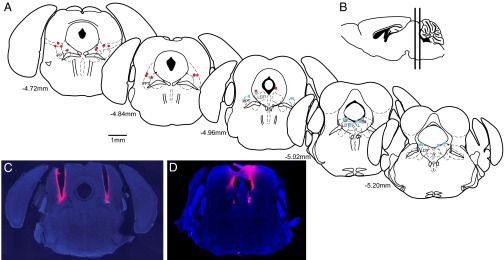
Tips of the fiber optics were localized to the top of the PPT or LDT. (A) Location of each fiber optic tip above the PPT (●) and LDT (■) is shown on coronal sections modified from the mouse brain atlas (33). Blue indicates ChR2+ mice, and red indicates ChR2− mice. (B) Vertical lines on the sagittal section show the span of the coronal sections. Coronal DAPI-stained sections show representative tracts over the PPT (C) and LDT (D) left by 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; pink)–coated fiber optics.
Wake and REM Sleep Stimulations.
Eighty-second (5 ms at 5 Hz) stimulations during wakefulness caused a small increase in the probability of wakefulness for the 4 min after the beginning of the stimulation [0.92 (95% CI: 0.91–0.93, n = 167) for ChAT-ChR2+ mice compared with 0.90 (95% CI: 0.90–0.91, n = 75) for ChAT-ChR2− mice; difference of the means = 1.6 (95% CI: 0.9–2.4)]. Eighty-second (5 ms at 5 Hz) stimulations during REM sleep did not change the duration of REM sleep between ChAT-ChR2+ mice 73.2-s (95% CI: 62.9–83.8, n = 87), ChAT-ChR2− mice 71.5-s (95% CI: 57.3–86.7, n = 51), and baseline days with no stimulation for 82.8 s in both ChAT-ChR2+ and ChAT-ChR2− mice (95% CI: 77–88.5, n = 333). Lack of a significant difference was calculated using the difference of the means and 98.3% (Bonferroni correction for three comparisons) CIs of the difference.
Discussion
PPT and LDT Cholinergic Neurons Are Important for REM Sleep Initiation but Not REM Sleep Maintenance.
Activation of cholinergic neurons in the PPT or LDT during NREM sleep increased the probability of REM sleep (Fig. 4) and the number of REM sleep episodes (Fig. 5) but not the duration of REM sleep episodes (Fig. 6). REM sleep probability for the PPT 180-s stimulation reached its peak of 0.30 (95% CI: 0.26–0.35) at 2 m and 25 s, and then started to decline (Fig. 4C). As has been suggested before (34), there are likely different mechanisms controlling REM sleep initiation vs. REM sleep maintenance. Activation of cholinergic cells in the basal forebrain during REM sleep was able to increase the duration of REM sleep episodes (35), whereas stimulation of the PPT during REM sleep in this study was not able to prolong REM sleep episodes. The present study suggests that cholinergic PPT and LDT neurons are important modulators of REM sleep initiation but not REM sleep maintenance. PPT 180-s stimulation increased REM sleep more than the LDT 180-s stimulation for ChR2+ mice (difference of means 15.72 s, 99% CI: 3.90–27.51 s). This difference suggests that although both areas contain cholinergic neurons, the PPT may be better situated in the REM sleep circuitry to control REM sleep initiation.
Implications for the Role of PPT and LDT Cholinergic Neurons in REM Sleep Modulation.
The PPT and LDT contain multiple subpopulations of REM-on neurons, which have made it difficult to distinguish the differential roles of each subtype. C-fos studies show both cholinergic and GABAergic REM-on neurons in rats (23), and juxtacellular recordings have found both GABAergic and glutamatergic REM-on neurons (13). Interestingly, this juxtacellular study did not find any cholinergic REM-on neurons. Instead, the identified cholinergic neurons were active during both wake and REM sleep, with the majority firing the highest during REM sleep (13). The cholinergic agonist carbachol induced prolonged REM sleep by inhibiting presumably cholinergic REM-on neurons and exciting presumably noncholinergic REM-on neurons in the cat pons (10). The present study demonstrated that selective optogenetic activation of cholinergic neurons in the PPT and LDT induced REM sleep in mice.
Monoamines are thought to inhibit REM sleep, and cholinergic neurons in the LDT are inhibited by serotonin in both rat (36) and guinea pig (37) brain slices. The selective 5-hydroxytryptamine 1A (5-HT1A) receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) selectively inhibits presumably cholinergic PPT REM-on neurons but not wake-on/REM-on neurons in cats (8). Therefore, it was surprising that Grace et al. (20) found that local PPT delivery of 8-OH-DPAT increased REM sleep in rats. In theory, this manipulation should inhibit cholinergic REM-on cells, which would have been expected to decrease REM sleep. In light of the multiple cell types that are now known to exhibit a REM-on firing pattern in the LDT and PPT (13), it is possible that serotonin inhibited GABAergic or glutamatergic REM-on neurons. In addition, it is possible that serotonin inhibition of cholinergic PPT occurs through other receptor subtypes or that selectivity for the 5-HT1A receptor by 8-OH-DPAT is lost at high concentrations. Also, 5HT1A serotonin receptor mRNA has only been found in GABAergic but not cholinergic neurons in the PPT of the mouse (38). Taken together, these data suggest that it is possible Grace et al. (20) inhibited GABAergic or glutamatergic REM-on neurons to get an increase in REM sleep, whereas we selectively activated cholinergic REM-on neurons to get an increase in REM sleep. Interestingly, both manipulations resulted in a change in the number of REM sleep episodes and did not change REM sleep episode duration, suggesting that the PPT is involved in REM sleep initiation but not REM sleep maintenance. Additional studies are needed to tease apart the differential roles of cholinergic, GABAergic, and glutamatergic REM-on cells in the PPT and LDT. There are about 50% fewer cholinergic cells compared with GABAergic cells in the PPT and LDT (29), in addition to intermingled glutamatergic cells. The fact that activation of a small number of cholinergic cells can elicit a strong increase in REM sleep adds to the strength of the evidence that those cholinergic neurons are important for REM sleep initiation.
Basal forebrain cholinergic neurons also contribute to the regulation of sleep and wakefulness. Carbachol injection into the basal forebrain of cats increased wakefulness (39). A recent study optogenetically activated cholinergic neurons in the basal forebrain during wake, NREM sleep, and REM sleep (35). Activation of basal forebrain cholinergic neurons during NREM sleep induced transitions to wake and REM sleep. However, these states were shorter in duration than natural wake and REM sleep episodes, and the stimulation did not influence whether wake or REM sleep was induced. Stimulation of the cholinergic basal forebrain during wakefulness in Han et al. (35) decreased NREM sleep. In the present study, stimulation of cholinergic neurons in the PPT during wakefulness caused a small increase in the probability of wakefulness. Optogenetic stimulation of cholinergic basal forebrain neurons during REM sleep increased the duration of REM sleep episodes, whereas stimulation of PPT cholinergic neurons during REM sleep in the present study did not prolong REM sleep. Basal forebrain carbachol injection decreased the amount of pontine carbachol injection-induced REM sleep-like state (39), suggesting that the basal forebrain arousal promoting system interacts with the pontine REM promoting system. Taken together, these findings suggest that there may be a forebrain component to REM sleep maintenance. Han el al. (35) concluded that the basal forebrain cholinergic neurons are responsible for terminating NREM sleep. This interpretation is complementary to our interpretation that brainstem cholinergic neurons are important for initiating REM sleep.
Implications for the Role of PPT and LDT Cholinergic Neurons in Wakefulness.
Given the wakefulness and REM sleep firing profile of cholinergic PPT and LDT neurons (7–13), we would have expected a larger effect on wakefulness. Instead, we only found two conditions that slightly increased wakefulness: the 60-s PPT stimulations during NREM sleep and the 80-s PPT stimulations during wakefulness. The PPT and LDT are well positioned to activate the cortex during both wakefulness and REM sleep. However, when activated during NREM sleep, the brainstem cholinergic neurons preferentially shift an animal to REM sleep vs. wakefulness.
Possible Explanations for the Delay in REM Sleep Onset After Stimulation.
The probability of REM sleep builds over the time course of the stimulation. A few possibilities exist for why the REM sleep transition is not an immediate switch with short latency: (i) cholinergic tone must build up to a certain level and meet a network threshold for the transition to occur; (ii) incomplete activation of the nucleus due to limited spread of the light out of the fiber optic or nonoptimal placement of the fibers; (iii) only one cholinergic brainstem center was activated at a time due to the small size of the mouse brain and limited space to target both the PPT and LDT simultaneously; and (iv) cholinergic cells in the PPT and LDT are modulators of REM sleep and exert their effect via projections to other REM-on areas, such as the sublaterodorsal nucleus. Slice recordings support cholinergic modulation of the sublaterodorsal nucleus, where carbachol activated spinally projecting sublaterodorsal nucleus neurons (40). Animals with fibers that were optimally positioned over the PPT and had the highest fiber transmittance had the strongest REM induction effect.
Limitations.
The transgenic mice used in the present study have been found to express extra copies of the vesicular ACh transporter gene and have increased cholinergic tone (41). Behaviorally, these mice have prolonged motor endurance and impaired attention and memory. These mice were reported to be more active at night than WT mice but had no difference in activity level during the day. In our hands, we recorded sleep during the day and found that the ChR2+ mice had 4% REM sleep compared with 3.7% REM sleep in the ChR2− mice, and this difference was not statistically different. All of our experiments were performed during the day, when locomotor activity and percentage of REM sleep were the same between experimental groups; therefore, we think our results are representative of the true effect. The transgenic mice used in the present study expressed ChR2 in cholinergic neurons throughout the brain. Hence, it is possible that activation of cholinergic fibers of passage from other brain regions to the PPT or LDT contributed to REM sleep induction. Future studies that selectively inhibit cholinergic neurons in the PPT and LDT of nonhypercholinergic mice are needed to determine if cholinergic neurons are necessary for REM sleep generation.
Conclusions
The present findings demonstrate that activation of cholinergic neurons in the PPT or LDT during NREM sleep is sufficient to increase REM sleep in mice. The induced REM sleep state closely resembles natural REM sleep. Selectively increasing cholinergic tone in the PPT or LDT increases the number of REM sleep episodes but not the duration of REM sleep episodes. Therefore, cholinergic neurons in the PPT and LDT remain potent modulators of REM sleep initiation. This modulation of REM sleep expression may occur via activation of other REM-on neuron populations, such as the pontine reticular formation and sublaterodorsal nucleus.
Materials and Methods
Adult male mice (n = 23) expressing ChR2 under the ChAT promoter (stock no. 014546; The Jackson Laboratory) (31) and their WT littermates were implanted with EEG and EMG electrodes and bilateral fiber optics in the PPT or LDT. All experiments were approved by the Massachusetts Institute of Technology Committee on Animal Care. The mice were placed in a recording chamber while EEGs and EMGs were recorded for 6–8 h. Blue light from a laser was used to stimulate the cholinergic PPT or LDT neurons optogenetically during NREM sleep. Stimulations were 60 s, 80 s, or 180 s long, separated by at least 1 min, and each experiment consisted of ∼25 stimulations. EEGs and EMGs were used to score sleep manually. Variables calculated included the amount of time spent in NREM sleep, REM sleep, and wake within the stimulation; REM sleep episode duration; and percentage of REM sleep induced. The probability of REM sleep over the time course of the stimulation was also plotted. Fiber placement and specific expression of ChR2 in cholinergic neurons were confirmed by histology. Patch-clamp of a ChAT-ChR2+ mouse LDT slice was performed to confirm light-induced action potentials. Statistical inferences were determined by calculating 95% CIs for each group. The decision rule was to reject the null hypothesis if zero is not in the 95% CI of the difference between the groups. Details of the experiment procedures are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by NIH Grant DP1-OD003646 (to E.N.B.), NIH Grant TR01-GM104948 (to E.N.B. and M.A.W.), and NIH Grant T32-HL07901 (to C.J.V.); a Massachusetts General Hospital Executive Committee on Research Fellowship (to C.J.V.); and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423136112/-/DCSupplemental.
References
- 1.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: Reciprocal discharge by two brainstem neuronal groups. Science. 1975;189(4196):55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 2.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8(4):302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Amatruda TT, 3rd, Black DA, McKenna TM, McCarley RW, Hobson JA. Sleep cycle control and cholinergic mechanisms: Differential effects of carbachol injections at pontine brain stem sites. Brain Res. 1975;98(3):501–515. doi: 10.1016/0006-8993(75)90369-8. [DOI] [PubMed] [Google Scholar]
- 4.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414(2):245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 5.George R, Haslett WL, Jenden DJ. A cholinergic mechanism in the brainstem reticular formation: Induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127(3):133–164. [PubMed] [Google Scholar]
- 7.el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76(3):519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 8.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: A simultaneous unit recording and microdialysis study. J Neurosci. 1998;18(14):5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569(2):210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 10.Sakai K, Koyama Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport. 1996;7(15-17):2449–2453. doi: 10.1097/00001756-199611040-00009. [DOI] [PubMed] [Google Scholar]
- 11.Grant SJ, Highfield DA. Extracellular characteristics of putative cholinergic neurons in the rat laterodorsal tegmental nucleus. Brain Res. 1991;559(1):64–74. doi: 10.1016/0006-8993(91)90287-6. [DOI] [PubMed] [Google Scholar]
- 12.Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10(8):2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucetta S, Cissé Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34(13):4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar M, Portas C, McCarley RW. Chronic low-amplitude electrical stimulation of the laterodorsal tegmental nucleus of freely moving cats increases REM sleep. Brain Res. 1996;723(1-2):223–227. doi: 10.1016/0006-8993(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 15.Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77(6):2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 16.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458(2):285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 17.Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: Integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571(1):50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 19.Boissard R, et al. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: A combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16(10):1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 20.Grace KP, Liu H, Horner RL. 5-HT1A receptor-responsive pedunculopontine tegmental neurons suppress REM sleep and respiratory motor activity. J Neurosci. 2012;32(5):1622–1633. doi: 10.1523/JNEUROSCI.5700-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglis WL, Semba K. Discriminable excitotoxic effects of ibotenic acid, AMPA, NMDA and quinolinic acid in the rat laterodorsal tegmental nucleus. Brain Res. 1997;755(1):17–27. doi: 10.1016/s0006-8993(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 22.Petrovic J, Ciric J, Lazic K, Kalauzi A, Saponjic J. Lesion of the pedunculopontine tegmental nucleus in rat augments cortical activation and disturbs sleep/wake state transitions structure. Exp Neurol. 2013;247:562–571. doi: 10.1016/j.expneurol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19(8):3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verret L, Léger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21(9):2488–2504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 25.Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007;584(Pt 3):735–741. doi: 10.1113/jphysiol.2007.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luppi PH, et al. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100(5-6):271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29(2):340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29(14):4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: Codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363(2):177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 31.Zhao S, et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8(9):745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 3rd Ed Academic; San Diego: 2007. [Google Scholar]
- 34.Luppi PH, Clément O, Fort P. Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr Opin Neurobiol. 2013;23(5):786–792. doi: 10.1016/j.conb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Han Y, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24(6):693–698. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Luebke JI, et al. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci USA. 1992;89(2):743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard CS, Llinás R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: An in vitro electrophysiological study. Neuroscience. 1994;59(2):309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 38.Bonnavion P, Bernard JF, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518(14):2744–2770. doi: 10.1002/cne.22331. [DOI] [PubMed] [Google Scholar]
- 39.Baghdoyan HA, Spotts JL, Snyder SG. Simultaneous pontine and basal forebrain microinjections of carbachol suppress REM sleep. J Neurosci. 1993;13(1):229–242. doi: 10.1523/JNEUROSCI.13-01-00229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng FJ, et al. Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord. J Physiol. 2014;592(Pt 7):1601–1617. doi: 10.1113/jphysiol.2013.261800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolisnyk B, et al. ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. J Neurosci. 2013;33(25):10427–10438. doi: 10.1523/JNEUROSCI.0395-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.