Abstract
Background
Patients with chronic kidney disease (CKD) have a high prevalence of sleep disorders. The association between sleep duration and self-reported CKD was examined in a population of Americans who participated in a national survey over a 3-year period.
Study Design
A cross-sectional study using survey data from the National Health Interview Survey (NHIS) from the year 2004-2006 was carried out. A retrospective examination of data from a community-based survey of 128,486 noninstitutionalized US civilian residents over the age of 18 years was conducted. Self-reported CKD was defined as having ‘weak or failing kidneys'. The sleep duration was defined by a self-reported estimate of habitual sleep duration.
Results
The prevalence of participants self-reporting kidney disease was higher in those with short (≤6 h per night) and long (≥8 h per night) sleep durations when compared to those sleeping 7 h per night. Self-reported information about sleep, demographic information, and information on comorbidities were assessed using standardized validated questionnaires which reported no kidney disease. A multivariate logistic regression analysis showed increased odds of self-reported kidney disease in study participants with both short and long sleep durations compared to healthy sleepers (sleeping >7-8 h per night). Observational data do not permit examination of causality, although possible confounders in observations of interest can be adjusted.
Conclusion
Among Americans surveyed in the NHIS (2004-2006), those with short or long sleep duration had higher odds of reporting that they had CKD.
Key Words: Chronic kidney disease, Sleep duration, Race/ethnicity
Introduction
There is increasing evidence indicating that habitual sleep duration affects morbidity and mortality. In large observational studies, both short and long sleep durations have been associated with an increased risk of hypertension [1,2], type 2 diabetes [2,3], obesity [2,4], cardiovascular disease [2,5], and all-cause mortality [6].
Chronic kidney disease (CKD) affects an estimated 10-13% of noninstitutionalized adults in the United States [7] and has serious implications for public health. A high prevalence of sleep apnea, restless leg syndrome, and overall poor sleep quality has been reported in patients with CKD [8,9,10,11,12]. The majority of studies that examined sleep health in CKD had a small sample size and focused on patients with advanced disease requiring renal replacement therapy. More recently, however, large observational studies have identified an association between predialysis CKD and sleep apnea or other indices of poor sleep quality [13,14].
While there is a growing body of evidence that sleep disorders are common in patients with CKD, the question of whether sleep duration is independently associated with CKD has not been extensively studied. It is critical to answer this question for several reasons: first, sleep duration has been associated with conditions that lead to CKD as noted above; second, sleep duration is a potentially modifiable factor, and third, short sleep duration has been associated with proteinuria [15], a key prognostic factor of CKD. In this study, we used data from the National Health Interview Survey (NHIS) to examine whether there is an association between inadequate sleep duration and self-reported CKD.
Methods
Study Design
The NHIS is a cross-sectional interview survey conducted annually by the National Center for Health Statistics at the Centers for Disease Control and Prevention. The survey employs a multistage probability design to select representative samples of the civilian, noninstitutionalized population of the United States. In addition to household information, trained interviewers collect individual data on sociodemographic factors, overall health status, health behavior, health care access and utilization, and the history of chronic conditions from a randomly selected adult member of a household. The questionnaire is administered using computer-assisted personal interviewing, which enables data validation through rules embedded in the software system. Details about the design and conduct of the NHIS have been published previously [16]. This analysis is limited to subjects older than 18 years of age who participated in surveys conducted between 2004 and 2006 and had a valid response to the question: ‘During the past 12 months, have you been told by a doctor or other health professional that you had weak or failing kidneys?' The beginning of the study period corresponds to the year when habitual sleep duration was included as a routine item in the Adult Sample Core questions.
Definitions
CKD and Sleep Duration
CKD was defined as a response of ‘yes' to the question of whether the subject had ‘weak or failing kidneys' diagnosed by a physician or other health care provider in the 12 months preceding the interview. The habitual sleep duration in the NHIS was recorded in full hour units. Short and long sleep durations were defined as a sleep duration of 6 h or less and 8 h or longer, respectively. A sleep duration of 7 h was used as the reference. This categorization scheme is consistent with the results of a prospective study by Kripke et al. [17] in which a sleep duration of 7 h was associated with the lowest risk of mortality.
Other Covariates
Predictor variables were selected a priori based on known associations with CKD or sleep disorders in the extant literature and consensus opinion of content experts amongst the investigators [1,2,3,4,5]. Self-reported chronic diseases were identified by the answers of ‘yes' to the question ‘Have you ever been told by a doctor or other health professional that you have [disease or condition]?' Obesity was defined as body mass index (BMI) of 30 or higher; volunteers with a BMI 25-29.9 were grouped as overweight. Categories of annual income, educational attainment, smoking, and alcohol use were collapsed into dichotomous values as follows: annual income USD >35,000 or USD ≤35,000; completion versus noncompletion of high school; ‘yes' versus ‘no' response to ever smoking 100 cigarettes in lifetime, and current use of alcohol versus no current alcohol use. A racial comparison was made between Blacks and all other races. Psychological distress was assessed by a score computed from the K6 scale, a validated measure of mental health that has been included in the NHIS questionnaires since 1997; a score of 13 or higher defined the presence of psychological distress as previously reported [18]. Individuals who responded ‘yes' to the question ‘Do you have any trouble seeing, even when wearing glasses or contact lenses?' were identified as having visual problems. Functional limitation due to health problems was assessed by a ‘yes/no' response to the difficulty walking 1/4 of a mile without assistive devices.
Statistical Analysis
Participants with and without self-reported CKD were compared using the Rao-Scott χ2 or t test for categorical and continuous variables, respectively. Unadjusted and adjusted odds ratios were calculated using multiple logistic regression, with adjustment for covariates that were selected a priori as described above and prespecified interaction terms between diabetes status and visual problems, and smoking and emphysema. The variance was estimated with Taylor series linearization. The person-level NHIS sample weights were divided by the number of years in the pooled dataset to accurately reflect the total population the pooled sample represents. Statistical significance was considered at two-tailed α <0.05. Statistical analysis was performed with SAS 9.3 (SAS Institute, Carey, N.C., USA) using the appropriate SURVEY procedures for complex sampling.
Results
Table 1 describes the characteristics of the NHIS participants. The prevalence of cardiovascular comorbidities was significantly higher in individuals self-reporting kidney disease. Of particular interest are reports of hypertension, coronary heart disease, diabetes, myocardial infarction/heart attack, and cerebrovascular accidents/stroke. There was also a higher prevalence of CKD among respondents with cancer, emphysema, visual problems, psychological stress, and difficulty walking 1/4 of a mile. Another interesting observation is the fact that participants who self-reported CKD were less likely to have completed high school education and also more likely to be earning USD ≤35,000. Female sex was a significant contributor to self-reported CKD. A significant number of Blacks were also found to be reporting CKD.
Table 1.
Comparison of sociodemographic and health characteristics of Americans participating in the 2004–2006 NHIS
| Variables | CKD (2,480) | No CKD (125,999) | p value |
|---|---|---|---|
| Age (mean ± SE), years | 59.6 ± 0.7 | 47 ± 0.2 | 0.002 |
| Females | 58.2 | 55 | 0.006 |
| Income, USD >35,000 | 35.5 | 59.2 | <0.001 |
| BMI | <0.001 | ||
| Overweight: 25-29.9 | 32.3 | 35.5 | |
| Obese: ⩾30 | 36.7 | 28.2 | |
| Education (completed high school) | 67.7 | 84.2 | <0.001 |
| Smoker | 54.5 | 43.2 | <0.001 |
| Current alcohol use | 68.8 | 76.4 | <0.001 |
| Cancer | 21.1 | 7.7 | <0.001 |
| Coronary heart disease | 22.6 | 4.5 | <0.001 |
| Diabetes | 34.2 | 8.4 | <0.001 |
| Emphysema | 7.7 | 1.9 | <0.001 |
| Heart attack | 17.9 | 3.4 | <0.001 |
| Hypertension | 68 | 27.2 | <0.001 |
| Stroke | 15.9 | 2.6 | <0.001 |
| Visual problems | 31.4 | 9.5 | <0.001 |
| Psychological distress | 11.5 | 2.5 | <0.001 |
| Difficulty walking 1/4 mile | 60.9 | 16.5 | <0.001 |
| Black | 15.4 | 12.1 | <0.001 |
| Sleep duration | <0.001 | ||
| Reference (7 h) | 16.6 | 30.7 | |
| Short sleepers (≤6 h) | 36.2 | 40.1 | |
| Long sleepers (≥8 h) | 40.1 | 47.2 |
Variables represent percentages unless otherwise specified.
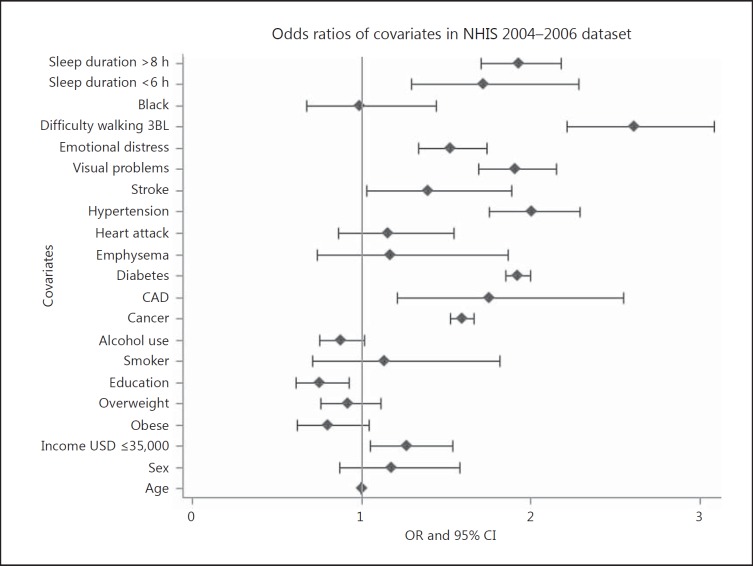
There was a higher prevalence of CKD among participants reporting short sleep (≤6 h per night) and long sleep (≥8 h per night) durations compared with healthy sleep durations (7 h per night). As shown in figure 1, after multivariate adjustment, participants reporting long sleep duration (≥8 h per night) also had an almost 2-fold odds ratio for reporting that they were diagnosed with CKD compared to participants with healthy sleep duration (7 h per night). Figure 1 also shows that participants with short sleep duration (≤6 h per night) had >2-fold increased odds for self-reported CKD compared to participants with healthy sleep.
Fig. 1.
Predictors of CKD among participants surveyed in the 2004-2006 NHIS. CAD = Coronary artery disease; 3BL = 3 blocks.
Discussion
This study showed that (1) study participants with a short habitual sleep duration had >2-fold odds of self-reported CKD compared to healthy sleepers and (2)study participants with habitual long sleep duration had almost 2-fold increased odds of self-reporting that they were given the diagnosis of CKD. It also showed that race/ethnicity may not be contributing significantly to this association between sleep duration and self-reported CKD.
To our knowledge, this is one of the first population-based studies to find a strong association between extremes of sleep duration and CKD. Our results extend the findings of the limited number of population-based studies on the topic. In a study of the relationship of race with physical and psychological well-being, Kutner et al. [19] reported that the prevalence of restless sleep was higher among community-dwelling dialysis patients than among their nondialysis counterparts. The main focus of the study, however, was the interplay of race, physical disease burden, and psychosocial well-being, and as such, the study did not specifically report differences in sleep duration.
A more recent study by Plantinga et al. [14] examined the association of sleep-related problems with CKD using data from the National Health and Nutrition Examination Survey (NHANES). The study participants were grouped into 3 categories based on the estimated glomerular filtration rate (eGFR): no CKD (eGFR >90 ml/min), stage 1 and 2 CKD (eGFR ≥60 ml/min and proteinuria), and stage 3 and 4 CKD (eGFR 15-59 ml/min). The study found that the sleep disorders and the use of sleep medications were more common among individuals with CKD but the relationship of sleep duration with CKD was not uniform across all stages of CKD. The adjusted odds of inadequate sleep (≤6 h) were not different between subjects with stage 1 and 2 CKD and those with no CKD. Subjects with stage 3 and 4 CKD, however, had lower adjusted odds of inadequate sleep as compared to those without CKD. The authors attributed this paradoxical finding to the higher use of sleep medications observed among those with CKD. Finally, Yamamoto et al. [15] retrospectively analyzed data on employees of the Osaka University in Japan to identify predictors of proteinuria. A short sleep duration was identified as an independent risk factor for proteinuria. Furthermore, there was a graded increase in the risk of proteinuria as sleep duration decreases.
The cross-sectional design of our study does not permit causal inferences. Although the direct renal effects of aberrant sleep duration have not been extensively investigated, emerging evidence suggests that sleep deprivation could interfere with normal renal physiology. Acute sleep deprivation in humans reduces plasma renin, angiotensin, and aldosterone levels, and is associated with increased urinary excretion of sodium and potassium [20,21]. In addition, the normal nocturnal dipping of blood pressure is attenuated. Similarly, animal studies of acute sleep deprivation have shown increased renal sympathetic nerve activity and reduced plasma angiotensin II levels; mean arterial pressure remained unchanged [22]. Taken together, these observations suggest that the changes in the renin-angiotensin-aldosterone system and solute excretion are most likely hormonal and renal adaptations to blunt the hypertensive response of renal sympathetic hyperactivity. Sustained disruption of the circadian rhythm may have more permanent consequences, as observed in hamsters with a mutation of the circadian regulatory gene casein kinase-1ε [23]. Animals heterozygote for the mutation exhibit phase-advanced and shortened circadian rhythms and were shown to develop proteinuria, renal tubular atrophy, and cardiac dysfunction.
As mentioned above, several studies have described an association of aberrant sleep duration with hypertension, diabetes, and cardiovascular disease, conditions that frequently accompany or cause CKD [1,2,3,4,5]. Our data demonstrate an association between sleep duration and CKD that is independent of these conditions. It is, therefore, possible that CKD and aberrant sleep duration share common pathophysiologic mechanisms. Indeed, both short and long sleep durations have been associated with markers of inflammation, a process now recognized to play an important role in the pathophysiology of chronic conditions such as diabetes, hypertension, and cardiovascular disease [24,25,26,27,28,29,30]. Sleep deprivation may also activate endothelin, a powerful vasoconstrictor involved in the pathogenesis of hypertension [31].
Once CKD is established, the uremic environment would further disturb the circadian rhythm, manifesting with more severe sleep disorders such as sleep apnea or periodic limb movement disorder. In addition to the clinical associations with these sleep disorders, advanced CKD has been linked to the suppression of the melatonin surge that is integrally linked to the regulation of the circadian rhythm [32,33]. Further evidence that advanced CKD exacerbates sleep disturbance is the observation that kidney transplantation and long nocturnal hemodialysis partially correct sleep apnea in dialysis patients [34,35,36].
The results of our study should be interpreted with several limitations in mind. First, both sleep duration and the presence or absence of any clinical condition including CKD was self-reported, which could lead to recall bias and misclassification of the disease status. Since individuals were asked to provide a subjective report of habitual sleep duration, data might have been influenced by recall/response bias, which is often observed in epidemiologic studies. These may on occasion be affected by the educational status. Although we did not specifically ascertain potential recall/response bias, our regression model adjusted for educational status. Second, our measure of depression was calculated from the limited data available in the survey. Third, even though we have adjusted for several variables that could explain the relationship between CKD and sleep duration, it is possible that our analysis has not accounted for other factors not captured in the survey.
Our study also has a number of strengths. Its findings are based on a large, nationally representative sample of adults. The NHIS is a rich data source, and standardized training of interviewers and the checks used in the computerized data collection system allow for the collection of highly accurate information. We minimized the likelihood of a spurious association by validating our findings in a training dataset on a separate cohort. Finally, we used a validated score to assess depression employing available survey data.
Sleep is an extremely important part of our daily lives. The finding of increased odds of self-reported CKD in Americans with aberrant sleep duration is a very important observation with major public health implications. Clinicians should, therefore, consider routinely assessing sleep complaints, and investigations into possible mechanisms underlying this association need to be pursued in the future.
Acknowledgment
This research was supported by funding from the National Institutes of Health (R01HL095799 and R01MD004113).
References
- 1.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 2.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 4.Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167:321–329. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037–1042. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Pierratos A, Hanly PJ. Sleep disorders over the full range of chronic kidney disease. Blood Purif. 2011;31:146–150. doi: 10.1159/000321859. [DOI] [PubMed] [Google Scholar]
- 9.Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Gigli GL, Adorati M, Dolso P, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309–315. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 12.Unruh ML, Sanders MH, Redline S, et al. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52:305–313. doi: 10.1053/j.ajkd.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sim JJ, Rasgon SA, Kujubu DA, et al. Sleep apnea in early and advanced chronic kidney disease: Kaiser Permanente Southern California cohort. Chest. 2009;135:710–716. doi: 10.1378/chest.08-2248. [DOI] [PubMed] [Google Scholar]
- 14.Plantinga L, Lee K, Inker LA, et al. Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis. 2011;58:554–564. doi: 10.1053/j.ajkd.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto R, Nagasawa Y, Iwatani H, et al. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis. 2012;59:343–355. doi: 10.1053/j.ajkd.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Design and estimation for the National Health Interview Survey, 1995-2004. Vital Health Stat. 2000;2:1–31. [PubMed] [Google Scholar]
- 17.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60:184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 19.Kutner NG, Bliwise DL, Zhang R. Linking race and well-being within a biopsychosocial framework: variation in subjective sleep quality in two racially diverse older adult samples. J Health Soc Behav. 2004;45:99–113. doi: 10.1177/002214650404500107. [DOI] [PubMed] [Google Scholar]
- 20.Charloux A, Gronfier C, Chapotot F, Ehrhart J, Piquard F, Brandenberger G. Sleep deprivation blunts the night time increase in aldosterone release in humans. J Sleep Res. 2001;10:27–33. doi: 10.1046/j.1365-2869.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–F411. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 22.Perry JC, Bergamaschi CT, Campos RR, Andersen ML, Montano N, Casarini DE, Tufik S. Sympathetic and angiotensinergic responses mediated by paradoxical sleep loss in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:146–152. doi: 10.1177/1470320310391504. [DOI] [PubMed] [Google Scholar]
- 23.Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 31.Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol. 2010;88:777–781. doi: 10.1139/Y10-046. [DOI] [PubMed] [Google Scholar]
- 32.Viljoen M, Steyn ME, van Rensburg BW, Reinach SG. Melatonin in chronic renal failure. Nephron. 1992;60:138–143. doi: 10.1159/000186729. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri ND, Oveisi F, Reyes GA, Zhou XJ. Dysregulation of melatonin metabolism in chronic renal insufficiency: role of erythropoietin-deficiency anemia. Kidney Int. 1996;50:653–656. doi: 10.1038/ki.1996.361. [DOI] [PubMed] [Google Scholar]
- 34.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 35.Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol Dial Transplant. 2007;22:3028–3033. doi: 10.1093/ndt/gfm309. [DOI] [PubMed] [Google Scholar]
- 36.Jurado-Gámez B, Martin-Malo A, Rodriguez-Benot A, Muñoz-Cabrera L, Cosano Povedano A, Aljama P. Kidney transplantation improves sleep-related breathing in hemodialysis patients. Blood Purif. 2008;26:485–490. doi: 10.1159/000157373. [DOI] [PubMed] [Google Scholar]