Significance
Chronic low-grade inflammation plays a causative role in obesity-associated metabolic diseases. However, the mechanisms underlying chronicity of low-grade inflammation remain elusive. Herein, we identify inhibitor of κB kinase epsilon (IKBKE) as a new modulator of chronic inflammation in macrophages. We first show that IKBKE is clinically relevant in distinct tissues associated with metabolic diseases including obesity, nonalcoholic steatohepatitis, and atherosclerosis. In all cases, IKBKE expression predominates in macrophages and associates with chronic inflammation. In macrophages, IKBKE affects chronicity of inflammation by temporally limiting inflammasome priming. Both global IKBKE ablation and reconstitution with hematopoietic IKBKE reciprocally alters metaflammation in adipose tissue, liver, and the composition of advanced atherosclerotic plaques. These findings establish a nonpathogenic role for hematopoietic IKBKE in limiting inflammasome priming and metaflammation.
Keywords: IKBKE, inflammasome, metaflammation, metabolic disease, immunometabolism
Abstract
Obesity increases the risk of developing life-threatening metabolic diseases including cardiovascular disease, fatty liver disease, diabetes, and cancer. Efforts to curb the global obesity epidemic and its impact have proven unsuccessful in part by a limited understanding of these chronic progressive diseases. It is clear that low-grade chronic inflammation, or metaflammation, underlies the pathogenesis of obesity-associated type 2 diabetes and atherosclerosis. However, the mechanisms that maintain chronicity and prevent inflammatory resolution are poorly understood. Here, we show that inhibitor of κB kinase epsilon (IKBKE) is a novel regulator that limits chronic inflammation during metabolic disease and atherosclerosis. The pathogenic relevance of IKBKE was indicated by the colocalization with macrophages in human and murine tissues and in atherosclerotic plaques. Genetic ablation of IKBKE resulted in enhanced and prolonged priming of the NLRP3 inflammasome in cultured macrophages, in hypertrophic adipose tissue, and in livers of hypercholesterolemic mice. This altered profile associated with enhanced acute phase response, deregulated cholesterol metabolism, and steatoheptatitis. Restoring IKBKE only in hematopoietic cells was sufficient to reverse elevated inflammasome priming and these metabolic features. In advanced atherosclerotic plaques, loss of IKBKE and hematopoietic cell restoration altered plaque composition. These studies reveal a new role for hematopoietic IKBKE: to limit inflammasome priming and metaflammation.
The metabolic syndrome is defined by the coexistence of central obesity, deregulated carbohydrate, and lipid metabolism and/or hypertension. Collectively these features increase the risk of developing type 2 diabetes, nonalcoholic fatty liver diseases, and atherosclerosis. These metabolic diseases have additional features in common; they are chronic disorders characterized by a state of persistent inflammation and tissue remodeling. In particular, chronic low-grade inflammation or “metaflammation” is now established as an important causative factor driving metabolic disease. Much progress has been made in our understanding of how metabolic stress or overnutrition induces metaflammation. However, the molecules involved in maintaining chronicity of low-grade inflammation remain unclear.
As specialized mediators of host defense, macrophages express danger-sensing pattern recognition receptors (PRRs) that include transmembrane receptors of the Toll-like receptor/interleukin-1 receptor (TLR/IL-1R) superfamily and intracellular cytosolic receptors such as RIG-I–like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors (1, 2). Numerous TLR/IL-1Rs and their downstream mediators have been implicated in the pathogenesis of obesity-associated diabetes (3, 4), fatty liver disease (5, 6), and atherosclerosis (7, 8). In some cases, putative nonmicrobial, host-derived sterile ligands have also been identified. For example, TLR4 is a putative sensor for dietary saturated fatty acids (SFAs) (4, 9) and TLR2 together with CD36 recognize oxidized low-density lipoproteins (OxLDL) (10). Recently, the NOD-like receptor NLRP3 has also been implicated in the pathogenesis of metabolic disease (11, 12) and atherosclerosis (13, 14). NLRP3 senses intracellular defects such as oxidative stress, ER stress, impaired mitophagy, impaired autophagy, or lysosome dysfunction, and responds by assembling the NLRP3 inflammasome and activating caspase-1. Active caspase-1 promotes both the proteolytic maturation of proinflammatory interleukins (IL)-1β and IL-18, and inactivation of antiinflammatory IL-33. Caspase-1 activity is also linked to the release of alarmins (15, 16) and excessive activation to pyroptotic cell death (17).
Stimulation of NLRP3-dependent inflammation is established as a “two-step” process. The first is a priming step that involves TLR/NFκB-mediated induction of NLRP3 and pro–IL-1β mRNA. The second step promotes NLRP3 inflammasome assembly and activity through additional stress-related signals. In contrast, the mechanism that maintains chronicity of inflammasome activity is not known. Here, we identify a new modulator of inflammasome priming and chronic inflammation: the inhibitor of κB kinase epsilon (IKBKE, IKKε, or IKKi). IKBKE is best characterized for its role in type 1 antiviral responses downstream of TLR4, TLR3/TRIF-dependent or MAVS-dependent signals (18–21). However, aberrant induction of IKBKE has also been linked to oncogenic signals (22) and in regulating energy balance in murine models of obesity (23, 24). This latter role remains incompletely understood because contrasting effects on weight gain have also been reported (25). Importantly, the clinical relevance of IKBKE induction has not been determined in human disease, nor has the role of hematopoietic IKBKE in metaflammation. Since IKBKE is predominantly expressed in immune cells, mediates TLR signals, and is a known NFκB target gene, we hypothesize that IKBKE is induced in parallel with inflammasome priming transcripts and as such is a good candidate to mediate inducible counterregulation of immune activity during chronic inflammation. As a regulator of inflammatory tone, IKBKE would be required to limit inflammation in a context and time-dependent manner. Herein, we report that IKBKE is required to temporally limit NLRP3 inflammasome priming in macrophages. Furthermore, we demonstrate that IKBKE expression associates with the pathogenesis of metabolic disease in humans where it colocalizes with proinflammatory macrophages, and that hematopoietic IKBKE is sufficient to limit metaflammation.
Results
IKBKE Associates with Clinical Pathology and Colocalizes with Proinflammatory Tissue Macrophages.
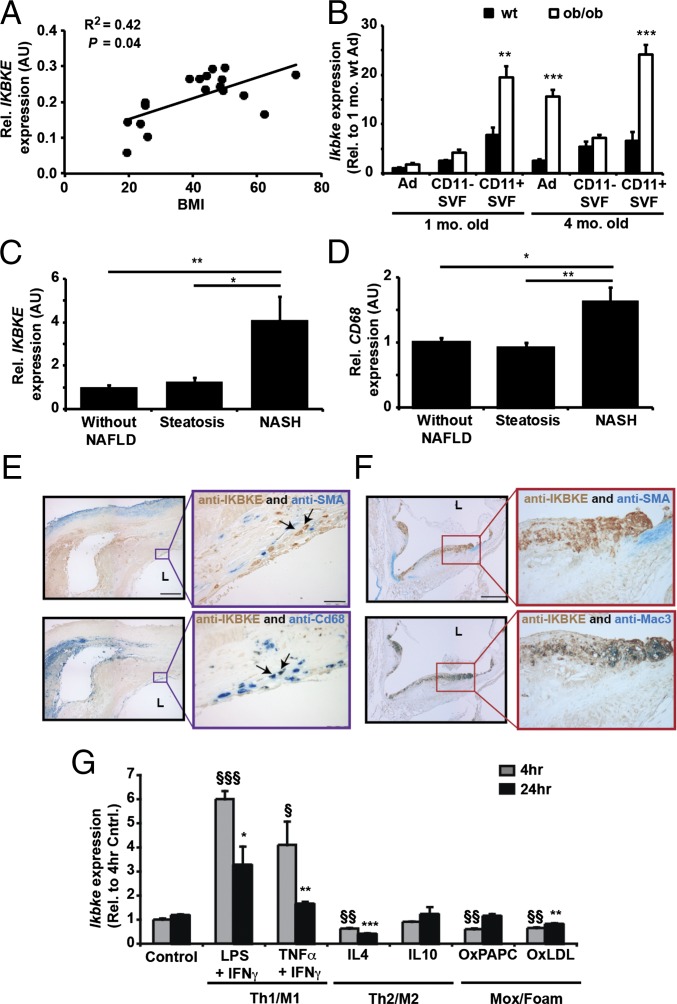
To explore the relevance of IKBKE in clinical pathology, we first examined its expression in three tissues commonly associated with metaflammation and dyslipidemia: adipose tissue, liver and vasculature. In human adipose tissue, IKBKE mRNA correlated positively with body mass index (Fig. 1A) and was induced in two murine models of obesity (Fig. S1 A and B). Adipose Ikbke also associated with increased macrophage and inflammatory markers (Fig. S1 A and B). It was elevated in macrophage-enriched CD11+ fractions, and this induction preceded the increase in adipocytes from obese tissues (Fig. 1B). Adipose Ikbke was also reduced by antiinflammatory thiazolidione treatment (Fig. S1C). In obese subjects with nonalcoholic steatohepatitis (NASH), hepatic IKBKE was significantly induced and correlated positively with expression of the macrophage marker CD68 (Fig. 1 C and D, Table 1, and Table S1). Furthermore, hepatic IKBKE expression correlated strongly with expression of multiple inflammatory genes and indicators of hepatic dysfunction (Table 1). In the vasculature, where chronic inflammation promotes atherosclerosis, IKBKE was readily detected in human atherosclerotic plaques and also in advanced plaques from proatherogenic, apolipoprotein E-deficient (Apoe−/−) mice (Fig. 1 E and F). Double labeling indicated that IKBKE colocalized primarily with infiltrating macrophages and not with SMA+ vascular smooth muscle cells (Fig. 1 E and F). These findings indicate that IKBKE is located in three pathologically relevant, anatomical locations where it is closely associated with macrophage infiltration and chronic inflammation.
Fig. 1.
IKBKE is expressed in pathogenic tissues, colocalizes with macrophages, and is induced in proinflammatory macrophages. (A) IKBKE mRNA expression in scWAT associates with human BMI. (B) Expression of Ikbke in adipose tissue fractions from wild-type and ob/ob mice at 1 and 4 mo of age. Adipocytes (Ad) and macrophage-enriched CD11+ve stromavascular fractions (SVF) were isolated as described (33). (C and D) Hepatic IKBKE and CD68 expression in obese subjects with NAFLD and/or NASH. Adjacent sections of a human carotid plaque (E) or aortic root plaque of an Apoe−/− mouse (F), coimmuno-stained for IKBKE (E and F; brown), and either SMA for smooth muscle cells (E and F, Upper; blue) or anti-CD68 (E and F, Lower; blue) or Mac3 (F, Upper; blue) for macrophages; L, lumen. (Scale bars: 200 µm.) (G) Expression of Ikbke in BMDMs after indicated treatments: LPS (100 ng/mL) + IFN-γ (100 ng/mL), TNFα (10 ng/mL) + IFN-γ (100 ng/mL), IL-4 (10 ng/mL), IL-10 (10 ng/mL), OxPAPC (50 µg/mL), and OxLDL (50 µg/mL). Statistical significance is indicated by *P < 0.05, **P < 0.01 compared with corresponding WT fractions (B), NASH patients (C and D) or 24-h control (G) and §P < 0.05, §§P < 0.01, §§§P < 0.001 compared with 4 h control (G).
Table 1.
Hepatic IKBKE expression correlates with indices of liver dysfunction and inflammatory gene expression in obese patients
| IKBKE expression | |||
| Parameter measured | rs | P | n |
| Hepatic histology | |||
| Steatosis | 0.507 | 0.004 | 30 |
| NASH | 0.542 | 0.002 | 30 |
| NAFLD activity score (NAS) | 0.559 | 0.001 | 30 |
| Plasma biochemistry | |||
| Alanine aminotransferase (ALT) | 0.412 | 0.024 | 30 |
| Aspartate transaminase (AST) | 0.375 | 0.041 | 30 |
| HOMA-IR | 0.404 | 0.037 | 27 |
| HbA1c, % | 0.430 | 0.022 | 28 |
| Hepatic gene expression | |||
| CD68 | 0.710 | <0.001 | 26 |
| IL1B | 0.570 | 0.001 | 29 |
| NLRP3 | 0.804 | <0.001 | 30 |
| CASP1 | 0.737 | <0.001 | 30 |
| CASP4 | 0.777 | <0.001 | 30 |
IKBKE Is Induced in Proinflammatory Macrophages and Its Ablation Prolongs NLRP3 Inflammasome Priming.
To investigate the involvement of IKBKE in inflammatory macrophages, we performed temporal profiling of IKBKE expression in stimulated macrophages. Classic proinflammatory (M1/Th1) activators robustly induced Ikbke expression within 4 h (Fig. 1G). Although this induction was transient, Ikbke levels remained significantly elevated for up to 24 h. This increase was observed in stimulated macrophages from both wild-type and proatherogenic Apoe−/− mice (Fig. S1 D–G). Induction of Ikbke was a common response to stimulation by multiple proinflammatory ligands, including TNFα, IL-1β, IFN-γ, LPS, and peptidoglycan (PGN), albeit with differing magnitude and temporal kinetics (Fig. S1 D–G). Conversely, Ikbke expression was inhibited by the antiinflammatory (M2a/Th2) cytokine IL-4 (Fig. 1G and Fig. S1G). IL-10, an activator of M2c macrophages, had no effect on Ikbke expression (Fig. 1G). Induction of Mox macrophages and foam cells by oxidized lipids (OxPAPC) and lipoproteins (OxLDL) transiently reduced Ikbke expression (Fig. 1G). These findings indicate that IKBKE is temporally regulated by proinflammatory mediators and may be a good candidate for cell autonomous regulation of chronic inflammation.
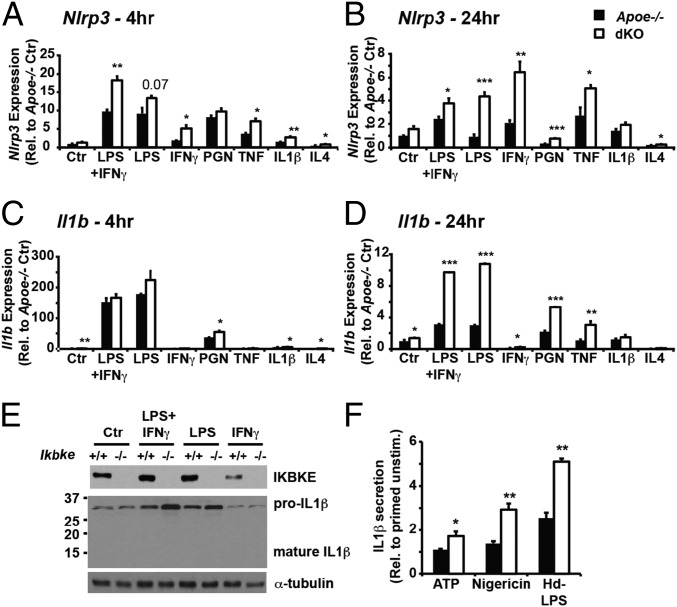
To investigate the requirement for IKBKE during an inflammatory response by proatherogenic macrophages, we first generated bone marrow differentiated macrophages (BMDMs) from Apoe−/− mice that also lacked IKBKE (dKO) together with wild-type IKBKE controls (Apoe−/−). BMDMs from both genotypes appeared morphologically indistinguishable and expressed similar levels of macrophage differentiation markers (Fig. S2A). IKBKE ablation did not alter the ability of macrophages to polarize into classical/proinflammatory (M1) or alternative/antiinflammatory (M2) subtypes following chronic treatment with LPS+IFN-γ or IL-4, respectively (Fig. S2B). However, M1-activated macrophages lacking IKBKE expressed significantly elevated levels of NLRP3 inflammasome-related transcripts. Specifically, the expression of Nlrp3 was higher in dKO macrophages following proinflammatory stimulation, and remained significantly greater for up to 24 h, when expression in Apoe−/− control cells was returning back down to unstimulated levels (Fig. 2 A and B). The expression of other NLR/PYD inflammasomes including Nlrp1 and Aim2 was not consistently greater in chronically stimulated dKO macrophages (Fig. S2C), indicating an IKBKE-dependent selectivity toward NLRP3 inflammasome priming. IKBKE-dependent enhanced Nlrp3 expression was not limited to proatherogenic Apoe−/− macrophages because it was also observed in chronically activated Ikbke−/− BMDMs from C57BL/6 and mixed background mice (Fig. S2 D and E). The expression and induction of IL1-related cytokines, Il1a, Il1b, and Il33, was more restricted to specific stimuli and time-dependent. Most notably, the induced expression of Il1b was dramatically greater in chronically stimulated dKO macrophages (Fig. 2D and Fig. S2 D and E). The expression of Il1a was less striking but greater in dKO macrophages at both 4 and 24 h (Fig. S3 A and B), whereas Il33 was significantly greater only at 4 h after stimulation (Fig. S3 C and D). Other NLRP3 inflammasome-associated transcripts, namely Il18, Pycard, and Casp1, did not show consistently elevated expression in dKO macrophages (Fig. S3 E–J). Immunoblotting for both pro–IL-1β and mature IL-1β proteins in cell lysates confirmed that, in the absence of exogenous inflammasome activators, IKBKE ablation did not directly alter IL-1β processing but resulted in prolonged pro–IL-1β availability (Fig. 2E). However, in the presence of additional NLRP3 activation (by ATP, nigericin, or high-dose LPS) chronically primed Ikbke−/− macrophages secreted greater amounts of IL-1β (Fig. 2F). Collectively, these data indicate that IKBKE is required to limit both the magnitude and temporal dynamics of NLRP3 inflammasome priming in macrophages. Since the expression of endogenous IKBKE is itself dynamically induced by the same inflammatory stimuli in BMDMs, our data supports the notion that in wild-type macrophages, the sustained expression of endogenous IKBKE is required to counterregulate chronic NLRP3 inflammasome priming.
Fig. 2.
Enhanced NLRP3 inflammasome priming in macrophages lacking IKBKE. Effect of IKBKE ablation on expression profiles of NLRP3 inflammasome-related genes in BMDMs following 4-h (A and C) or 24-h (B and D) treatment with LPS (100 ng/mL), IFN-γ (100 ng/mL), PGN (50 µg/mL), TNFα (10 ng/mL), IL-1β (10 ng/mL), IL-4 (10 ng/mL), or indicated combinations. (E) Western blot of IKBKE, IL-1β, and α-tubulin in BMDM whole-cell lysates collected at 24 h after treatment without NLRP3 activation. (F) IL-1β secretion was determined in conditioned media from BMDMs primed with LPS (100 ng/mL) and IFN-γ (100 ng/mL) for up to 24 h and treated with NLRP3 activators: ATP (5 mM for 1 h), Nigericin (10 µM for 1 h) or high-dose LPS (10 µg/mL for 16 h). Statistical significance is indicted by *P < 0.05, **P < 0.01, and ***P < 0.001 relative to corresponding Apoe−/− BMDMs.
IKBKE Deficiency Enhances Inflammasome Priming in Adipose Tissue.
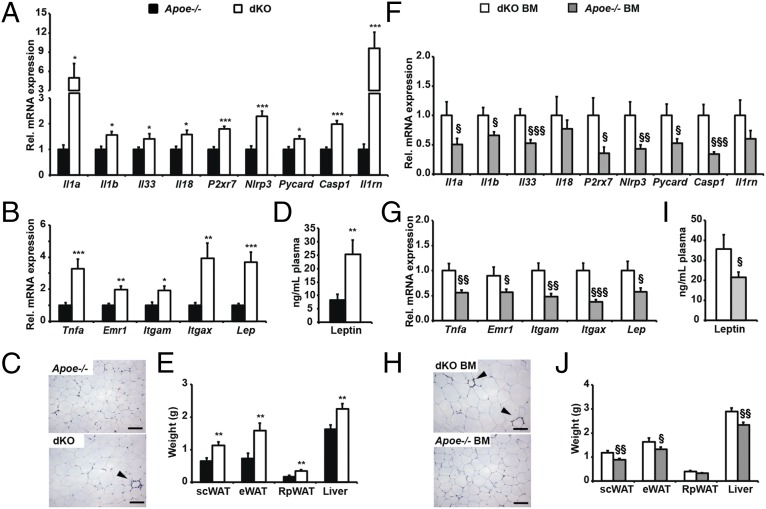
We next investigated the in vivo consequences of IKBKE ablation. Since IKBKE ablation may prevent the development of high fat diet-induced obesity and associated metaflammation, we investigated the impact of IKBKE ablation in Apoe−/− mice. This model is protected from diet-induced obesity but develops metaflammation in adipose tissue, liver steatosis, hypercholesterolemia, and readily develops atherosclerotic plaques (26, 27). Moreover, macrophages from these mice can be primed for NLRP3 activity in vivo following feeding with a high-fat, high-cholesterol Western diet (28). We reasoned that in this model, the absence of IKBKE would result in enhanced NLRP3 inflammasome priming and activity, which, in turn, would promote a greater inflammatory response and exacerbated metaflammation. To test this hypothesis, we fed Apoe−/− mice and dKO littermates a Western-type diet (WD) for 16 wk before killing and tissue collection. Expression profiling of adipose tissue revealed that multiple NLRP3 inflammasome-related genes, (i.e., Il1a, Il1b, Il33, Il18, P2rx7, Nlrp3, Pycard, and Casp1) were all elevated in WD-fed female dKO mice (Fig. 3A). Furthermore, the IL-1β–responsive IL-1 receptor antagonist (Il1rn) was elevated in dKO mice, suggesting that inflammasome activity was enhanced. However, this observation coincided with an increased presence and expression of the macrophage markers Emr1, Itgam, and Itgax and the proinflammatory cytokine Tnfa (Fig. 3 B and C). This proinflammatory profile was also associated with increased leptin production, adipocyte hypertrophy, white adipose tissue (WAT) mass, and generalized diet-induced adiposity (Fig. 3 B–E and Fig. S4 A–D), suggesting that IKBKE may also be important in regulating energy balance and subsequent obesity-related metabolic deregulation. Indeed, food intake but not energy expenditure was significantly increased in female dKO mice (Fig. S5 A–C). Despite increased adiposity, proadipogenic markers were unaltered (Fig. S5D) and dKO mice remained euglycemic, glucose- and insulin-tolerant (Table S2 and Fig. S5 E and F). However, elevated insulin levels in dKO mice indicated mild systemic insulin resistance (Table S2). In contrast to female dKO mice, male dKO mice did not exhibit differential weight gain or adiposity following WD feeding, nor was there evidence of increased inflammatory cell recruitment in adipose tissue (Fig. S6). Nonetheless, the expression of NLRP3 mRNA was significantly elevated and Il1a, Il1b, and Il33 all showed trends toward increased expression (Fig. S6H).
Fig. 3.
Effects of global IKBKE deficiency and hematopoietic restoration on inflammasome-related gene expression and metaflammation in adipose tissue. Apoe−/− and dKO mice (A–E), and dKO mice (F–J) transplanted with either dKO BM (white) or Apoe−/− BM (gray), were fed a WD for 16 wk before sacrifice and tissue collection. Adipose tissue expression of NLRP3 inflammasome-related genes (A and F), macrophage markers (Emr1, Itgam, and Itgax), and adipokines (Lep and Tnf) (B and G). (C and H) Histological sections of Epididymal WAT (eWAT) stained with anti-EMR1. (Scale bars: 100 μm.) (D and I) Plasma leptin levels. (E and J) Weights of Interscapular brown adipose tissue (BAT), Inguinal s.c. white adipose tissue (scWAT), eWAT, Retroperitoneal WAT (RpWAT), and liver. All data are means ± SE (n = 5–9 per group). Statistical significance is indicted by * or § P < 0.05, ** or §§ P < 0.01 and *** or §§§ P < 0.001 relative to Apoe−/− mice (*) or dKO BM > dKO mice (§).
Hematopoietic Restoration of IKBKE Limits Inflammasome Priming in Adipose Tissue.
Since the effects of global IKBKE ablation may be due to removal of IKBKE in nonimmune cell types such as adipocytes, we next sought to determine whether restoration of hematopoietic IKBKE was sufficient to reverse elevated NLRP3 inflammasome priming in vivo. To test this hypothesis, recipient dKO female mice were irradiated, and reconstituted with bone marrow (BM) from age-and sex-matched Apoe−/− mice that either expressed endogenous wild-type IKBKE (Apoe−/− BM) or lacked IKBKE (dKO BM). Following BM reconstitution, all chimeric mice were fed WD for 16 wk. Transplant with Apoe−/− (Ikbke+/+) BM was sufficient to reduce adipose tissue expression of NLRP3-related inflammasome genes (Fig. 3F). However, proinflammatory cytokines and macrophage markers were also reduced (Fig. 3G) and coincided with a reduction in all indicators of adiposity including, leptin production, adipocyte hypertrophy, WAT mass, and generalized adiposity (Fig. 3 G–J and Fig. S4 E–H). Indicators of energy expenditure were not altered (Fig. S5 G and H), and the expression of proadipogenic genes in WAT remained similar (Fig. S5I). These mice also remained euglycemic and glucose- and insulin-tolerant (Table S2 and Fig. S5 J and K). However, insulin levels were reduced by hematopoietic IKBKE reconstitution, suggesting protection from the development of mild insulin resistance (Table S2). Hence, whereas mice transplanted with dKO bone marrow reproduced the diet-induced increase in body weight, adiposity, and adipose tissue inflammation, transplant with Ikbke+/+ marrow protected dKO mice from developing excessive diet-induced obesity and also reduced inflammasome priming and metaflammation in adipose tissue.
IKBKE Deficiency and Hematopoietic Restoration Alters Hepatic Inflammasome Priming, APR, and Hypercholesterolemia.
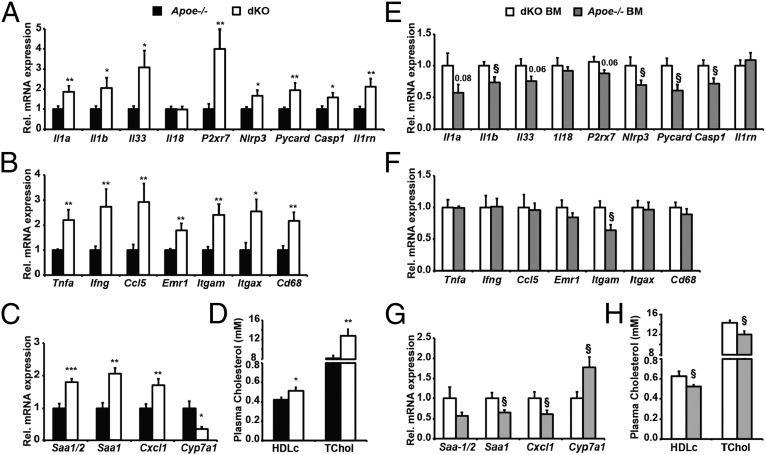
Since IKBKE is induced in chronically inflamed livers, and WD-fed Apoe−/− mice also develop hepatic steatosis, we next investigated the impact of IKBKE ablation on hepatic NLRP3 inflammasome expression in dKO mice. The expression of Il1a, Il1b, Il33, P2rx7, Nlrp3, Pycard, and Casp1 were all elevated in dKO livers (Fig. 4A). This induction also coincided with increased levels of Il1rn (Fig. 4A), proinflammatory (Th1/M1) genes, and macrophage markers in livers of dKO mice (Fig. 4B and Fig. S7A). However, antiinflammatory (Th2/M2) expression profiles were similar in both genotypes (Fig. S7B). These data suggest IKBKE ablation promotes priming and functional activation of hepatic NLRP3 inflammasomes and metaflammation.
Fig. 4.
Effects of global IKBKE deficiency and hematopoietic restoration on hepatic inflammasome-related gene expression and metaflammation. Experimental conditions were as described for Fig. 3. Hepatic transcript expression was determined for NLRP3 inflammasome-related genes (A and E), proinflammatory and macrophage marker genes (B and F), and acute phase response genes (C and G). (D and H) Plasma HDL cholesterol and total cholesterol levels. All data are means ± SE (n = 5–9 per group). Statistical significance is indicted by * or § P < 0.05, ** or §§ P < 0.01 and *** or §§§ P < 0.001 relative to Apoe−/− mice (*) or dKO BM > dKO mice (§).
In rodents, hepatic IL-1β production and inflammation promotes a class 1 acute phase response (APR) and impairs cholesterol clearance. Indeed, dKO livers expressed greater levels of class 1 APR transcripts (Fig. 4C): serum amyloid A 1/2 (Saa1/2) and Chemokine C-X-C motif ligand 1 (Cxcl1). Hepatic expression of the inflammation-responsive, cholesterol-clearing enzyme Cyp7a1 was reduced (Fig. 4C), and circulating levels of total cholesterol and HDLc were further elevated in dKO mice (Fig. 4D). IKBKE ablation also promoted increased liver mass (Fig. 3D) and histologically proven hepatic steatosis (Fig. S7A). Similar findings were also observed in male mice (Fig. S8). Taken together, these data demonstrate that IKBKE ablation in WD-fed Apoe−/− mice enhanced hepatic expression of NLRP3 inflammasome-related genes, enhanced inflammation, enhanced APR, and decreased cholesterol clearance. These features are characteristic of increased hepatic metaflammation.
We next determined the effect of restoring hematopoietic IKBKE on hepatic inflammasome priming and hepatic metaflammation. In mice transplanted with Apoe−/− (Ikbke+/+) marrow, the expression of hepatic Il1b, Nlrp3, Pycard, and Casp1 were all significantly reduced (Fig. 4E). However, this reduction did not coincide with a generalized improvement in inflammation or macrophage infiltration. Global M1/M2 inflammatory profiles and immune cell markers also remained similar (Fig. 4F and Fig. S7 C and D). Nonetheless, hepatic expression of the class 1 APPs Saa1 and Cxcl1 was reduced (Fig. 4G), suggesting that these APPs may be more sensitive to changes in IL-1β expression and reduced NLRP3 inflammasome activity. Furthermore, the repression of Cyp7a1 was prevented, and circulating cholesterol levels (TChol and HDLc) were reduced (Fig. 4 G and H), indicating improved cholesterol clearance. Taken together, these data strongly suggest that in this hyperlipidemic model, hematopoietic IKBKE limits hepatic inflammasome priming, hepatic acute phase response, and cholesterol catabolism.
IKBKE Deficiency and Hematopoietic Restoration Alters Atherosclerotic Lesion Composition.
Our observations demonstrate that IKBKE ablation in Apoe−/− mice enhanced NLRP3 inflammasome priming, metaflammation, adiposity, hepatosteatosis, APR, and hypercholesterolemia, all of which are proatherogenic risk factors. Since IKBKE is also present in atherosclerotic plaques, we reasoned that IKBKE ablation might also promote atherosclerosis and that reconstitution with hematopoietic IKBKE might elicit beneficial effects. To test this hypothesis, we investigated the impact of both global IKBKE ablation and reconstitution with hematopoietic IKBKE on atherosclerotic plaque morphology and composition in the same mice. Following 16 wk of WD feeding, all female Apoe−/− mice had advanced lesions. However, IKBKE ablation did not impact on plaque size, macrophage area, or SMA+ area, although necrotic core sizes were smaller in dKO mice (Table 2). Following restoration of IKBKE in hematopoietic cells, plaque sizes and macrophage areas remained similar but plaque composition was markedly altered. Apoe−/− BM mice had lesions with significantly larger necrotic cores, an increased number of TUNEL-positive cells, and a reduced SMA+ area (Table 2). Collectively, these features may indicate changes in plaque stability and contrast with improvements in proatherogenic metabolic risk factors in these mice. Nonetheless, in advanced atherosclerotic lesions, hematopoietic IKBKE can influence local vascular remodeling.
Table 2.
Effects of global IKBKE deficiency and hematopoietic restoration on atherosclerotic plaque morphology and composition
| Study 1 | Study 2 | |||
| Measurement | Apoe−/− | dKO | dKO BM | Apoe−/− BM |
| Lesion area (× 103 µm2) | 275.3 ± 13.3 | 255.9 ± 9.5 | 199.1 ± 16.8 | 231.3 ± 15.6 |
| Mac3 + ve area, % | 13.3 ± 0.8 | 13.0 ± 2.0 | 21.3 ± 2.0 | 21.5 ± 2.1 |
| SMA + ve area, % | 21.0 ± 2.2 | 19.8 ± 1.5 | 28.7 ± 1.2 | 23.1 ± 1.0† |
| Necrotic area (× 103 µm2) | 127.6 ± 8.7 | 99.4 ± 7.2* | 53.5 ± 7.2 | 89.9 ± 13.0* |
| Tunnel + ve cells (no. per mm2) | 30.9 ± 6.0 | 26.1 ± 4.5 | 69.7 ± 9.2 | 190.1 ± 12.0‡ |
Statistical significance is indicated (*P < 0.05; †P < 0.01; ‡P < 0.001) relative to study-specific control mice.
Discussion
Great strides have been made in understanding how inflammation is induced in immune cells and its defining role in inflammatory diseases. In contrast, little is known about the cell-autonomous mechanisms that govern the tone and chronicity of metaflammation. In this study we show that IKBKE, a downstream mediator of TLR and cytokine signals, plays important dual roles in regulating the tone and temporal kinetics of inflammation. It does so through counterregulation of key stress-response genes involved in NLRP3 inflammasome priming. This conclusion is supported by three key findings: First, IKBKE is induced in parallel with genes that prime the NLRP3 inflammasome in PRR-stimulated macrophages. Second, the absence of IKBKE results in both enhanced and prolonged expression of Nlrp3, Il1a, and Il1b in stimulated proinflammatory macrophages. Third, in WD-fed Apoe−/− mice, in which multiple endogenous inflammasome activators are generated, IKBKE ablation results in enhanced expression of inflammasome-related genes and exacerbated metaflammation.
The pathological relevance of these findings is supported by the fact that IKBKE is aberrantly expressed in three distinct human tissues associated with metaflammation: i.e., in adipose tissue from obese subjects, in livers of obese subjects with NASH, and in atherosclerotic plaques where it also colocalized with macrophages. The specific role of hematopoietic IKBKE was confirmed in bone marrow transplant studies wherein wild-type IKBKE in hematopoietic cells alone was sufficient to rescue the exacerbated adiposity, metaflammation, hyperinsulinemia, APR, and hypercholesterolemia. Unexpectedly, IKBKE ablation and reconstitution with hematopoietic IKBKE does not alter the size of advanced atherosclerotic lesions, rather it influences atherosclerotic plaque composition.
Chronic inflammation in the vasculature is a hallmark of atherosclerotic plaques and is implicated in promoting plaque development (in early plaques) and instability (in clinically relevant advanced plaques). However, the requirement for NLRP3 inflammasome activity in atherosclerosis is hotly debated and contrasting findings have been reported (13, 29). Recent studies have also revealed additional complexities, suggesting IL-1R signaling is beneficial and promotes plaque stability in advanced atherosclerosis (30). Our studies show that IKBKE ablation in Apoe−/− female mice further enhances proatherogenic metabolic profiles and has minor effects on plaque composition. Although hematopoietic IKBKE rescues the systemic metabolic features, these improvements do not translate into an advantageous plaque composition. This finding reinforces the notion that tissue localized hematopoietic signals can override systemic metabolic cues and highlights a potential to uncouple the pathological association between metabolic features and atherosclerosis.
The observation that IKBKE ablation can promote obesity in female mice contrasts with previous studies, which were all performed in male mice (23–25). Although our study is not directly comparable (owing to model-specific differences in genotypes, sex, diet, and housing), we also performed parallel studies in male littermates. Here, we reproduced a lack of effect of IKBKE ablation on diet-induced adiposity in Apoe−/− mice. This finding suggests that the functions of IKBKE in energy balance are influenced by sex and warrants further investigation. Whether sex dimorphism also plays a role in metabolic stress and inflammasome activation remains to be formally addressed. However, in the present study, the impact of IKBKE ablation on NLRP3 inflammasome priming occurs in both genders.
In conclusion, this study demonstrates that IKBKE is relevant to clinical pathologies that are associated with metaflammation. It is induced by inflammatory signals in tissue macrophages where it functions as a brake to limit the chronicity of NLRP3 inflammasome priming and subsequent inflammatory response. Whereas global IKBKE deficiency in a disease model results in enhanced and prolonged inflammasome priming and metaflammation, restoring hematopoietic IKBKE is sufficient to rescue these features. These findings provide new molecular insights into how innate immunity may be self-regulated during low-grade chronic inflammation. Moreover, the possibility that hematopoietic IKBKE may play a nonpathogenic role in metaflammation is an important consideration for therapeutic strategies aimed at treating metabolic diseases such as NAFLD, obesity-related diabetes, and atherosclerosis.
Materials and Methods
Human Studies.
Adipose tissue (scWAT) biopsies were collected during bariatric surgery from female patients with a range of body mass indices (BMI) as reported (31). The institutional review board at Indiana University approved all protocols and patient consent was given. For liver biopsies, morbidly obese patients undergoing bariatric surgery gave their informed written consent to participate in this study in accordance with French legislation regarding ethics and human research (Huriet-Serusclat law). The “Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de Nice” approved the study (07/04:2003, N° 03.017). See SI Materials and Methods for additional details and Table S1 for characteristics of the study groups.
Murine Studies.
High fat diet fed C57BL/6 and ob/ob mice were generated and treated as described (32). Apoe−/− (Apoetm1Unc × N10 onto C57BL/6 background) mice were from Jackson Laboratories. Ikbke−/−(Ikbketm1Aki) mice were generated as reported (20). The UK Home Office and University of Cambridge approved all animal procedures. See SI Materials and Methods for additional experimental details.
BMDM Studies.
Bone marrow cells (BMCs) were collected from tibia of 11- to 13-wk-old female mice and treated in vitro as described in SI Materials and Methods. Western blotting details are also described in SI Materials and Methods.
Targeted Gene Expression Profiling by Quantitative RT-PCR.
Total RNA was isolated by using STAT60 for tissues samples, and RNAeasy kits (Qiagen) for BMDMs, according to the manufacturers’ instructions. Total RNA (500 ng) was reverse-transcribed by using standard protocols. See SI Materials and Methods for further details.
Statistical Analysis.
Statistical analysis used two-tailed unpaired Student’s t tests with P < 0.05 considered significant, with § or * for P < 0.05, §§ or ** for P < 0.01, and §§§ or *** for P < 0.001. Clinical correlations were analyzed by using the Spearman’s rank correlation test.
Supplementary Material
Acknowledgments
We thank Gökhan Hotamisligil for critical reading of the manuscript and acknowledge excellent technical assistance from C. Marciano, A. Superville, L. Baker, J. Harrison, M. Dale, A. Lukasik, and core facilities staff at University of Cambridge Central Biomedical Services, Medical Research Council Centre for Obesity and Related Metabolic Diseases (MRC-CORD) Phenomics Centre, MRC-CORD Biochemistry Lab and MRC-CORD Microscopy and Histology Labs. Funding was provided by a British Heart Foundation Project Grant PG/10/38/28359 (to J.K.S.), a PhD Studentship (to W.G.B.), and in part by Programme Grants RG/12/13/29853 (to A.V.-P.) and RG/13/14/30314 (to M.R.B.); Gates Cambridge PhD Scholarship (to M.N.P.); a Wellcome Trust PhD Studentship (to N.B.M.); and the National Institute for Health Research Cambridge Biomedical Research Centre. Clinical hepatic studies were funded by INSERM, the University of Nice, the Programme Hospitalier de Recherche Clinique (Centre Hospitalier Universitaire of Nice), a European Foundation for the Study of Diabetes/Lilly Fellowship (to P.G.), and the French Government (National Research Agency) through “Investments for the Future” LABEX SIGNALIFE Grant ANR-11-LABX-0028-01.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414536112/-/DCSupplemental.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Könner AC, Brüning JC. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22(1):16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera CA, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura K, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–334, e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5(10):975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 8.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4(8):444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 9.Weatherill AR, et al. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174(9):5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Dalmaroni MJ, et al. Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS ONE. 2009;4(10):e7411. doi: 10.1371/journal.pone.0007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108(37):15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 13.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajamäki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 16.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Labbe K, Saleh M. Pyroptosis: A caspase-1-dependent programmed cell death and a barrier to infection. In: Couilin I, editor. Progress in Inflammation Research. Springer; Basel: 2011. pp 18–36. [Google Scholar]
- 18.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 19.tenOever BR, et al. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78(19):10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199(12):1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 22.Boehm JS, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138(5):961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly SM, et al. An inhibitor of the protein kinases TBK1 and IKK-ϵ improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheja L, Heese B, Seedorf K. Beneficial effects of IKKε-deficiency on body weight and insulin sensitivity are lost in high fat diet-induced obesity in mice. Biochem Biophys Res Commun. 2011;407(2):288–294. doi: 10.1016/j.bbrc.2011.02.137. [DOI] [PubMed] [Google Scholar]
- 26.Lohmann C, et al. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009;207(2):360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bartelt A, et al. Altered endocannabinoid signalling after a high-fat diet in Apoe(-/-) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 2011;54(11):2900–2910. doi: 10.1007/s00125-011-2274-6. [DOI] [PubMed] [Google Scholar]
- 28.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander MR, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122(1):70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carobbio S, et al. Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity. Diabetes. 2013;62(11):3697–3708. doi: 10.2337/db12-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagathu C, et al. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes. 2009;58(3):609–619. doi: 10.2337/db08-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prieur X, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.