Significance
Dietary specialization determines an organism’s resource base as well as impacts on host or prey species. There are important basic and applied reasons to ask why some animals have narrow diets and others are more generalized, and if different regions of the Earth support more specialized interactions. We investigated site-specific host records for more than 7,500 species of insect herbivores. Although host specialists predominate, the proportion of specialists is affected by the diversity of hosts and shifts globally, supporting predictions of more exclusive tropical interactions. These results not only affect our understanding of the ecology of food webs, but also have implications for how they respond to environmental change, as well as for ecosystem management and restoration.
Keywords: host range, latitudinal gradient, niche width, Pareto distribution, specialization
Abstract
Understanding variation in resource specialization is important for progress on issues that include coevolution, community assembly, ecosystem processes, and the latitudinal gradient of species richness. Herbivorous insects are useful models for studying resource specialization, and the interaction between plants and herbivorous insects is one of the most common and consequential ecological associations on the planet. However, uncertainty persists regarding fundamental features of herbivore diet breadth, including its relationship to latitude and plant species richness. Here, we use a global dataset to investigate host range for over 7,500 insect herbivore species covering a wide taxonomic breadth and interacting with more than 2,000 species of plants in 165 families. We ask whether relatively specialized and generalized herbivores represent a dichotomy rather than a continuum from few to many host families and species attacked and whether diet breadth changes with increasing plant species richness toward the tropics. Across geographic regions and taxonomic subsets of the data, we find that the distribution of diet breadth is fit well by a discrete, truncated Pareto power law characterized by the predominance of specialized herbivores and a long, thin tail of more generalized species. Both the taxonomic and phylogenetic distributions of diet breadth shift globally with latitude, consistent with a higher frequency of specialized insects in tropical regions. We also find that more diverse lineages of plants support assemblages of relatively more specialized herbivores and that the global distribution of plant diversity contributes to but does not fully explain the latitudinal gradient in insect herbivore specialization.
Variation in dietary specialization among individuals, populations, and species drives numerous ecological and evolutionary processes. Differences in diet breadth and composition mediate the coexistence of competitors (1), the persistence of species in the face of environmental disturbance (2), the diversity of interactions, and the stability of entire networks of interacting species (3, 4). At the ecosystem level, the top-down effect of predators on primary productivity can be controlled by the level of herbivore specialization (5). At the scale of evolutionary diversification, differences among lineages in rates of speciation and extinction can be understood in terms of variation in dietary specialization (6). Progress in addressing all of these issues has been limited by disparity in the methods used to quantify specialization (7) and the rarity of comparative datasets encompassing large numbers of species and regions (8).
Herbivorous insects have long served as models for the study of specialization (9), because they use a variety of plant resources in many different ways, and their host plants are discrete resources; thus, diet breadth of a given herbivore species can be quantified as the number of plant taxa that it eats. In addition to serving as models for the study of diet breadth, herbivorous insects are important in their own right as one of the most abundant and diverse forms of life on Earth, and the consumption of plant material by insect herbivores is a dominant mover of energy and matter through terrestrial ecosystems (10). Although herbivorous insects are known collectively for narrow diet breadth, species vary significantly in the number of host plant taxa that they attack, and this variation has scarcely been described in a quantitative manner (11). It is unclear, for example, whether species with relatively specialized and generalized diets anchor the ends of a continuum or if diet breadth is discontinuous, with specialists and generalists forming distinct modes. Researchers have most commonly treated diet breadth as bimodal, distinguishing only generalists and specialists (7). In some cases, this distinction is a simplification for theoretical discussion (12), and in other cases it is simply a convenience (13, 14).
A quantitative description of the relative frequencies of specialists and generalists is important for understanding the evolution and ecology of plant–insect interactions. Because specialists can evolve from generalists and vice versa (6), species presumably pass through stages of intermediate diet breadth; thus, a bimodal distribution of the number of hosts attacked would suggest that intermediate levels of diet breadth are maladaptive. Similarly, if the distribution of diet breadth is continuous, the relative frequencies of herbivores in different diet breadth categories could be examined in the context of environmental determinants of niche width. It has also been suggested that treating herbivores as either specialists or generalists creates errors in estimates of the diversity of ecological communities, including the number of species of arthropods on Earth (15). Here, we quantify herbivore host ranges from field collections of larval and adult insects. We focus many of our analyses on larval Lepidoptera (caterpillars), for which we have the most geographically extensive data, but also include herbivorous insects from other feeding guilds as well as limited samples of parasitoids (flies and wasps) that feed on caterpillars. Parasitoids potentially offer an informative contrast with insect herbivores, because they are also highly specialized but feed at a higher trophic level (16).
Our global dataset of plant–insect interactions encompasses thousands of species of herbivores from 17 localities spanning 63° latitude from Canada to Brazil in the Western Hemisphere and from the United Kingdom and Japan to Papua New Guinea in the Eastern Hemisphere (Fig. 1A and SI Appendix, section S1). In addition to our primary objective of characterizing the distribution of specialists and generalists among insect herbivores, the latitudinal breadth of our samples allows us to address hypotheses concerning global patterns of ecological interactions. In particular, several explanations for the higher diversity of species at lower latitudes assume that interactions in tropical regions are more specialized (17).
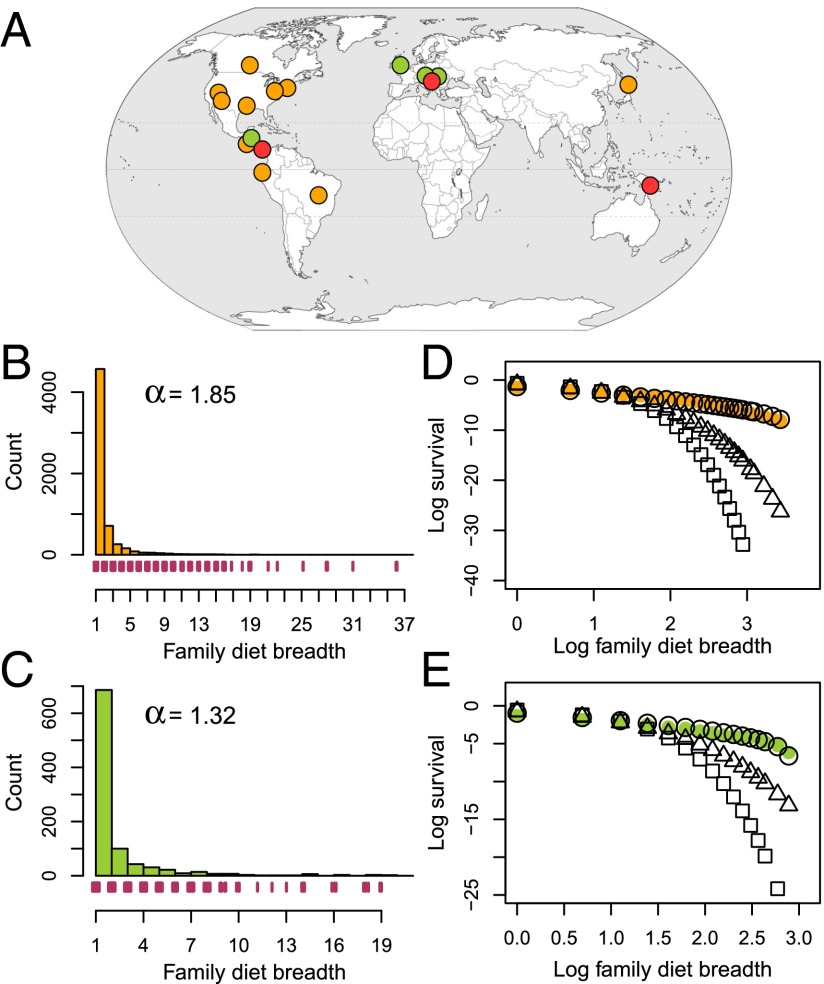
Fig. 1.
(A) Study locales and the distribution of diet breadth for (B) Lepidoptera and (C) all other herbivores. Points on the globe are shown in orange for Lepidoptera study sites, green for other herbivore study sites, and red for study sites for both. Histograms in B and C illustrate counts of the numbers of herbivores associated with different numbers of host plant families; also shown is the shape parameter (α) from the discrete, truncated Pareto distribution. Tick marks under histograms indicate individual observations for visualization in the thin tail of the distributions. (D and E) Survival plots illustrate the fit of the Pareto (white circles), geometric (triangles), and Poisson (squares) distributions to the data (colored symbols). Log survival on the y axis is ln(P(X > x)), which is the natural logarithm of the probability of herbivores having a greater diet breadth (X) than the corresponding value (x) on the x axis. Note that most analyses focus on diet breadth at the scale of individual sites; for simplicity, diet breadth is shown here across sites.
For example, a greater presence of specialists at lower latitudes could promote diversification of plant traits (18), and herbivory by specialists can contribute to coexistence among competing plants (19). However, evidence both for and against global gradients in interaction specificity has been reported (20–22), and we do not know if latitudinal trends in specificity might result from heterogeneity of resources or other factors.
Results
The distribution of taxonomic diet breadth is characterized by a highly skewed, concave distribution that is well-fit by a discrete, truncated Pareto power law. Examples of diet breadth distributions for both Lepidoptera and other herbivores are shown in Fig. 1 B and C. Also shown in Fig. 1 are visualizations of fit to other distributions that are commonly used for ecological count data (Fig. 1 D and E). Compared with the truncated Pareto distribution, the geometric and Poisson distributions fit the diet breadth data poorly. We use the discrete, truncated Pareto, because the long tail of the empirical distributions of diet breadth (Fig. 1B) suggests a power law, and the discrete, truncated formulation is appropriate to the particular data being modeled (the number of hosts attacked by herbivores is a discrete count, and truncation results from the number of plant taxa attacked by any one herbivore always being less than the total number of plant taxa sampled). Furthermore, the shape parameter (α) of the discrete, truncated Pareto is more informative than measures of central tendency, such as the mean, for highly nonsymmetrical distributions. Higher values of α indicate a greater proportion of more specialized herbivores.
The distributions in Fig. 1 B and C illustrate family-level diet breadth (the number of host plant families attacked), which is highly correlated with species-level diet breadth (the number of host plant species attacked): Pearson’s correlation coefficient between family- and species-level diet breadth across all herbivores = 0.89 (P < 0.001) (S1 Appendix, section S2). Species-level diet breadth is also closely fit by the discrete, truncated Pareto (SI Appendix, Fig. S2); high values of α, indicating a concentration of specialists and a long tail of generalists, characterize most of the taxonomic and functional subsets of the global data (SI Appendix, Table S3). Based on these data, an average of 69% of caterpillar species are associated with a single host family at each site; above 25° latitude, this value is 60%, and at sites less than 25° latitude, it is 83%. Among the sampled guilds of herbivorous insects, 76% of species associate with a single host family, although values for individual guilds vary widely (species-level diet breadth for the different feeding guilds as well as Lepidoptera shows similar patterns) (SI Appendix, Table S3).
Geographically, the discrete, truncated Pareto shows a consistently good fit across latitudes (SI Appendix, Fig. S4), whereas the shape parameter (α) increases toward the equator (P = 0.0046; F2,10 = 8.87; R2 = 0.64) (Fig. 2A). The increase in α toward the equator for larval Lepidoptera corresponds to a greater relative frequency of specialized herbivores. In contrast, maximum diet breadth does not change with latitude, although a greater number of potential hosts is available in the tropics (white symbols in Fig. 2B). Although the upper limit of the distribution is unchanged with latitude, there is a shift throughout the distribution toward more specialized diets, such that most insect herbivores become more specialized toward the equator, which is illustrated in Fig. 2B (SI Appendix, Figs. S5 and S6).
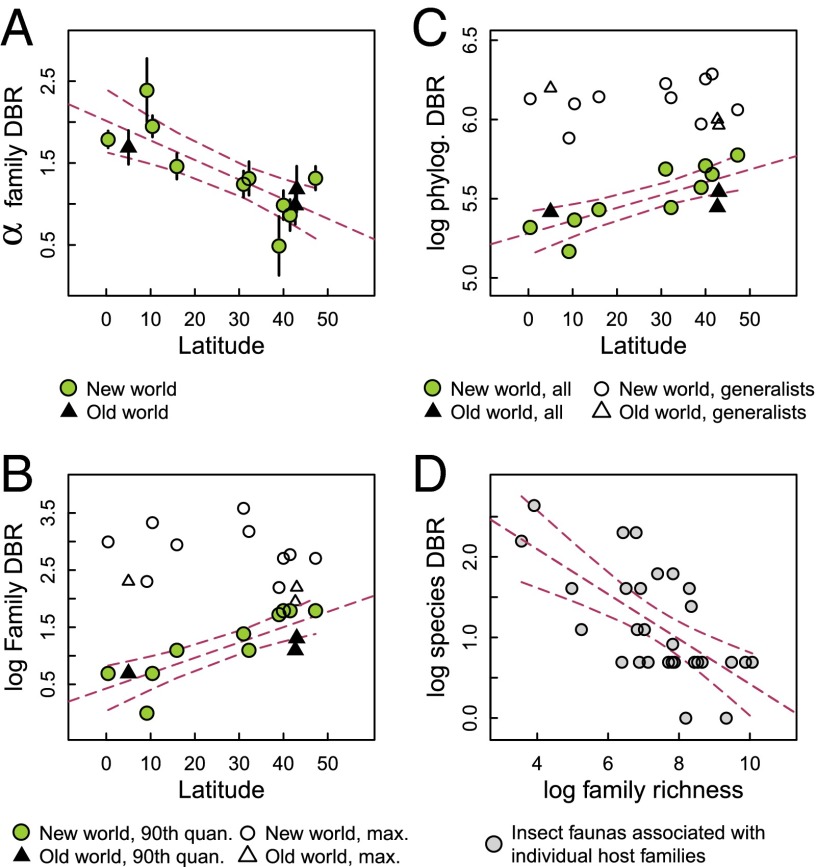
Fig. 2.
Patterns in the distribution of diet breadth (DBR) for Lepidoptera. (A) Latitudinal trend in the shape parameter (α) for family-level DBR with bootstrapped SEs for individual sites; larger values of α indicate distributions with a higher density of herbivores having more narrow DBRs. (B) Maximum observed DBR (white symbols) and DBR in the 90th quantile of the DBR distributions (colored symbols) vs. latitude. Lower values of the 90th quantile correspond to a distribution in which more herbivores are compressed toward lower, more specialized DBRs. (C) Latitudinal patterns in phylogenetic DBR among sites for all species are shown with colored symbols, and more generalized species that attack more than one host family are shown with white symbols (log units on the y axis are in millions of years). (D) Relationship between median species-level DBR (as the number of host species attacked; y axis) for herbivores associated with particular families and the species richness of the plant families (x axis); more species-rich plant families host more specialized herbivores (SI Appendix, section S4 shows analyses on latitudinal subsets of herbivores). For all panels, 95% confidence limits are shown around linear relationships, and the natural logarithm is used in all cases. In A–C, circles (white and green) are New World sites, and triangles (white and black) are Old World sites; differences between white and colored symbols in B and C are specific to B and C.
Taxonomic diet breadth is a convenient but incomplete index of host range or dietary niche width, because herbivores attack particular plants for a variety of reasons (e.g., phytochemistry or geography) that may or may not be captured by simply counting the number of species eaten. As a complementary approach to investigating the latitudinal gradient in specialization, we calculated phylogenetic diet breadth (23) for Lepidoptera species associated with angiosperms, for which a robust phylogeny is available at the family level (24). Phylogenetic family-level diet breadth, as measured by phylogenetic distance (PD) among hosts, changes globally: mean PD declines toward lower latitudes (P < 0.001; F2,10 = 11.58; R2 = 0.70) (Fig. 2C), despite the greater phylogenetic diversity of tropical compared with temperate plants (25). This result is driven by the increase in the number of specialists (species with low PD) at lower latitudes. Thus, when specialists (i.e., herbivores associated with a single host family) are removed from the analysis, PD does not change with latitude (white symbols in Fig. 2C).
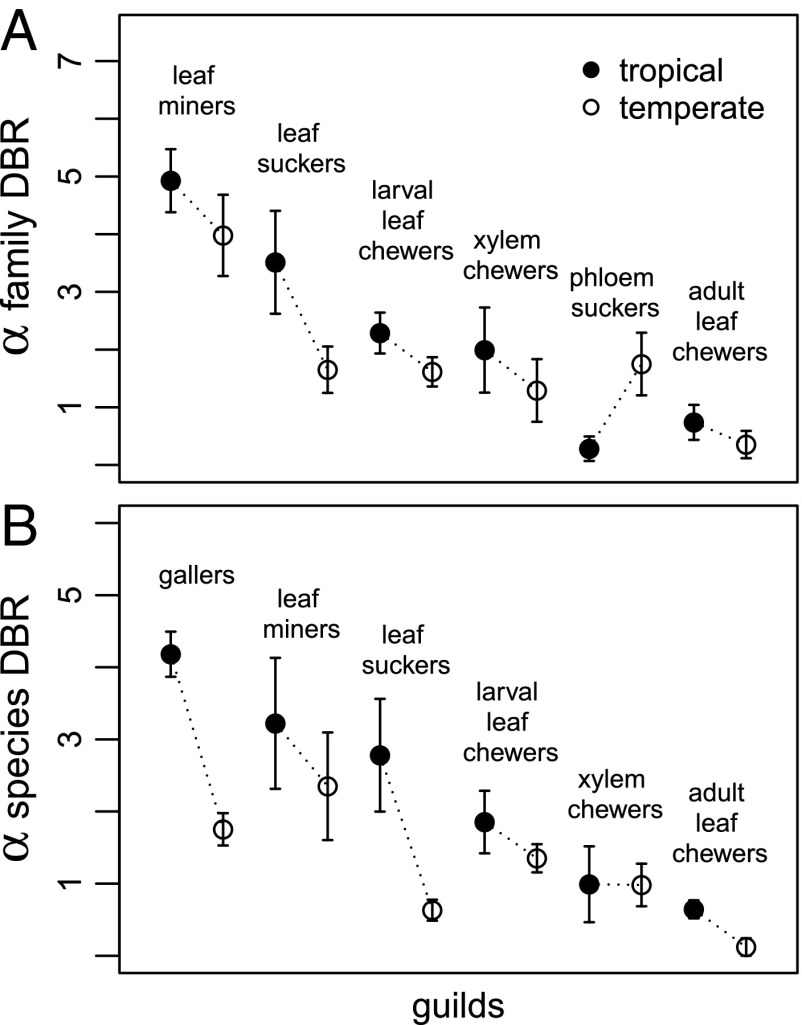
Previously, insect herbivores were reported to be more specialized at lower latitudes in the Western Hemisphere (21), whereas no latitudinal trend in specialization was found in the Eastern Hemisphere, where the sampled vegetation had been standardized for latitudinal differences in phylogenetic diversity (20). Results from this analysis for both taxonomic and phylogenetic diet breadth suggest that data from both hemispheres conform to a single global latitudinal gradient (Fig. 2 A and C). These analyses differ from previous studies in a number of ways: we have focused on a single life history stage (larvae), we have included phylogenetic information for insect hosts from all sites, and we have increased the number of sites from both hemispheres. The inference of a global gradient in herbivore specialization is supported by temperate and tropical comparisons among different feeding guilds, which is illustrated by variation in α for family- and species-level diet breadth in Fig. 3. Average α across samples of species-level diet breadth rarefied to the lowest number of hosts in each comparison is significantly higher in tropical than temperate samples (Wilcoxon signed rank test, P = 0.016), and the same is true for family-level diet breadth if the one apparent exception, phloem suckers, is removed (P = 0.031; without phloem suckers removed, P = 0.16). Guilds with the most intimate host plant associations (gallers and leaf miners) show the highest levels of specialization (26), and it is noteworthy that such interguild differences are evident, despite latitudinal variation (Fig. 3). Among the most generalized guilds are mobile adult chewers, such as leaf beetles (family Chrysomelidae), that are able to move among individual plants to a greater extent than sessile or wingless life stages and may achieve a broader diet than larvae through selection of plant tissues that limit exposure to phytochemicals (27).
Fig. 3.
Diet breadth (DBR) comparisons for herbivore guilds from tropical and temperate communities for (A) family-level DBR and (B) species-level DBR. Higher values of the shape parameter (α) indicate more specialized diets. Means and SDs are based on rarefaction to the lowest number of host taxa sampled in each pairwise comparison connected by dotted lines. More than one community is represented by some but not all of the points (SI Appendix, section S1). Note that not all guilds could be analyzed for both species- and family-level DBR (Materials and Methods and SI Appendix contain more details).
Variation in specialization among lineages and regions is addressed by theories of adaptive radiation and coevolution (28), which predict a negative relationship between the diversity of available resources and the diet breadth of consumers. Unexploited resources, for example, allowed the Hawaiian honeycreepers to evolve into a large number of specialized species (29). Thus, for herbivorous insects, it has been hypothesized that lower latitudes might harbor more specialized herbivores because of the diversity of hosts; similarly, the most species-rich lineages of plants might allow for the evolution of a greater number of specialists (30–32). However, these patterns have been difficult to evaluate empirically given the scope of many previous studies. Based on more than 6,000 species of Lepidoptera, we investigated connections between herbivore diet breadth and plant diversity and found that host plant families with high global species richness are associated with assemblages of herbivores with relatively narrow species-level diet breadth (P < 0.001; R2 = 0.56) (Fig. 2D). This relationship holds when linear models contain covariates associated with plant families, including sample size (the number of experimental rearings from a family), phylogenetic age, and latitudinal extent of geographic range (SI Appendix, Table S4 shows analyses of latitudinal subsets of herbivores). The relationship between host richness and dietary specialization could inform our understanding of the latitudinal gradient in herbivore specialization if lower latitudes contain more diverse lineages of hosts. This possibility was addressed with path analysis, including a direct effect of latitude on specialization as well as an indirect effect of latitude mediated through plant richness (the number of plant families and species encompassed by insect sampling at each site). We found that the effect of latitudinal variation in plant richness on specialization is approximately one-fourth the direct effect of latitude on the global trend in specialization (SI Appendix, Fig. S7), indicating that plant diversity may contribute to variation in specialization but does not fully explain the global pattern of interactions (33).
Discussion
In summary, the distribution of diet breadth for insect herbivores conforms to a power law, with a majority of species associated locally with a single plant taxonomic family or species and a long tail of more generalized herbivores. The distribution of specialization shifts globally with latitude (Fig. 2A), which confirms the long-standing expectation that interactions are more specific at lower latitudes (17) and contributes to our understanding of the latitudinal diversity gradient. Plant diversity affects insect diet breadth (Fig. 2D and SI Appendix, Fig. S7), which may, in turn, feed back onto plant diversity through either coevolutionary (30) or ecological (12, 34) interactions, although the investigation of such feedback processes is beyond the scope of this study (35).
When interpreting the frequencies of insects in different diet breadth categories (Fig. 1), it is important to note that we have focused on local estimates of diet breadth derived from collections and rearings at focal sites. The alternative approach (using host records that encompass entire geographic ranges of herbivores) has less relevance for understanding ecological determinants of diet breadth (36) and faces the additional challenge that widespread generalists may often be composed of cryptic, localized specialists (37). Clearly, the distribution of herbivore diet breadth is continuous (specialists and generalists are not separated by a gap in frequency distributions) (Fig. 1), although it may be useful to consider herbivores as belonging to either the great majority of specialists or the long, thin tail of generalists. A similar frequency distribution is apparent in the diet breadth of insect parasitoids (SI Appendix, Fig. S3), suggesting that the highly concave, skewed distribution could be inherent to the parasitic habit, of which insect herbivores are only one example, albeit the most well-studied (38). In general, the predominance of specialists is relevant to issues in natural resource management and challenges the idea that, in human-impacted systems, the interactions among novel suites of co-occurring species will be comparable in structure with less-degraded systems (39, 40).
Recent approaches to studying biotic networks include comparisons of distributions of natural interactions with theoretical and mathematical predictions. In randomly assembled networks of interactions, the frequency of highly connected nodes drops rapidly beyond the mode, such as in a Poisson distribution (41, 42), and some natural networks (for example, plant–pollinator interactions) deviate from that pattern by having a small portion of relatively overconnected nodes, which we have observed with generalist herbivores (41, 43, 44). For these more heterogeneous networks, it has been suggested that facilitating processes underlie the long, thin tail of the frequency distribution (45). In the case of herbivore diet breadth, the process of host range expansion could be such a process: initial expansion of diet breadth might be rare, but after more than one host plant family has been colonized, adaptations for generalized feeding could facilitate the colonization of new hosts (46). This possibility is consistent with the observation that highly polyphagous species of butterflies are more likely to use novel hosts (47, 48), and the ubiquity of the highly peaked, skewed distribution of diet breadth suggests that any processes facilitating dietary generalization operate not only in different regions of the globe, but also within different lineages and guilds of herbivores. An additional mechanism for the observed frequencies of diet breadth could be disruptive selection, favoring either extreme specialization or increasing generalization. However, the biological factors that favor dietary generalism as an ever-present but relatively infrequent life history strategy in herbivorous insects must await additional study.
Materials and Methods
Data Collection.
Rearing of field-collected caterpillars (Lepidoptera) to establish consumer–host relationships was undertaken at 13 sites in North, Central, and South America, Papua New Guinea, Japan, and central Europe (Fig. 1A and SI Appendix, Table S1). Collections and rearings focused on externally feeding caterpillars, mostly macrolepidopterans, with broader sampling at some sites. Site-specific details are provided in SI Appendix for each dataset and site or a collection of sites when more than one site has been managed in a similar way. We tabulated data for other herbivores to study variation in diet breadth within and among ecological guilds, with the goal of encompassing both sucking and chewing feeding modes, species using various plant resources (including leaves, phloem sap, and wood), and species having diverse life histories, from species intimately connected with plants, such as miners and gallers, to those that are mobile, either as larvae or adults (Fig. 3). Data were compiled from seven sites (three sites in common with the Lepidoptera data and four additional sites) (Fig. 1A and SI Appendix, Table S2). As with Lepidoptera, all data for these herbivores are based on field collections for both larval and adult stages (depending on the herbivore taxa).
Heterogeneity among sites in methodology and sampling effort is almost always present in global datasets, which emphasizes the importance of statistical controls (covariates) to account for variation in sampling effort. Although this approach has a well-established history in ecology, covariates introduce complexity to models and reduce degrees of freedom for main effects; however, substantial power is gained by the inclusion of additional sites, despite differences in design. Variation in sampling effort could be particularly important when considering variation in diet breadth: an understudied herbivore community might seem more specialized, because not all plant–insect interactions will have been sampled, or less specialized if rare species are mostly specialists (and missed by sampling). Furthermore, the discovery of interactions will necessarily take more time in areas with more diverse floras and faunas. However, our most diverse sites are tropical and extremely well-sampled, with 71 site-years of sampling below 30° latitude just for Lepidoptera (SI Appendix, Table S1). We use the number of years of study along with other appropriate covariates (including the number of rearing records and the geographical area of study sites) to address sampling issues throughout our analyses, which are described below and in SI Appendix. Ultimately, biological signal is evident at multiple scales (in latitudinal patterns and the consistent fit of the Pareto distribution among sites), despite differences in methodologies and sampling effort. It can also be noted that the number of rearing records per Lepidoptera species does not vary with latitude (F1,11 = 0.84; R2 = 0.0071; P = 0.38). Although we have been successful in detecting relationships among our focal variables, future research in the area of global interactions could reasonably strive for a greater number of sites with standardized methodologies that would potentially increase explained variance in analyses, such as the path models in SI Appendix, Fig. S7.
To determine host associations for each herbivore species, data from each site were subjected to site-specific preanalysis filters appropriate to the methods of collection and taxonomic knowledge at each site (SI Appendix, section S1). Data for Lepidoptera from some of the sites were analyzed previously in the works by Dyer et al. (21) and Novotny et al. (20). Here, we updated those previous datasets with additional years (more than double the previously examined number of years for some sites) and added new locations for Lepidoptera (Ohio, Great Basin, and Japan). We also added 18 datasets for other herbivores that had not previously been brought together into one analysis (SI Appendix, section S1). Identification procedures varied among sites, but voucher specimens have been deposited at appropriate museums, and both insect and plant species were identified by knowledgeable taxonomists in so far as possible; several sites additionally used DNA barcoding to validate species identifications.
Pareto Distribution.
Because the distribution of diet breadth is highly skewed (Fig. 1), the core of our statistical approach is the use of a theoretical distribution appropriate to the structure of the data. We have used a version of the Pareto distribution, from which a shape parameter (α) can be extracted that serves as a useful summary statistic; higher values of α correspond to diet breadth distributions that are more highly peaked, with a greater density of specialists. The Pareto distribution (49) is widely used in a number of fields of science, and the truncated Pareto has been proposed as an important extension (50). Here, we use a form of the Pareto that is truncated and discrete, and thus, it is appropriate for ecological count data (51); in our case, we are interested in counts of host plant families and host plant species associated with specific herbivore species. We say that a random variable X has a truncated discrete Pareto distribution with parameters α, β, and γ if
for any natural number of host plant taxa (x), such that γ ≤ x ≤ β. Here, α is any real number, and γ and β are positive integers. Parameters γ and β are the lower and upper truncation parameters, respectively, for which maximum likelihood estimates were used: the sample minimum for γ and the sample maximum for β. We estimated α by minimizing the sum of squares of the differences between the model survival function and the empirical survival function. The distribution was fit to data using custom R scripts (SI Appendix, section S5).
As a measure of goodness of fit or closeness of the modeled discrete truncated Pareto distribution to the empirical distribution of the data, we used the maximum absolute difference between the modeled and the empirical cumulative distribution functions. This approach provides the largest difference between probabilities computed using the model and the relative frequency from the data, and it gives an intuitive notion of closeness (52). Fits of the discrete, truncated Pareto to our data were visualized using plots of survival functions, as in the work by Aban et al. (50), which are useful for comparing values in the tail of the distribution with predictions generated by theoretical distributions (53–55). We compared the discrete, truncated Pareto with the geometric and Poisson distributions; in Fig. 1, it can be seen that the Pareto (white circles) predict the observations (colored symbols) throughout the tail of the distribution.
Relationships between parameters from the Pareto distribution fitted to diet breadth (e.g., the shape parameter, α) and latitude were investigated for Lepidoptera with linear models that included sample size (the number of experimental rearings per site), area (of sampled locations), the number of herbivore species, and the number of years (over which sampling was conducted) as covariates. For other herbivores, we did not have the same well-sampled latitudinal gradient but were able to make pairwise comparisons between temperate and tropical sites (SI Appendix, Table S2). Because these analyses did not involve multiple regression models, we did not include covariates associated with sampling effort or other factors as in the Lepidoptera analyses. Instead, we used a rarefaction approach based on the number of plant taxa (families and species) sampled, which is a key axis along which datasets differ: when one dataset includes 15 hosts and another includes only 10 hosts, the latter will potentially be biased toward a more narrow observed diet breadth among herbivores. For each set of comparisons (for example, matrices of plant–insect associations for leaf miners from tropical and temperate communities), we subsampled each matrix down to the lowest number of plant taxa sampled for any of the matrices involved in the comparison, and we did this 1,000 times using the sample function in R and inspected means and variances across resampled replicates.
Phylogenetic Analyses.
To investigate phylogenetic diet breadth of Lepidoptera species, we used PD among host plant families calculated with the R package picante and using the angiosperm phylogeny from the work by Davies et al. (24), which was the most complete angiosperm phylogeny available at the time of analysis (nonangiosperm host records were excluded from these analyses). As with parameters from the fitted Pareto distributions (see above), the relationship between PD and latitude was investigated using linear models and covariates to account for sampling effort.
Plant Diversity.
Diversity of resources (host plants) can affect the evolution of consumer traits by providing opportunities for local adaptation and specialization, a possibility that we have investigated in two complementary ways: using plant lineages (for which resource diversity is global species richness of plant families) followed by using geographic sites as replicates (for which resource diversity is richness of sampled plant taxa). For the first approach, multiple regression used median species-level diet breadth of insect herbivores as the response variable and the following independent variables: species richness (number of species within plant families), relative age (extracted from the angiosperm phylogeny) (24), latitudinal range, and sample size (the number of times in which an insect was reared from any species in a host plant family). Median diet breadth was used here instead of α from the Pareto, because comparisons were being made among subsets of data (insects associated with plant families) that varied greatly in sample size, and the Pareto could not always be fit to the smallest sample sizes. However, the use of the median is conservative, because shifts in the tail of the distribution may not be reflected in the median value when comparing two distributions that have similar numbers of extreme specialists. Species richness (the total number of species within plant families) for these analyses was taken from the angiosperm diversity website maintained by the Missouri Botanical Garden (www.mobot.org/MOBOT/research/APweb/), which was also used as a reference to standardize family names from the different databases used in this project. Latitudinal range (the maximum extent of north–south latitude occupied by any species in the plant family) was taken from family-level distribution maps in Heywood (56). Only plant families for which all measures were available (richness, relative age, and latitudinal range) were included in analyses. Furthermore, a sample size cutoff was used to avoid plant families that were less well-characterized from the perspective of insect rearings: we considered families from which insects had been reared at least 100 times. To investigate the robustness of results, the multiple regression using these variables was repeated with subsets of the data, specifically only using insects for which rearing records were restricted to sites either greater than 25° or less than or equal to 25° latitude.
Our second approach to understanding the influence of plant diversity on dietary specialization involved path analyses and variation among sites in dietary specialization and plant richness. Path analysis is useful in this context, because it allows for the simultaneous analysis of direct and indirect effects. Specifically, path analyses included the α shape parameter from the Pareto distributions and plant richness per site as endogenous variables (plant richness for each site is the number of plant families and species associated with the sampled herbivores). The exogenous variable was simply latitude, which pointed directly to α and indirectly to α through plant richness (allowing for the possibility that latitude affects α through resource diversity but also has effects that are not explained by global variation in plant richness). The model was evaluated using plant richness as both the number of plant species and the number of plant families sampled at sites.
Supplementary Material
Acknowledgments
For helpful suggestions on the manuscript, we thank Douglas Futuyma and three anonymous reviewers. We also thank Jonathan Davies for the angiosperm phylogeny. Funding was provided by the US National Science Foundation, the Earthwatch Institute, the Czech Science Foundation, the University of Missouri–St. Louis, Wesleyan University, the Howard Hughes Medical Institute, the Brazilian Council for Scientific and Technological Development, and the Brazilian Federal District Foundation for Science and Research. Additional acknowledgments are SI Appendix, section S6.
Footnotes
The authors declare no conflict of interest.
Data deposition: The diet breadth distribution data have been deposited in the Dryad database, datadryad.org (doi:10.5061/dryad.hg549).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423042112/-/DCSupplemental.
References
- 1.Büchi L, Vuilleumier S. Coexistence of specialist and generalist species is shaped by dispersal and environmental factors. Am Nat. 2014;183(5):612–624. doi: 10.1086/675756. [DOI] [PubMed] [Google Scholar]
- 2.Devictor V, Julliard R, Jiguet F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos. 2008;117(4):507–514. [Google Scholar]
- 3.Mougi A, Kondoh M. Diversity of interaction types and ecological community stability. Science. 2012;337(6092):349–351. doi: 10.1126/science.1220529. [DOI] [PubMed] [Google Scholar]
- 4.Lever JJ, van Nes EH, Scheffer M, Bascompte J. The sudden collapse of pollinator communities. Ecol Lett. 2014;17(3):350–359. doi: 10.1111/ele.12236. [DOI] [PubMed] [Google Scholar]
- 5.Singer MS, et al. Herbivore diet breadth mediates the cascading effects of carnivores in food webs. Proc Natl Acad Sci USA. 2014;111(26):9521–9526. doi: 10.1073/pnas.1401949111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy NB, Otto SP. Specialization and generalization in the diversification of phytophagous insects: Tests of the musical chairs and oscillation hypotheses. Proc Biol Sci. 2014;281(1795):20132960. doi: 10.1098/rspb.2013.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devictor V, et al. Defining and measuring ecological specialization. J Appl Ecol. 2010;47(1):15–25. [Google Scholar]
- 8.Forister ML, Dyer LA, Singer MS, Stireman JO, 3rd, Lill JT. Revisiting the evolution of ecological specialization, with emphasis on insect-plant interactions. Ecology. 2012;93(5):981–991. doi: 10.1890/11-0650.1. [DOI] [PubMed] [Google Scholar]
- 9.Futuyma DJ, Moreno G. The evolution of ecological specialization. Annu Rev Ecol Syst. 1988;19:207–233. [Google Scholar]
- 10.Seastedt T, Crossley D., Jr The influence of arthropods on ecosystems. Bioscience. 1984;34(3):157–161. [Google Scholar]
- 11.Jaenike J. Host specialization in phytophagous insects. Annu Rev Ecol Syst. 1990;21:243–273. [Google Scholar]
- 12.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104(940):501–528. [Google Scholar]
- 13.Erwin TL. Tropical forests: Their richness in Coleoptera and other arthropod species. Coleopt Bull. 1982;36(1):74–75. [Google Scholar]
- 14.Dyer LA. Tasty generalists and nasty specialists? Antipredator mechanisms in tropical lepidopteran larvae. Ecology. 1995;76(5):1483–1496. [Google Scholar]
- 15.Ødegaard F. How many species of arthropods? Erwin's estimate revised. Biol J Linn Soc Lond. 2000;71(4):583–597. [Google Scholar]
- 16.Wiegmann BM, Mitter C, Farrell B. Diversification of carnivorous parasitic insects: Extraordinary radiation or specialized dead end? Am Nat. 1993;134(5):737–754. [Google Scholar]
- 17.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annu Rev Ecol Evol Syst. 2009;40:245–269. [Google Scholar]
- 18.Agrawal AA, Fishbein M. Phylogenetic escalation and decline of plant defense strategies. Proc Natl Acad Sci USA. 2008;105(29):10057–10060. doi: 10.1073/pnas.0802368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer LA, Letourneau DK, Chavarria GV, Amoretti DS. Herbivores on a dominant understory shrub increase local plant diversity in rain forest communities. Ecology. 2010;91(12):3707–3718. doi: 10.1890/08-1634.1. [DOI] [PubMed] [Google Scholar]
- 20.Novotny V, et al. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313(5790):1115–1118. doi: 10.1126/science.1129237. [DOI] [PubMed] [Google Scholar]
- 21.Dyer LA, et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature. 2007;448(7154):696–699. doi: 10.1038/nature05884. [DOI] [PubMed] [Google Scholar]
- 22.Morris RJ, Gripenberg S, Lewis OT, Roslin T. Antagonistic interaction networks are structured independently of latitude and host guild. Ecol Lett. 2014;17(3):340–349. doi: 10.1111/ele.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 24.Davies TJ, et al. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc Natl Acad Sci USA. 2004;101(7):1904–1909. doi: 10.1073/pnas.0308127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerkhoff AJ, Moriarty PE, Weiser MD. The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc Natl Acad Sci USA. 2014;111(22):8125–8130. doi: 10.1073/pnas.1308932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novotny V, et al. Guild-specific patterns of species richness and host specialization in plant-herbivore food webs from a tropical forest. J Anim Ecol. 2010;79(6):1193–1203. doi: 10.1111/j.1365-2656.2010.01728.x. [DOI] [PubMed] [Google Scholar]
- 27.Wäckers FL, Romeis J, van Rijn P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu Rev Entomol. 2007;52:301–323. doi: 10.1146/annurev.ento.52.110405.091352. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann M, Doebeli M. Evolution of niche width and adaptive diversification. Evolution. 2004;58(12):2599–2612. doi: 10.1111/j.0014-3820.2004.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 29.Lovette IJ, Bermingham E, Ricklefs RE. Clade-specific morphological diversification and adaptive radiation in Hawaiian songbirds. Proc Biol Sci. 2002;269(1486):37–42. doi: 10.1098/rspb.2001.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18(4):586–608. [Google Scholar]
- 31.Janz N, Nylin S, Wahlberg N. Diversity begets diversity: Host expansions and the diversification of plant-feeding insects. BMC Evol Biol. 2006;6(1):4. doi: 10.1186/1471-2148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fordyce JA. Host shifts and evolutionary radiations of butterflies. Proc Biol Sci. 2010;277(1701):3735–3743. doi: 10.1098/rspb.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewinsohn TM, Roslin T. Four ways towards tropical herbivore megadiversity. Ecol Lett. 2008;11(4):398–416. doi: 10.1111/j.1461-0248.2008.01155.x. [DOI] [PubMed] [Google Scholar]
- 34.Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dynamics of populations. In: den Boer PJ, Gradwell GR, editors. Proceedings of the Advanced Study Institute on Dynamics of Numbers in Populations. Centre for Agricultural Publishing and Documentation; Wageningen, The Netherlands: 1971. pp. 298–312. [Google Scholar]
- 35.Bagchi R, et al. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature. 2014;506(7486):85–88. doi: 10.1038/nature12911. [DOI] [PubMed] [Google Scholar]
- 36.Fox LR, Morrow PA. Specialization: Species property or local phenomenon? Science. 1981;211(4485):887–893. doi: 10.1126/science.211.4485.887. [DOI] [PubMed] [Google Scholar]
- 37.Bickford D, et al. Cryptic species as a window on diversity and conservation. Trends Ecol Evol. 2007;22(3):148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Price PW. Evolutionary Biology of Parasites. Princeton Univ Press; Princeton: 1980. pp. xi–237. [Google Scholar]
- 39.Murcia C, et al. A critique of the ‘novel ecosystem’ concept. Trends Ecol Evol. 2014;29(10):548–553. doi: 10.1016/j.tree.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Tallamy DW. Do alien plants reduce insect biomass? Conserv Biol. 2004;18(6):1689–1692. [Google Scholar]
- 41.Bascompte J, Jordano P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu Rev Ecol Evol Syst. 2007;38(38):567–593. [Google Scholar]
- 42.Erdös P, Rényi A. On random graphs. Publ Math. 1959;6:290–297. [Google Scholar]
- 43.Proulx SR, Promislow DE, Phillips PC. Network thinking in ecology and evolution. Trends Ecol Evol. 2005;20(6):345–353. doi: 10.1016/j.tree.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Lewinsohn TM, Prado PI, Jordano P, Bascompte J, Olesen JM. Structure in plant-animal interaction assemblages. Oikos. 2006;113(1):174–184. [Google Scholar]
- 45.Newman ME. Power laws, Pareto distributions and Zipf's law. Contemp Phys. 2005;46(5):323–351. [Google Scholar]
- 46.Janz N, Nylin S. The oscillation hypothesis of host-plant range and speciation. In: Tilmon KJ, editor. Specialization, Speciation and Radiation: The Evolutionary Biology of Herbivorous Insects. Univ of California Press; Berkeley, CA: 2008. pp. 203–215. [Google Scholar]
- 47.Courtney SP. The ecology of pierid butterfies: Dynamics and interactions. Adv Ecol Res. 1986;15:51–131. [Google Scholar]
- 48.Jahner JP, Bonilla MM, Badik KJ, Shapiro AM, Forister ML. Use of exotic hosts by Lepidoptera: Widespread species colonize more novel hosts. Evolution. 2011;65(9):2719–2724. doi: 10.1111/j.1558-5646.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 49.Arnold BC. Encyclopedia of Statistical Sciences. 2008. Pareto distribution. [DOI] [Google Scholar]
- 50.Aban IB, Meerschaert MM, Panorska AK. Parameter estimation for the truncated Pareto distribution. J Am Stat Assoc. 2006;101(473):270–277. [Google Scholar]
- 51.Clauset A, Shalizi CR, Newman ME. Power-law distributions in empirical data. SIAM Rev. 2009;51(4):661–703. [Google Scholar]
- 52.D'Agostino RB. Goodness-of-Fit-Techniques. CRC; Boca Raton, FL: 1986. [Google Scholar]
- 53.Zaninetti L, Ferraro M. On the truncated Pareto distribution with applications. Centr Eur J Phys. 2008;6(1):1–6. [Google Scholar]
- 54.Deidda R. A multiple threshold method for fitting the generalized Pareto distribution to rainfall time series. Hydrol Earth Syst Sci. 2010;14(12):2559–2575. [Google Scholar]
- 55.Schoenberg FP, Patel RD. Comparison of Pareto and tapered Pareto distributions for environmental phenomena. Eur Phys J Spec Top. 2012;205(1):159–166. [Google Scholar]
- 56.Heywood VH. Flowering Plants of the World. New York: Mayflower Books; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.