Significance
Voltage-gated calcium channels are essential for diverse cellular functions. For example, CaV1.1 channels trigger skeletal muscle contraction and CaV1.2 channels regulate neural gene expression in response to neuronal activity. Thus, it is important to understand the cellular mechanisms that regulate delivery of these channels to the plasma membrane and that govern calcium movements via the membrane-inserted channels. Here we show that the cellular adapter protein “Stac3” participates in both processes. Specifically, Stac3 binds to both CaV1.1 and CaV1.2. This binding is essential for efficient delivery of CaV1.1 to the plasma membrane, but not for CaV1.2. However, binding of Stac3, or the related protein Stac2, to CaV1.2 causes a dramatic slowing of inactivation, thereby increasing calcium entry via CaV1.2.
Keywords: Stac adaptor protein, L-type Ca2+ channel, excitation–contraction coupling
Abstract
Excitation–contraction (EC) coupling in skeletal muscle depends upon trafficking of CaV1.1, the principal subunit of the dihydropyridine receptor (DHPR) (L-type Ca2+ channel), to plasma membrane regions at which the DHPRs interact with type 1 ryanodine receptors (RyR1) in the sarcoplasmic reticulum. A distinctive feature of this trafficking is that CaV1.1 expresses poorly or not at all in mammalian cells that are not of muscle origin (e.g., tsA201 cells), in which all of the other nine CaV isoforms have been successfully expressed. Here, we tested whether plasma membrane trafficking of CaV1.1 in tsA201 cells is promoted by the adapter protein Stac3, because recent work has shown that genetic deletion of Stac3 in skeletal muscle causes the loss of EC coupling. Using fluorescently tagged constructs, we found that Stac3 and CaV1.1 traffic together to the tsA201 plasma membrane, whereas CaV1.1 is retained intracellularly when Stac3 is absent. Moreover, L-type Ca2+ channel function in tsA201 cells coexpressing Stac3 and CaV1.1 is quantitatively similar to that in myotubes, despite the absence of RyR1. Although Stac3 is not required for surface expression of CaV1.2, the principle subunit of the cardiac/brain L-type Ca2+ channel, Stac3 does bind to CaV1.2 and, as a result, greatly slows the rate of current inactivation, with Stac2 acting similarly. Overall, these results indicate that Stac3 is an essential chaperone of CaV1.1 in skeletal muscle and that in the brain, Stac2 and Stac3 may significantly modulate CaV1.2 function.
Voltage-gated calcium channels serve to couple membrane electrical activity to various downstream signaling cascades, whose properties are strongly influenced by the spatial interrelationships between the calcium channels, effector proteins, and cellular organelles that comprise these signaling pathways. For example, in skeletal muscle the link between muscle excitation and contraction [excitation–contraction (EC) coupling] depends upon triadic or dyadic junctions between the plasma membrane and the sarcoplasmic reticulum (SR), with L-type Ca2+ channels (dihydropyridine receptors, DHPRs) and type 1 ryanodine receptors (RyR1) localized in junctional domains of the plasma membrane and the SR, respectively. The DHPRs, which contain CaV1.1 as their principal subunit, are arranged into groups of four (“tetrads”), evidently as a consequence of yet-to-be identified, physical links between the DHPRs and the four homomeric subunits of RyR1 (1, 2). These links are thought to be necessary for the bidirectional functional interactions between the DHPR and RyR1. In particular, the DHPR is thought to be conformationally coupled to RyR1 with the result that movements of the CaV1.1 S4 helices in response to depolarization of the plasma membrane cause RyR1 to release Ca2+ from the SR. In addition to this orthograde signal, which is necessary for EC coupling, there is also a retrograde signal whereby the association with RyR1 causes the L-type Ca2+ current via CaV1.1 to be larger and to activate more rapidly. Thus, Ca2+ currents are substantially reduced in size in dyspedic myotubes genetically null for RyR1 (3, 4).
Given the structural and functional interactions between CaV1.1-containing DHPRs and RyR1, it would seem necessary that the trafficking of CaV1.1 to the plasma membrane be precisely regulated. As for other high-voltage activated Ca2+ channels, the auxiliary β-subunit is important for membrane trafficking (5, 6). However, it is clear that an additional factor(s) is also crucial. Specifically, CaV1.1 expresses poorly (7) or not at all in mammalian cells that are not of muscle origin (e.g., tsA201 cells), whereas the closely related cardiac/neuronal L-type Ca2+ channel, CaV1.2, is readily expressed in such cells (8–10). The absence of RyR1 seems insufficient to account for the difficulty of expressing CaV1.1 in tsA201 cells because CaV1.1 expresses well in dyspedic myotubes, albeit producing less current per channel (3). Here we explored the hypothesis that the adaptor protein Stac3 is important for membrane trafficking of CaV1.1. Stac3 is one of three isoforms of the Stac family of adaptor proteins, so named because they contain src homology 3 (SH3) and cysteine-rich domains. Based on quantitative PCR of adult mice, transcripts for Stac3 are found at high levels in skeletal muscle and at low levels in the cerebellum, forebrain, and eye, whereas transcripts for Stac2 are found in these same, three, neuronally rich regions at levels comparable to those of Stac3 in skeletal muscle (11). Transcripts for Stac1 (often designated simply as Stac) are present in cerebellum, fore-/midbrain, and eye (at levels 1/10th or less those of Stac2) and also present in the bladder and adrenal gland (11). In skeletal muscle, the absence of Stac3 causes the failure of EC coupling in both mice (11) and fish (12). Moreover, Stac3 appears to interact with CaV1.1 based on both localization at triad junctions (11, 12) and coimmunoprecipitation (12). Our results now indicate that Stac3 is a chaperone protein that binds directly to CaV1.1 and is necessary for its plasma membrane expression. Stac3 and Stac2 also bind to CaV1.2, which is not required for membrane trafficking but is functionally important, greatly slowing the inactivation of current.
Results
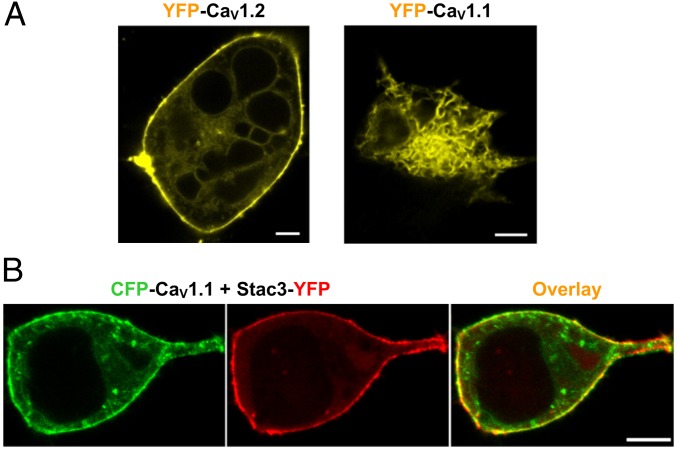
Fig. 1A compares the subcellular distribution of fluorescently tagged CaV1.2 and CaV1.1, after coexpression in tsA201 cells with the auxiliary β1a- and α2δ-subunits. CaV1.2 appeared to be associated with the surface membrane (Fig. 1A, Left), but CaV1.1 had a reticular distribution (Fig. 1A, Right) consistent with retention in the endoplasmic reticulum (ER) (13). Recently, Stac3 was reported to localize to plasma membrane/SR junctions (triads) in skeletal muscle, to be necessary for skeletal-type EC coupling (not requiring entry of extracellular Ca2+), and to be highly expressed in skeletal muscle but not in cardiac muscle. Thus, we decided to test whether Stac3 would affect the trafficking of CaV1.1. Strikingly, the presence of Stac3 caused CaV1.1 to associate with the cell surface (Fig. 1B). To determine whether this surface-associated CaV1.1 was actually inserted into the plasma membrane, we used a monoclonal antibody to CaV1.1 (14). This antibody stained intact tsA201 cells coexpressing CaV1.1 and Stac3, but not cells expressing only CaV1.1 (Fig. S1). An obvious feature of cells like those illustrated in Fig. 1B was that Stac3, like CaV1.1, was associated with the surface. On the basis of photobleaching analysis, such surface binding was not an intrinsic property of Stac3, but depended instead on CaV1.1 also being present (Fig. S2). Moreover, even with neither β1a nor α2δ, Stac3 appeared able to colocalize at the surface with CaV1.1 and to promote the insertion of CaV1.1 into the plasma membrane (Fig. S3). Taken together, these results strongly suggest that Stac3 binds directly to CaV1.1 and that this binding is necessary for efficient delivery of CaV1.1-containing DHPRs to the plasma membrane.
Fig. 1.
CaV1.1 is retained in the ER of tsA201 cells but traffics to the surface when Stac3 is present. (A) Subcellular distribution of YFP-labeled CaV1.2 and CaV1.1 in tsA201 cells. (Scale bars, 5 μm.) (B) Fluorescence images demonstrating that CFP-CaV1.1 (green) and Stac3-YFP (red) traffic to the surface after coexpression in tsA201 cells. (Scale bar, 2 μm.) The auxiliary β1a- and α2δ-subunits were also present here and in all other tsA201 cell experiments, unless otherwise mentioned.
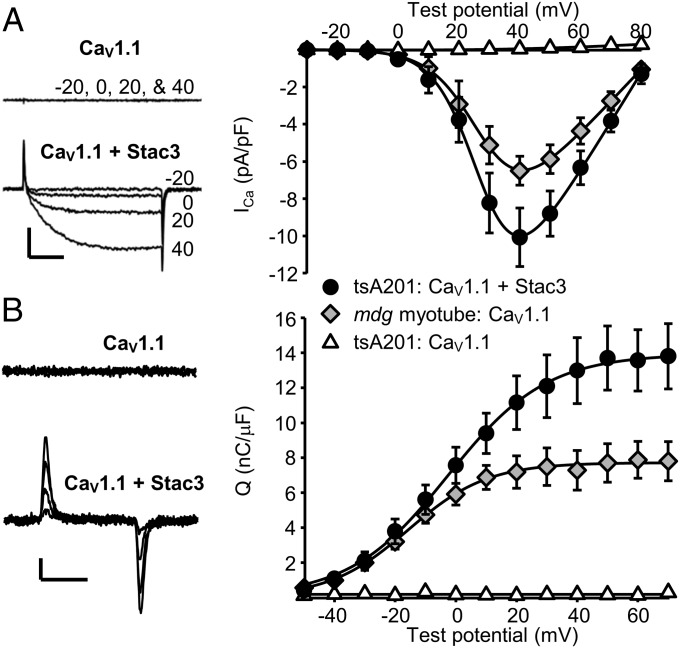
Whole-cell patch clamping was used to test whether CaV1.1 was functional after its cotransfection with Stac3 in tsA201 cells. As shown in Fig. 2, CaV1.1 in the absence of Stac3 produced neither Ca2+ currents nor membrane-bound charge movements (gating currents), whereas, in the presence of Stac3, CaV1.1 produced robust Ca2+ currents and charge movements. Fig. 2 also compares the peak current–voltage and charge–voltage relationships for CaV1.1 in tsA201 cells with those of CaV1.1 expressed in dysgenic myotubes (“mdg,” null for endogenous CaV1.1). We had expected that CaV1.1 function would differ between the two cell types because RyR1 is present in dysgenic myotubes but not in tsA201 cells and because the absence of RyR1 in dyspedic myotubes (null for endogenous RyR1) has been shown to cause Ca2+ currents via CaV1.1 to activate more rapidly (4) and to be severalfold smaller in relationship to charge movement (Gmax/Qmax) (3, 4). However, double-exponential fits (Eq. 3) revealed no significant differences between tsA201 cells and dysgenic myotubes in the rate of Ca2+ current activation (Fig. 3). Additionally, based on fits of Boltzmann expressions (Eqs. 1 and 2), there were no significant differences between the two cell types in the magnitude of the ratio Gmax/Qmax (Fig. 3). Thus, the function of CaV1.1 coexpressed with Stac3/β1a/α2δ in tsA201 cells is remarkably similar to that of CaV1.1 as part of the DHPR complex in myotubes.
Fig. 2.
Stac3 promotes robust functional expression of CaV1.1 in tsA201 cells. (A) Representative ionic currents and peak I–V relationships for YFP-CaV1.1 in tsA201 cells either without Stac3 (open triangles, n = 10) or with Stac3 (solid circles, n = 14). (Right) Shown for comparison is the peak I–V relationship for dysgenic myotubes (null for endogenous CaV1.1), which were injected with cDNA encoding YFP-CaV1.1 (gray diamonds, n = 12). Calibrations: 5 pA/pF (vertical), 50 ms (horizontal). (B) Representative charge movements (depolarizations from −50 mV to −30 mV, −10 mV, 10 mV, and 30 mV) for YFP-CaV1.1 expressed in tsA201 cells either without or with Stac3. Calibrations: 1 pA/pF (vertical), 10 ms (horizontal). (Right) Average Q–V relationships for tsA201 cells expressing YFP-CaV1.1 with or without Stac3 and YFP-CaV1.1-expressing dysgenic myotubes are shown (symbols and numbers of cells as in A). The smooth curves (A and B, Right) are plots of Eqs. 1 and 2 (Materials and Methods) with the average parameters determined by fits of these equations to data from individual cells. Throughout, error bars represent ± SEM.
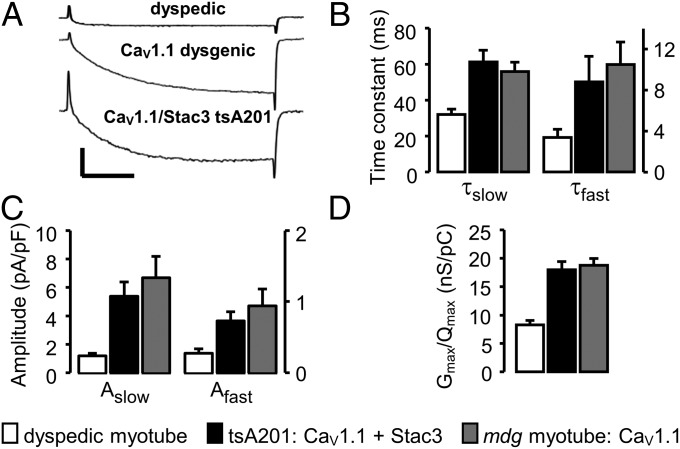
Fig. 3.
L-type Ca2+ currents are similar for YFP-CaV1.1 expressed in dysgenic myotubes and tsA201 cells. (A) Comparison of peak Ca2+ currents (Vtest = +40 mV) in a naive dyspedic (RyR1-null) myotube; a dysgenic myotube transfected with YFP-CaV1.1; and a tsA201 cell cotransfected with YFP-CaV1.1, α2δ, β1a, and Stac3. Calibrations: 5 pA/pF, vertical; 50 ms, horizontal. (B and C) Activation was fitted with a double-exponential function (Eq. 3) for Ca2+ currents at +40 mV in naive dyspedic myotubes (open bars, n = 10), YFP-CaV1.1 expressing dysgenic myotubes (solid bars, n = 10), and in YFP-CaV1.1/ Stac3 expressing tsA201 cells (shaded bars, n = 12). Based on one-way ANOVA, there was no statistically significant difference between the tsA201 cells and dysgenic myotubes in either the time constants (B, τslow, P = 0.461; τfast, P = 0.421) or amplitudes (C, Aslow, P = 0.394; Afast, P = 0.400) of the slow and fast components of current activation. (D) Peak current–voltage (peak I–V) and charge–voltage (Q–V) relationships were fitted with Eqs. 1 and 2, respectively, to yield values of Gmax, Qmax, and Gmax/Qmax for individual cells. Mean values (±SEM) are shown for dyspedic myotubes (open bars, n = 6), YFP-CaV1.1 expressing dysgenic myotubes (solid bars, n = 6), and YFP-CaV1.1/ Stac3 expressing tsA201 cells (shaded bars, n = 8). Data shown were obtained only from cells in which both peak I–V and Q–V relationships were measured. The Gmax/Qmax ratios for dyspedic and dysgenic myotubes were not corrected for any background charge (unrelated to CaV1.1) because this was small in the current experiments: 0.87 ± 0.09 nC/µF, n = 7, measured at +40 mV in naive dysgenic myotubes.
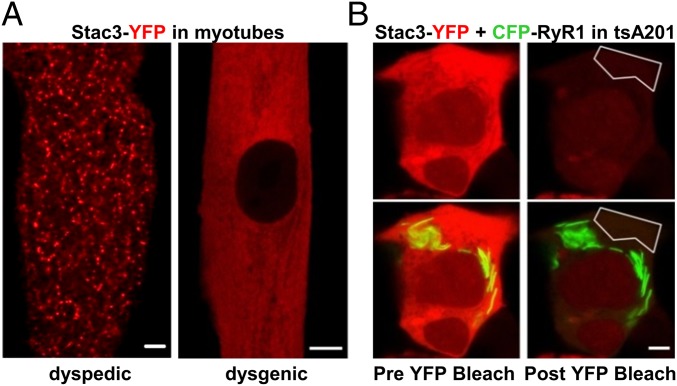
In addition to binding to the DHPR, it has been suggested that Stac3 may also bind to RyR1 and thus serve as a functional link between the two proteins (11, 12). As a test of this possibility, we determined whether Stac3-YFP had the distribution expected for targeting to plasma membrane/SR junctions, which are present with a punctate distribution in both dyspedic (15, 16) and dysgenic myotubes (17). Stac3-YFP had a punctate distribution in dyspedic myotubes (Fig. 4A, Left), which contain DHPRs but lack RyR1. This result is consistent with the apparent interaction between Stac3 and DHPRs in tsA201 cells (Fig. 1B, extended data in Fig.2). By contrast, Stac3-YFP had a diffuse distribution in dysgenic myotubes (Fig. 4A, Right), which contain RyR1 but not CaV1.1. This latter result could mean either that Stac3-YFP does not bind to RyR1 or that the RyR1 binding sites are occluded in dysgenic myotubes by endogenous Stac3. Thus, we also tested for an interaction between Stac3-YFP and RyR1 after expression of both in tsA201 cells. Fig. 4B illustrates a cell in which the two proteins were expressed at high levels and shows images obtained before and after bleaching YFP within the indicated region of interest. This bleaching would not have been expected to affect any Stac3-YFP that remained bound to RyR1. Thus, the absence in the postbleach image of YFP fluorescence in regions containing RyR1 argues against a stable interaction between Stac3 and RyR1. This conclusion differs from that drawn from previous work on muscle tissue, in which Stac3 was found to coimmunoprecipitate with RyR1 (12). It is possible that we failed to detect such an interaction because the C-terminal YFP tag interfered with the binding of Stac3 to RyR1. Alternatively, it may be that the previously described coimmunoprecipitation was a consequence of a ternary complex of Stac3, the DHPR, and RyR1.
Fig. 4.
Stac3 does not appear to bind to RyR1 in either myotubes or tsA201 cells. (A) Stac3-YFP (red) had a punctate distribution in dyspedic (RyR1 null) myotubes (Left) and a diffuse distribution in dysgenic (CaV1.1 null) myotubes (Right), indicative of a lack of binding to RyR1 because punctate plasma membrane/SR junctions are present in both types of myotubes. (B) Images of a tsA201 cell coexpressing CFP-RyR1 (green) and Stac3-YFP (red) before (Left) and after (Right) bleaching of YFP within the indicated polygon. Note that CFP-RyR1 is localized in stacks of elongated ER. (Scale bars, 5 μm.)
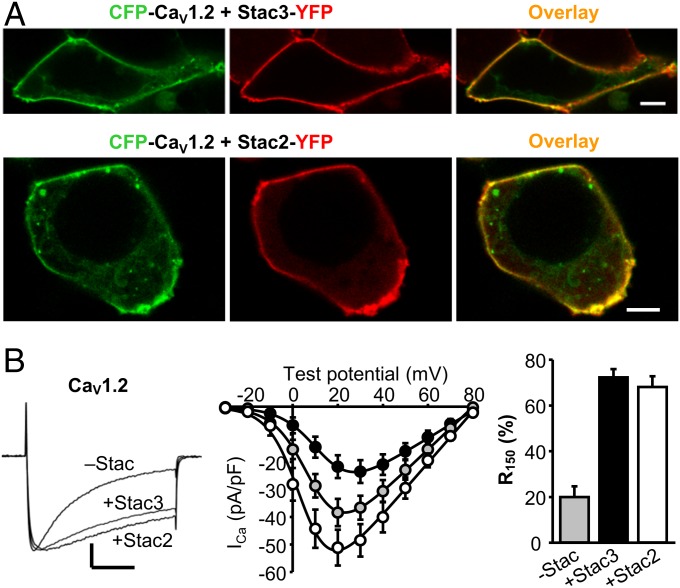
As described above, the membrane trafficking of CaV1.2 in tsA201 cells does not depend on the presence of Stac proteins (Fig. 1A). However, low levels of Stac3 transcripts and high levels of Stac2 are present in the cerebellum and fore-/midbrain (11), where CaV1.2 is also present (18). Thus, we tested whether Stac3 and/or Stac2 might interact with CaV1.2. As shown in Fig. 5A, both Stac3-YFP and Stac2-YFP colocalized with CFP-CaV1.2 at the surface of tsA201 cells. Furthermore, this interaction alters CaV1.2 function. Specifically, in addition to causing modest changes in Ca2+ current magnitude, both Stac2 and Stac3 caused a profound slowing of the rate of inactivation (Fig. 5B).
Fig. 5.
Stac3 and Stac2 associate with CaV1.2 in tsA201 cells and slow its inactivation rate. (A) Images of tsA201 cells coexpressing CFP-CaV1.2 (green) and either Stac3-YFP (red, Top) or Stac2-YFP (red, Bottom). (Scale bars, 5 μm.) (B, Left) Whole-cell Ca2+ currents in tsA201 cells expressing YFP-CaV1.2 without Stac 2 or Stac3 (Vtest = +20 mV) and YFP-CaV1.2 with either Stac3 (Vtest = +30 mV; vertically scaled 1.7-fold) or Stac2 (Vtest = +20 mV; vertically scaled 0.75-fold). Vertical calibration, 10 pA/pF (nonscaled current); horizontal, 50 ms. Peak I–V relationships (Center) and the fraction-of-peak current at 150 ms (Right) are indicated for YFP-CaV1.2 without Stac2 or Stac3 (gray symbols, +20 mV, n = 11) and YFP-CaV1.2 with either Stac3 (black symbols, +30 mV, n = 9) or Stac2 (white symbols, +20 mV, n = 10).
Discussion
If, as our results suggest, Stac3 is required for efficient membrane trafficking of CaV1.1, then it stands to reason that EC coupling would fail in animals that lack it. However, it seems likely that its continued presence is required for maintaining the EC coupling apparatus in a functional state. In particular, EC coupling is greatly impaired in muscle fibers of Stac3-null zebrafish embryos at 48 h postfertilization (hpf) even though there is a near-normal level of triadic CaV1.1 (12). Moreover, these Stac3-null embryos displayed some motile activity at 2 hpf, which could be attributed to the presence of Stac3 translated from maternally deposited mRNA. Thus, one could postulate that the maternally derived Stac3 supported the triadic insertion of CaV1.1, but that the subsequent loss of Stac3 protein caused CaV1.1 to become inoperative for EC coupling. An important goal for future research will be to identify the domains of CaV1.1 and Stac3 that are important for their interactions with one another. It will also be important to determine whether the modulation of Ca2+ channel function that we have shown to be produced by Stac2 and Stac3 also occurs for Stac1 and to determine whether such modulation actually occurs in specific neuronal populations.
The ability to achieve high-level expression of CaV1.1 in tsA201 cells provides a new experimental tool that will facilitate the study of skeletal muscle DHPRs without the time and expense involved in maintaining colonies of animals heterozygous for mutations of DHPR subunits. The relative absence of contaminating currents is also an advantage. For example, the ability to study how mutations alter the voltage dependence of CaV1.1 charge movements is hampered in dysgenic myotubes by the variable presence of gating currents arising from other ion channels, whereas background gating currents are essentially nonexistent in tsA201 cells (Fig. 2B, Right). Moreover, experiments on tsA201 cells may provide unexpected insights. For example, it was previously hypothesized that the loss of a “current-enhancing” signal from RyR1 to CaV1.1 accounted for the reduced size of Ca2+ currents in RyR1-null myotubes (3). However, this hypothesis appears to be incompatible with the near equivalence of CaV1.1 Ca2+ currents in tsA201 cells and dysgenic myotubes (Figs. 2 and 3). Thus, it may be that Ca2+ currents via CaV1.1 are inhibited by other junctional proteins unless RyR1 is present to relieve this inhibition. In principle, this idea and other aspects of DHPR signaling in skeletal muscle could be addressed by using tsA201 cells to manipulate the set of junctional proteins present in addition to CaV1.1 and Stac3. Of course, there are many questions about signaling interactions between the DHPR and RyR1 that will continue to require the use of skeletal muscle. However, even those experiments are likely to benefit from insights obtained in experiments on tsA201 cells.
Materials and Methods
Molecular Biology.
The construction of the expression plasmids for GFP-tagged, CFP-tagged, YFP-tagged, or unlabeled CaV1.1 was described previously (19, 20). The coding sequence of CaV1.2 (21) was inserted “in frame” and downstream of enhanced yellow or cyan fluorescent protein in a mammalian expression vector (pEYFP-C1, pECFP-C1; Clontech) to create YFP-CaV1.2 and CFP-CaV1.2. The plasmid CFP-RyR1, labeled at its N terminus with enhanced cyan fluorescent protein, was derived from the original GFP-RyR1 plasmid (22, 23) by excising the RyR1 encoding sequence from GFP-RyR1 and inserting it into the multiple-cloning site of ECFP-C1 (Clontech). PCR with the primers [forward (fw)] 5′-GAATTCATGACAGAAAAGGAAGTGGTG-3′ and [reverse (rev)] 5′-GGATCCCAAATCTCCTCCAGGAAGTCG-3′ was used to introduce EcoRI (5′) and BamHI (3′) sites flanking the coding sequence for Stac3 (gene ID 237611; plasmid kindly provided by Eric N. Olson, University of Texas Southwestern Medical Center, Dallas), from which the EcoRI-BamHI fragment was inserted into EYFP-N1 (Clontech) to create Stac3-YFP. To create Stac3-DsRed, the Stac3 fragment from Stac3-YFP was inserted into pDsRed1-N1 (Clontech), using the restriction enzymes HindIII and BamHI. To provide an unlabeled Stac3 construct with a comparable expression level, Stac3-YFP was digested with NdeI and BamHI, and the fragment containing the Stac3 sequence was then ligated to the 3,575-bp fragment of pEYFP-C1 that had been digested with the same enzymes. PCR with the primers (fw) 5′-CTGAGGTACCAACCATGACCGAAATGAGCGAGAAG-3′ and (rev) 5′-GTGGTACCTAGATCTCTGCCAAGGAGTCG-3′ was used to introduce KpnI sites and a stop codon flanking the coding sequence for Stac2 (gene ID 217154; OriGene Technologies), from which the KpnI-KpnI fragment was inserted into EYFP-N1. Afterward this construct was digested with NdeI and BamHI, and the fragment containing the Stac2 sequence was then ligated to the 3,575-bp fragment of pEYFP-C1 that had been digested with the same enzymes. Digestion of the plasmid β1a-YFP (24) Acc65I/BsrGI, followed by self-ligation of the compatible ends, was used to produce the expression vector for unlabeled β1a. Unlabeled α2δ-subunit was kindly provided by William A. Sather (University of Colorado).
Primary Skeletal Muscle Cell Culture and cDNA Microinjection.
All procedures involving mice were approved by the University of Colorado Denver–Anschutz Medical Campus Institutional Animal Care and Use Committee. Myoblasts were prepared from newborn dysgenic mice, homozygous for absence of CaV1.1 (25), or newborn dyspedic mice, homozygous for absence of RyR1 (26) as described before (27). For electrophysiological experiments, myoblasts were plated into 35-mm, plastic culture dishes (Falcon) coated with ECL (Millipore). Myoblasts destined for imaging were plated into 35-mm culture dishes with ECL-coated glass-coverslip bottoms (MatTek). Cultures were grown for 4–5 d in a humidified 37 °C incubator with 5% (vol/vol) CO2 in high-glucose Dulbecco’s modified Eagle medium (DMEM) (Mediatech), supplemented with 10% (vol/vol) FBS and 10% (vol/vol) horse serum (both from HyClone Laboratories). After 4–5 d, this medium was replaced with differentiation medium [DMEM supplemented with 2% (vol/vol) horse serum]. Two to 4 d following the shift to differentiation medium, single nuclei were microinjected with plasmid cDNA (200 ng/μL in water). Forty-eight hours after injection, expressing cells were identified on the basis of YFP fluorescence.
tsA201 Cell Culture and Expression of cDNA.
tsA201 cells were propagated in high-glucose DMEM supplemented with 10% (vol/vol) FBS and 2 mM glutamine in a humidified incubator with 5% (vol/vol) CO2. Cells were plated at a density of 2 × 105 cells in 35-mm dishes and transfected by Lipofectamine 2000 (Life Technologies) 24 h later with equal concentrations (1 μg/μL) of various combinations of YFP-CaV1.2, CFP-CaV1.2, YFP-CaV1.1, CFP-CaV1.1, unlabeled CaV1.1, CFP-RyR1, β1a, α2δ, unlabeled Stac3, Stac3-DsRed, Stac3-YFP, and unlabeled Stac2 cDNA. Six hours following transfection, cells were removed from the dish, using Trypsin EDTA (Mediatech), and replated at ∼1 × 104 cells per 35-mm dish to obtain isolated cells for electrophysiological recording. Seventy-two hours following transfection, positively transfected cells were identified by fluorescence and used for electrophysiology or imaging.
Immunostaining.
Transfected tsA201 cells were washed twice with PBS containing 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH to 7.4 with HCl, and fixed in paraformaldehyde [4% (vol/vol) in PBS] for 20 min at room temperature. After fixation, the cells were washed three times with PBS and incubated in 10% (vol/vol) goat serum/1% BSA/PBS for 60 min at room temperature. Incubation in mouse CaV1.1 subunit primary antibody IIF7 (1:500 diluted in 1% BSA/PBS, kindly provided by Kevin P. Campbell, University of Iowa, Iowa City, IA) was overnight at 4 °C, after which the cells were washed three times for 10 min with 1% BSA/PBS. Cells were then incubated in Alexa Fluor 568-labeled secondary antibody (diluted 1:300 in 1% BSA/PBS) for 1h at room temperature, washed with 1% BSA/PBS (3 × 10 min, with gentle agitation), and imaged.
Confocal Microscopy and Photobleaching.
Myotubes or tsA201 cells were superfused with Rodent Ringer’s solution (146 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, pH 7.4, with NaOH) and examined using a Zeiss LSM 710 confocal microscope. Images were obtained as a single optical slice with a 40× [1.3 numerical aperture (NA)] or 63× (1.4 NA) oil immersion objective. Excitation and emission, respectively, were 440 nm (diode laser) and 465–495 nm for CFP, 488 nm (argon laser) and 498–544 nm for GFP, 514 nm (argon laser) and 530–565 nm for YFP, 543 nm (HeNe laser), and either 565–721 nm for DsRed or 596–664 nm for Alexa Fluor 568. Relative to full power output, the excitation was attenuated to ∼4% (440 nm), ∼2% (488 nm and 514 nm), ∼15% (543 nm, DsRed), and ∼3% (543 nm, Alexa Fluor 568). For photobleaching of Stac3-YFP in tsA201 cells, a prebleach image was obtained as described above. Within this image, a smaller region of interest was designated to avoid the cell surface and repeatedly scanned for 15–45 s with nonattenuated 514-nm excitation. About 5–10 s after completion of the bleaching, a postbleach image was then obtained with the same settings as those of the prebleach image.
Measurement of L-Type Ca2+ Currents and Intramembrane Charge Movements.
All experiments were performed at room temperature (∼25 °C). Pipettes (∼2.0 MΩ) were fabricated from borosilicate glass and were filled with internal solution, which consisted of 140 mM Cs-aspartate, 10 mM Cs2-EGTA, 5 mM MgCl2, and 10 mM Hepes, pH 7.4, with CsOH. The bath solution contained 145 mM tetraethylammonium-Cl, 10 mM CaCl2, and 10 mM Hepes, pH 7.4, with NaOH. For electrophysiological experiments on myotubes, 0.003 mM tetrodotoxin (TTX) and 0.1 mM N-benzyl-p-toluene sulphonamide (BTS) were added to the bath solution. To isolate L-type currents in myotubes, voltage was stepped from the holding potential (−80 mV) to −20 mV for 1 s to inactivate endogenous T-type current. For measurement of intramembrane charge movements attributable to CaV1.1, 0.1 M LaCl3 and 0.5 M CdCl2 were added to the bath solution and a prepulse protocol (28) was used to inactivate T-type Ca2+ channels and sodium channels. Electronic compensation was used to reduce the effective series resistance to <5 MΩ (time constant <400 μs). Linear components of leak and capacitive current were corrected with −P/4 online subtraction protocols. Filtering was at 2–5 kHz and digitization was either at 10 kHz (L-type currents) or at 25 kHz (charge movements). Cell capacitance was determined by integration of a transient elicited by stepping from the holding potential (−80 mV) to −70 mV, using Clampex 8.2 (Molecular Devices), and was used to normalize charge movements (nC/μF) and ionic currents (pA/pF). Peak current–voltage (I–V) curves were fitted according to
| [1] |
where I is the peal current for the test potential V, Vrev is the reversal potential, Gmax is the maximum Ca2+ channel conductance, V1/2 is the half-maximal activation potential, and kG is the slope factor. Plots of the integral of the ON transient (Qon) of intramembrane charge movement as a function of test potential (V) were fitted according to
| [2] |
where Qmax is the maximal Qon, VQ is the potential causing movement of half the maximal charge, and kQ is a slope parameter. The activation phase of macroscopic ionic currents was fitted as described in ref. 4, using the exponential function
| [3] |
where I(t) is the current at time t after the depolarization, Afast and Aslow are the steady-state current amplitudes of each component with their respective time constants of activation (τfast and τslow), and C represents the peak current. Fractional inactivation of current in 150 ms (R150) was determined by dividing the peak current into the current 150 ms after the peak.
Analysis.
Figures were made using the software program SigmaPlot (version 11.0; SSPS). All data are presented as mean ± SEM. Statistical comparisons were made by one-way ANOVA.
Supplementary Material
Acknowledgments
We thank Ms. O. Moua for expert technical assistance. This work was supported by grants from the National Institutes of Health (AR055104) and the Muscular Dystrophy Association (MDA277475) (to K.G.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423113112/-/DCSupplemental.
References
- 1.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protasi F, et al. RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys J. 2000;79(5):2494–2508. doi: 10.1016/S0006-3495(00)76491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakai J, et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 4.Avila G, Dirksen RT. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca(2+) channel. J Gen Physiol. 2000;115(4):467–480. doi: 10.1085/jgp.115.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg RG, et al. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci USA. 1996;93(24):13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren D, Hall LM. Functional expression and characterization of skeletal muscle dihydropyridine receptors in Xenopus oocytes. J Biol Chem. 1997;272(36):22393–22396. doi: 10.1074/jbc.272.36.22393. [DOI] [PubMed] [Google Scholar]
- 7.Johnson BD, et al. Modulation of the cloned skeletal muscle L-type Ca2+ channel by anchored cAMP-dependent protein kinase. J Neurosci. 1997;17(4):1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-García MT, Kamp TJ, Marbán E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of α1 and β subunits. J Gen Physiol. 1995;105(2):289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong X, Hofmann F. Ca(2+)-dependent inactivation of the class C L-type Ca2+ channel is a property of the α1 subunit. FEBS Lett. 1996;378(2):121–125. doi: 10.1016/0014-5793(95)01434-9. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda T, et al. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20(1):1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 11.Nelson BR, et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc Natl Acad Sci USA. 2013;110(29):11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstick EJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuhuber B, Gerster U, Mitterdorfer J, Glossmann H, Flucher BE. Differential effects of Ca2+ channel β1a and β2a subunits on complex formation with α1S and on current expression in tsA201 cells. J Biol Chem. 1998;273(15):9110–9118. doi: 10.1074/jbc.273.15.9110. [DOI] [PubMed] [Google Scholar]
- 14.Leung AT, Imagawa T, Campbell KP. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent Ca2+ channel from rabbit skeletal muscle. Evidence for two distinct high molecular weight subunits. J Biol Chem. 1987;262(17):7943–7946. [PubMed] [Google Scholar]
- 15.Protasi F, Franzini-Armstrong C, Allen PD. Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle. J Cell Biol. 1998;140(4):831–842. doi: 10.1083/jcb.140.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takekura H, Franzini-Armstrong C. Correct targeting of dihydropyridine receptors and triadin in dyspedic mouse skeletal muscle in vivo. Dev Dyn. 1999;214(4):372–380. doi: 10.1002/(SICI)1097-0177(199904)214:4<372::AID-AJA9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Flucher BE, et al. Triad formation: Organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J Cell Biol. 1993;123(5):1161–1174. doi: 10.1083/jcb.123.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hell JW, et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123(4):949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA. 1998;95(4):1903–1908. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos S, Leuranguer V, Bannister RA, Beam KG. Mapping sites of potential proximity between the dihydropyridine receptor and RyR1 in muscle using a cyan fluorescent protein-yellow fluorescent protein tandem as a fluorescence resonance energy transfer probe. J Biol Chem. 2004;279(42):44046–44056. doi: 10.1074/jbc.M405317200. [DOI] [PubMed] [Google Scholar]
- 21.Mikami A, et al. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 22.Takeshima H, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzon NM, Grabner M, Suda N, Beam KG. Structure and targeting of RyR1: Implications from fusion of green fluorescent protein at the amino-terminal. Arch Biochem Biophys. 2001;388(1):13–17. doi: 10.1006/abbi.2000.2263. [DOI] [PubMed] [Google Scholar]
- 24.Leuranguer V, Papadopoulos S, Beam KG. Organization of calcium channel β1a subunits in triad junctions in skeletal muscle. J Biol Chem. 2006;281(6):3521–3527. doi: 10.1074/jbc.M509566200. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 26.Takeshima H, et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369(6481):556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- 27.Beam KG, Franzini-Armstrong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol. 1997;52:283–306. doi: 10.1016/s0091-679x(08)60384-2. [DOI] [PubMed] [Google Scholar]
- 28.Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346(6284):569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.