Abstract
Merkel cell–neurite complexes are located in touch-sensitive areas of the mammalian skin and are involved in recognition of the texture and shape of objects. Merkel cells are essential for these tactile discriminations, as they generate action potentials in response to touch stimuli and induce the firing of innervating afferent nerves. It has been shown that Merkel cells originate from epidermal stem cells, but the cellular and molecular mechanisms of their development are largely unknown. In this study, we analyzed Merkel cell differentiation during development and found that it is a temporally regulated maturation process characterized by a sequential activation of Merkel cell-specific genes. We uncovered key transcription factors controlling this process and showed that the transcription factor Atoh1 is required for initial Merkel cell specification. The subsequent maturation steps of Merkel cell differentiation are controlled by cooperative function of the transcription factors Sox2 and Isl1, which physically interact and work to sustain Atoh1 expression. These findings reveal the presence of a robust transcriptional network required to produce functional Merkel cells that are required for tactile discrimination.
Keywords: Merkel cells, Skin, Stem cells, Mouse
INTRODUCTION
Of the five senses essential for our survival, the sense of touch is the least understood. Whereas in invertebrates free-ending nerves directly perceive touch, mammals have elaborate tactile organs to sense a wide range of touch stimuli. One of these organs, known as the Merkel cell–neurite complex, is responsible for tactile discrimination of the shape, curvature and texture of objects (Chalfie, 2009; Lumpkin et al., 2010; Maricich et al., 2012).
Merkel cell–neurite complexes are formed by Merkel cells and slow-adapting type I Aβ-afferent nerve fibers, and are located in touch-sensitive areas of the skin, such as whiskers, paw pads and back skin in mice and fingertips in humans. Due to the fact that tactile stimuli are directly received by afferent neurons in invertebrates, the role of Merkel cells in touch sensation has been debated (Chalfie, 2009; Lumpkin et al., 2010; Maksimovic et al., 2013; Maricich et al., 2012). Exciting recent studies in mammals, however, have shown that upon direct touch stimuli, Merkel cells display ionic currents that induce the release of neurotransmitters, which in turn trigger action potential firing of the Aβ-afferent fibers. These studies further showed that Piezo2, an ion channel expressed in Merkel cells, is required for touch-evoked ionic currents. Importantly, loss of Piezo2 in Merkel cells abrogates Aβ-afferent nerve firing and behavioral tactile sensitivity (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014).
Transcriptional profiling of Merkel cells revealed expression of a large number of genes of the neuronal lineage, such as components of presynaptic machinery, synaptic vesicle proteins, ion channel subunits and transcription factors involved in neuronal development (Haeberle et al., 2004). Surprisingly, however, lineage-tracing experiments have shown that Merkel cells are of epithelial origin and, during development, originate from keratin (Krt) 14+ epidermal stem cells located in the basal layer of the epidermis (Bardot et al., 2013; Morrison et al., 2009; Van Keymeulen et al., 2009; Woo et al., 2010). Despite the essential role of Merkel cells in touch sensation, the molecular mechanisms of epidermal stem cell differentiation to Merkel cells are vastly understudied.
Embryonic epidermal stem cells give rise to the Merkel cell lineage and the suprabasal cells that provide a barrier function between the body and the environment. The epidermal differentiation program for suprabasal cells is well characterized; once cells exit the basal layer, they downregulate proliferation-associated genes and execute a terminal differentiation program. This process is marked by a step-wise transcriptional transition from the early differentiation-stage spinous layers to the late differentiation-stage granular and stratum corneum layers. Differentiated suprabasal cells form an essential barrier against the external environment, providing protection from pathogens, mechanical and chemical stresses, and dehydration (Blanpain and Fuchs, 2009).
Despite the common origin, the Merkel cell differentiation process seems to be completely different. Mature Merkel cells express the intermediate filaments keratin 8, 18 and 20 (K8/Krt8, K18/Krt18, K20/Krt20) and components of presynaptic machinery, such as Rab3c, all of which are absent from epidermal suprabasal cells. Transcriptional profiling revealed that there are only a small number of transcription factors that are expressed in Merkel cells and are absent in epidermal cells (Haeberle et al., 2004), with the crucial functions of transcriptional regulators in Merkel cell differentiation being largely undefined. The first characterized Merkel cell-specific transcription factor, Atoh1, has been shown to be required for formation of mature Merkel cells (Maricich et al., 2009; Van Keymeulen et al., 2009). The role of the transcription factor Sox2 in Merkel cell development appears to be more complex. Our previous studies have reported complete absence of Krt20+ mature Merkel cells in Sox2-null epidermis, as detected by immunofluorescence (IF) and corroborated by electron microscopy (EM) (Bardot et al., 2013). Work by Fiona Watt's group, however, showed a decrease of only about 50% in the number of Krt8+ Merkel cells (Lesko et al., 2013). This raises the possibility that Sox2 might be required for the expression of some Merkel cell-specific genes but not all, thus being required for a subset of the differentiation steps. The Sox2-null epidermal phenotype also points toward potential synergism between transcription factors in control of the Merkel cell differentiation process.
In this paper, we first characterized the developmental program that leads to the formation of Merkel cells and showed that Merkel cell development is a temporally controlled maturation process. This process is characterized by the sequential activation and sustained expression of genes required to produce functional Merkel cells. Second, we uncovered key transcription factors that function at different stages of this maturation process. We showed that Atoh1 is required for the initial specification of Merkel cell lineage, and that Isl1 and Sox2 co-regulate the following maturation steps by sustaining expression of Atoh1. Together, these studies define a novel transcriptional regulatory network in place to control the differentiation of epidermal stem cells to Merkel cells.
RESULTS
Merkel cell differentiation is a temporally regulated maturation process
Recent studies revealed that, whereas Merkel and suprabasal cells originate from epidermal stem cells, their transcriptional signatures are very different. Differentiation of epidermal stem cells to suprabasal cells is well studied and has been shown to be a stepwise process. We hypothesized that Merkel cell differentiation might be a multi-step process and sought to characterize which genes are expressed during the different stages of differentiation.
We started by analyzing expression of Merkel cell genes in fully developed Merkel cells at postnatal day 0 (P0). For this analysis we employed Atoh1-GFP mice that contain an enhanced green fluorescent protein (GFP) fused to the 3′-end of the atonal homolog 1 gene (Atoh1). In these animals, Merkel cells are the only epidermal cells with detectible GFP expression. Merkel cells form touch-domes, which are located around guard hairs, consisting of 20-30 Atoh1-GFP+ Merkel cells clustered together to form a horseshoe-shaped structure (Fig. 1A,B). Immunostaining for the Merkel cell-specific genes Sox2, Krt8 and Krt20 revealed >97% overlap between Atoh1-GFP+ and Sox2+ cells, and >92% overlap between Atoh1-GFP+ and Krt8+ cells (Fig. 1A-C). Some heterogeneity in Krt20 expression was observed, as the overlap between Atoh1-GFP+ and Krt20+ cells was 72%, reflecting that some Atoh1-GFP+ cells were Krt20 negative; notably, Knt20+ cells were not observed without Atoh1-GFP labeling (Fig. 1A,C). Another Merkel cell-rich area is the whisker follicles, where similar results were observed (supplementary material Fig. S1A).
Fig. 1.
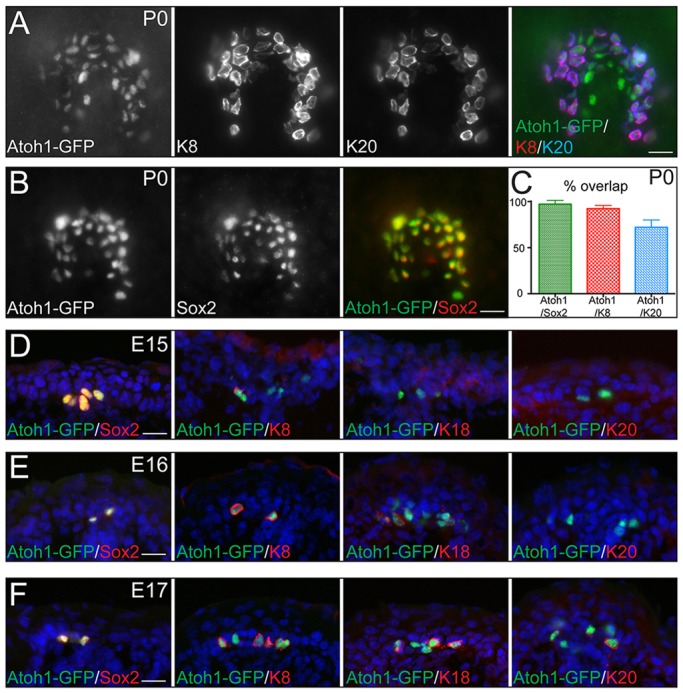
Merkel cell differentiation is a temporal maturation process. (A-C) Whole-mount IF (WMIF) staining showing overlap of Merkel cell-specific genes Atoh1, K8 (Krt8), K20 (Krt20) and Sox2 in neonatal (P0) mouse skin. Percentage of overlap is shown in C. Merkel cells show characteristic horseshoe-shaped touch-dome structure. (D-F) IF on tissue section co-staining of Atoh1-GFP and Sox2 (left), K8 (center-left), K18 (Krt18) (center-right) and K20 (right) at E15 (D), E16 (E) and E17 (F) shows progressive accumulation of markers through development. Scale bars: 25 µm.
We next speculated that the difference in expression of Atoh1-GFP, Sox2, Krt8 and Krt20 is due to temporal differences in expression of these genes during Merkel cell differentiation. In the back skin, the earliest expressed Merkel cell-specific genes were observed at E15 (Fig. 1D). At this time point, we observed expression of Atoh1-GFP and Sox2, and all Atoh1-GFP+ cells were Sox2 positive (Fig. 1D, left). Interestingly, some Atoh1-GFP+ cells started to express Krt8, but no Krt18 or Krt20 expression was observed (Fig. 1D). At E16, all Atoh1-GFP+ cells expressed Krt8 and a few cells began to express Krt18, but almost no Krt20 expression was observed (Fig. 1E). Finally, at E17, Krt18 expression in Atoh1-GFP+ cells was more robust, and a few Atoh1-GFP+ cells began to express Krt20 (Fig. 1F). A similar differentiation program was observed for whisker follicles, although it occurred one day earlier, with Atoh1 and Sox2 expression at E14, Krt8 and Krt18 at E15, and Krt20 at E16 (supplementary material Fig. S1B-D).
These data point toward temporal regulation of the Merkel cell differentiation process, with the sequential activation of genes that will form a mature Merkel cell. This is in contrast to epidermal suprabasal cell differentiation, which occurs as a stepwise process with marker substitution rather than accumulation (Blanpain and Fuchs, 2009). These differences are intriguing, as both Merkel cells and suprabasal cells originate from a common origin – epidermal stem cells.
The transcription factor Atoh1 is essential for Merkel cell specification
As the transcription factors Atoh1 and Sox2 are both expressed at the initial phase of Merkel cell differentiation, we decided to further investigate their functions during Merkel cell specification. We decided to ablate Atoh1 expression in epidermal stem cells prior to the first appearance of Atoh1 expression in the skin. To do so, we crossed Atoh1flox (fl) mice with mice expressing Cre recombinase under control of the Keratin 14 promoter, which is active in epidermal stem cells starting at E12.5 (Atoh1cKO). As previously reported, mice deficient for Atoh1 in the skin epidermis were born alive and did not have alterations in epidermal or hair follicle formation (Van Keymeulen et al., 2009). Moreover, as previously reported, no Krt8+ or Krt20+ cells were observed in Atoh1cKO compared with wild-type (WT) back skin and whisker follicles (Fig. 2A,C; and data not shown) (Maricich et al., 2009; Van Keymeulen et al., 2009). These genes, however, are expressed in the later phases of Merkel cell differentiation. To analyze whether Atoh1 function is required for the initial phase of Merkel cell specification, we analyzed Sox2 expression. IF analysis revealed complete absence of Sox2+ cells in the epidermis and whisker follicles of P0 and embryonic E15 Atoh1cKO animals (Fig. 2A-D), whereas the mesenchymal dermal papilla cells, which are not targeted by the Krt14-Cre ablation strategy, remained Sox2+ (supplementary material Fig. S2A). Importantly, no increase in apoptosis of Merkel cells was observed in Atoh1cKO epidermis at E15, when Sox2+ cells first appear, or at P0, as shown by IF with antibodies against activated caspase 3 (supplementary material Fig. S2B-E). Taken together, these data indicate that Atoh1 is required for Merkel cell specification.
Fig. 2.
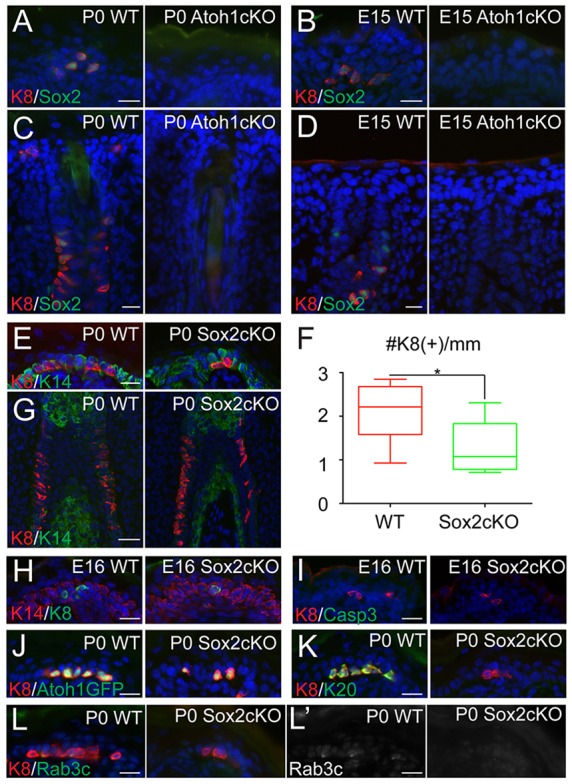
Atoh1 is essential for Merkel cell specification, whereas Sox2 is required for late-phase Merkel cell differentiation. (A-D) IF staining of WT and Atoh1cKO back skin (A,B) and whisker follicles (C,D) at P0 (A,C) and E15 (B,D) shows a complete loss of both Sox2 and K8 (Krt8). (E) Analysis of P0 WT and Sox2cKO back skin shows a reduction in the number of K8+ Merkel cells. Reduction is quantified in F (P=0.0477) and confirmed in whisker follicles (G) and in E16 back skin (H). (I) E16 WT and Sox2cKO mice show no differences in apoptosis, as measured by IF against activated caspase3 in the back skin. (J-L′) Further analysis shows that, in P0 Sox2cKO back skin, K8+ Merkel cells express the early marker Atoh1-GFP (J), but not the late differentiation markers K20 (Krt20) (K) or Rab3c (L,L′). Scale bars: 50 µm in G; 25 µm in all others.
The transcription factor Sox2 is essential to produce mature Merkel cells
Our previous work has shown that ablation of Sox2 in mouse epithelium leads to complete loss of Krt18+ and Krt20+ cells in all body regions analyzed (Bardot et al., 2013). As with IF, EM studies revealed a complete lack of mature Merkel cells in newborn skin, indicating that Sox2 is essential for Merkel cell maturation. Interestingly, however, the number of Atoh1-GFP+ cells in Sox2cKO animals was reduced but not absent, suggesting a differential requirement for Sox2 at each stage of differentiation (Bardot et al., 2013).
To follow up on that, we analyzed expression of Krt8 and observed a ∼50% decrease in the number of Krt8+ cells in P0 Sox2cKO compared with WT (Fig. 2E,F). The number of Krt8+ cells was also reduced in the whisker follicles (Fig. 2G) and at E16, when Krt8+ cells first appear (Fig. 2H). Importantly, no increase in apoptosis of Krt8+ cells was observed in Sox2cKO either at E16 or at P0, as shown by IF with antibodies against activated caspase 3 [Fig. 2I; and Bardot et al. (2013)]. The observed Krt8+ cells present in Sox2cKO were all Atoh1-GFP+ (Fig. 2J). However, the Krt8+ and Atoh1-GFP+ cells did not express markers of late differentiation stages, such as Krt20 or Rab3c (Fig. 2K-L′; supplementary material Fig. S2F). These data indicate differences in the requirements of Atoh1 and Sox2 in Merkel cell development. Despite being the earliest expressed transcription factors, they appear to play different roles in Merkel cell development. Atoh1 is required for Merkel cell specification, whereas Sox2 is required for Merkel cell maturation.
The transcription factor Isl1 is expressed at mid-stages of the Merkel cell differentiation process
Our obtained data show that Sox2 functions in Merkel cell maturation, but its function in this process is possibly redundant. To get insight into possible mechanisms of this redundancy, we revisited the list of transcription factors that are expressed in Merkel cells.
Isl1 became our top candidate. Isl1 is a LIM-homeodomain transcription factor that plays essential roles in cell proliferation and proper differentiation in many stem cells during development, including cardiac stem cells and secretory endocrine cells in the pancreas and small intestine (Habener et al., 2005; Laugwitz et al., 2008). Isl1 expression in the epidermis is restricted to Merkel cells and is not present in the basal or suprabasal layers (Bardot et al., 2013; Haeberle et al., 2004). During epidermal embryogenesis, Isl1 expression in the back skin is detected in some Atoh1-GFP+ cells in the initial phase of Merkel cell specification at E15 (Fig. 3A, left). However, during the mid phase of Merkel cell differentiation at E16, most Atoh1-GFP+ cells also express Isl1 (Fig. 3A, right). Its expression persists to the postnatal stage, at which it overlaps with Atoh1-GFP+ and Krt20+ cells (Fig. 3B,C). Similar conclusions were reached for the analysis of whisker follicles (supplementary material Fig. S3A,B). Consistent with Isl1 being a protein expressed in the mid phase of the Merkel cell differentiation process, Isl1+ cells were absent in Atoh1cKO back skin and whisker follicles during embryogenesis at E16 and postnatally at P0 (Fig. 3D,E; supplementary material Fig. S3C).
Fig. 3.
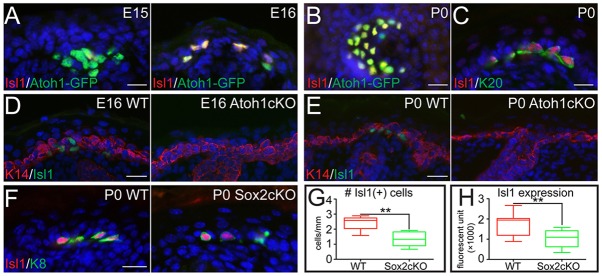
The transcription factor Isl1 is expressed during the middle stages of Merkel cell differentiation. (A) IF analysis showing the onset of Isl1 expression in Atoh1-GFP+ Merkel cells at E15 and full expression at E16. (B) WMIF shows overlap of Atoh1-GFP and Isl1 in P0 back skin touch-domes. (C) Further analysis shows that Isl1 expression overlaps with Krt20 at P0. (D,E) Analysis of WT and Atoh1cKO back skin at E16 (D) and P0 (E) reveals a complete absence of Isl1 in the Atoh1cKO epidermis. (F-H) IF studies of P0 WT and Sox2cKO epidermis show a partial reduction in both Isl1+ cells (G, P=0.0026) and the intensity of Isl1 fluorescent signal (H, P=0.0014) in Sox2cKO mice. Scale bars: 25 µm.
Our previous studies reported that the promoter sequence of the Isl1 gene does not contain canonical Sox2 binding sites; thus, Isl1 expression is unlikely to be under direct control of Sox2 (Bardot et al., 2013). Indeed, IF analysis revealed that Isl1+ cells were present in P0 Sox2cKO skin, although their numbers and the Isl1 fluorescence intensity in Krt8+ cells were reduced compared with WT (Fig. 3F-H). Together, these data suggest that Isl1 cooperates with Sox2 to control the mid-stage Merkel cell differentiation process.
Isl1 is not required for Merkel cell development
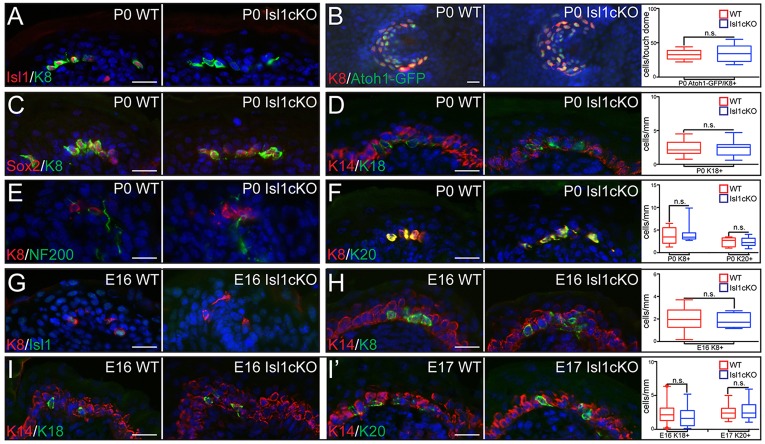
We first analyzed the role of Isl1 alone in control of the Merkel cell lineage. We conditionally ablated Isl1 in skin epithelium by crossing Isl1fl/fl mice with Krt14-Cre mice (Isl1cKO). IF studies confirmed that Isl1 expression was completely abolished in Krt8+ cells of P0 Isl1cKO epithelium (Fig. 4A; supplementary material Fig. S4A). Despite the complete loss of Isl1, no statistically significant differences were observed in the number of Atoh1+, Sox2+, Krt8+, Krt18+ or Krt20+ cells in both back skin and whisker follicles of P0 and in adult Isl1cKO animals (Fig. 4B-D,F; supplementary material Fig. S4B-D). As in WT skin, Merkel cells were also innervated by NF200+ (Nefh – Mouse Genome Informatics) neurons in Isl1cKO animals (Fig. 4E).
Fig. 4.
Isl1 is not required for Merkel cell development. (A) IF analysis of P0 WT and Isl1cKO epidermis confirms the loss of Isl1 from K+ (Krt8+) Merkel cells. (B) WMIF reveals no difference in the number of K8+/Atoh1-GFP+ cells per touch-dome in Isl1cKO epidermis (P=0.9283). (C) Sox2 expression is not lost from K8+ Merkel cells in Isl1cKO epidermis, as confirmed by IF at P0. (D-F) IF analysis of mature Merkel cell markers Krt18 (D, P=0.8993), NF200 (E) and Krt20 (F, P=0.8726) shows that Merkel cells are able to reach maturity in Isl1cKO epidermis. (G) IF analysis of E16 Isl1cKO epidermis confirms the loss of Isl1 from developing Merkel cells. (H,I) Analysis of WT and Isl1cKO mice at E16 (H,I) or E17 (I′), using IF against Merkel cell markers K8 (H, P=0.8545), K18 (Krt18) (I, 0.2245) and K20 (Krt20)(I′, 0.6417), shows that the Merkel cell maturation program is unaffected in Isl1cKO embryos. Scale bars: 25 µm.
Analysis of Merkel cell development during embryogenesis also did not reveal any significant abnormalities. Whereas Isl1 expression was completely diminished at E16 (Fig. 4G; supplementary material Fig. S4I), we did not observe differences in the appearance of Krt8+, Krt18+ and Krt20+ cells during Merkel cell maturation (Fig. 4H-I′; supplementary material Fig. S4E,I,J). No increase in apoptosis of Merkel cells was observed in P0 Isl1cKO back skin, as shown by IF with antibodies against activated caspase 3 (supplementary material Fig. S4F). Finally, the lack of a Merkel cell phenotype in Isl1cKO animals could not be explained by compensation by Isl-family members, as mRNA microarray analysis of genes expressed in Merkel cells did not reveal expression of other Isl family members (Haeberle et al., 2004), and IF studies showed that Isl2 is not expressed in WT or Isl1cKO Krt8+ Merkel cells (supplementary material Fig. S4G,H). Overall, Isl1 alone is not required for Merkel cell differentiation.
Sox2 and Isl1 cooperate to orchestrate Merkel cell differentiation
To investigate whether Sox2 and Isl1 cooperate to control the Merkel cell maturation program, we generated Sox2 Isl1 2cKO (2cKO) mice. Analysis of P0 2cKO skin by IF confirmed a complete loss of both Sox2 and Isl1 expression in the knockout epithelium (supplementary material Fig. S5A,B). Newborn 2cKO mice were viable with no apparent defects in epidermal differentiation and hair follicle formation (supplementary material Fig. S5C-E).
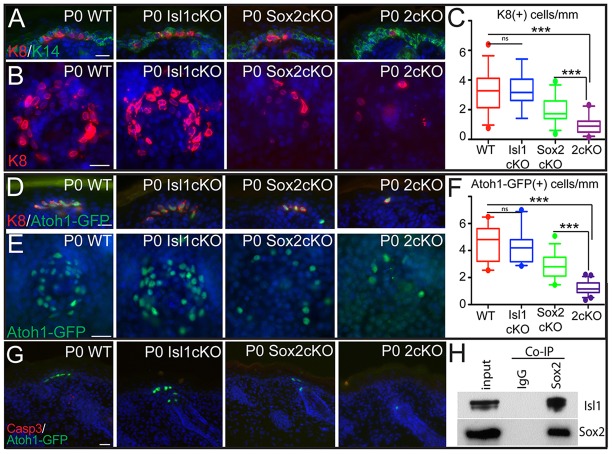
We next analyzed whether the Merkel cell maturation program is altered in 2cKO mice. Consistent with the loss of Sox2, Krt20+ cells were completely absent in 2cKO epithelium and whisker follicles (supplementary material Fig. S5F-I). Analysis by IF showed a drastic decrease in the number of Krt8+ cells in 2cKO epithelium compared with WT (Fig. 5A-C). This effect was also observed in the whisker follicle region, typically enriched with Merkel cells (supplementary material Fig. S6A). The reduction in the number of Krt8+ cells in 2cKO mice was not due to changes in NF200-neuronal innervations (supplementary material Fig. S6B) or apoptosis of Krt8 cells (supplementary material Fig. S6C). Importantly, the number of Krt8+ cells was significantly decreased in the 2cKO epidermis when compared with Sox2cKO (Fig. 5C), suggesting that Isl1 plays a redundant role with Sox2 to promote the formation of Krt8+ Merkel cells.
Fig. 5.
Sox2 and Isl1 cooperate to orchestrate Merkel cell differentiation. (A) IF analysis of WT, Isl1cKO, Sox2cKO and Isl1 Sox2 2cKO mice, showing a further reduction in the number of K8+ (Krt8+) cells present in the 2cKO epidermis. (B) WMIF analysis confirmation of the cooperative effect of Sox2 and Isl1. Quantification of the number of K8+ cells per mm of epidermis is provided in C (WT versus Isl1cKO, P=0.8902; WT versus Sox2cKO, P<0.0001; Sox2cKO versus Isl1 Sox2 2cKO, P<0.0001). (D-F) Abrogation of Isl1 and Sox2 in mice results in dramatic loss of Atoh1-GFP+ cells in the back skin. (D) IF analysis of WT, Isl1cKO, Sox2cKO and Isl1 Sox2 2cKO mice showing a further reduction in the number of Atoh1GFP+ K8+ cells present in the 2cKO epidermis. (E) WMIF analysis confirms the cooperative effect of Sox2 and Isl1 in specifying Atoh1-GFP+ cells. (F) Quantification of Atoh1-GFP+ cells per mm of epidermis (WT versus Isl1cKO, P=0.4579; WT versus Sox2cKO, P<0.0001; Sox2cKO versus Isl1 Sox2 2cKO, P<0.0001). (G) WT, Isl1cKO, Sox2cKO and Isl1 Sox2 2cKO epidermis showed no differences in apoptosis, as measured by IF against activated caspase3. (H) Isl1 co-immunoprecipitates with Sox2 when expressed in 293T cells in vitro. Scale bars: 25 µm.
We have previously shown that Sox2 binds to the enhancer region of the Atoh1 gene and promotes its transcription (Bardot et al., 2013). A great deal of evidence in the literature suggests that Sox2 requires interacting proteins to fully execute its transactivating activity (Ahmed et al., 2012; Gagliardi et al., 2013). In light of the observed genetic interaction, we investigated whether Sox2 cooperates with Isl1 to regulate transcription of the Atoh1 gene. To do this, we generated Sox2 Isl1 2cKO mice that also expressed Atoh1-GFP, and found that the number of Atoh1-GFP+ cells was dramatically reduced in the 2cKO when compared with WT and Sox2cKO (Fig. 5D-F). The reduction in Atoh1-GFP+ cells was also observed in the whisker pads of 2cKO mice (supplementary material Fig. S6D). Importantly, the decrease in the number of Atoh1-GFP+ cells was not due to the apoptosis, as we did not observe an increase in the number of activated caspase 3+ cells in Atoh1-GFP cells in 2cKO mice (Fig. 5G; supplementary material Fig. S6E). To investigate the mechanism by which Isl1 and Sox2 transcription factors cooperate, we performed biochemical assays and found that Isl1 co-immunoprecipitates with Sox2 (Fig. 5H), indicating that Isl1 and Sox2 physically interact. Together, these data show that Isl1 and Sox2 function together during Merkel cell development to sustain the transcription of Atoh1 required for Merkel cell differentiation.
DISCUSSION
Although Merkel cells were discovered more than 100 years ago, their function has remained enigmatic for a long time. Studies published this year, however, clearly show that Merkel cells are essential for discrimination of shape and texture, as they generate action potentials in response to tactile stimuli and induce the firing of afferent nerves innervating the Merkel cells (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014). This discovery leaves open questions on the biological processes leading to the formation of these important cells. Although it was known that Merkel cells originate from epidermal stem cells during embryogenesis, the molecular mechanisms of their development are still unknown.
In this paper, we show that Merkel cell development is a maturation process. It initiates at E15 and is characterized by activation of Atoh1 and Sox2 expression. The differentiation process proceeds to E16 with activation of Isl1 and Krt8 genes, but early differentiation genes continue to be expressed. By E17-E18, Krt18, Krt20 and Rab3c start to be expressed, and, in mature Merkel cells, genes of all differentiation stages are co-expressed. This is in striking contrast to the epidermal differentiation program that exists to form the suprabasal layers of the epidermis. In this program, the genes expressed during early differentiation of the spinous layer are switched off as expression of late differentiation genes of granular and stratum corneum layers are turned on (Blanpain and Fuchs, 2009). This stepwise differentiation program is reflected in the sequential expression of markers, but it has also been shown that transcriptional regulators crucial for early differentiation steps might not be required for late stages. This is perhaps best shown for the Notch pathway, in which loss of Rbpj expression completely abrogates the formation of the early differentiation-stage spinous layer, while the late differentiation-stage granular and stratum corneum layers are still formed (Blanpain et al., 2006). These differences are very intriguing, as both lineages originate from a common origin – epidermal stem cells.
The observed difference in differentiation programs of Merkel and epidermal cell lineages is not entirely surprising. Other sensory organs of our body, such as ear hair cells responsible for auditory sensations and photoreceptors responsible for visual perception, are also derived from the same cells that give rise to the epidermis. Despite the common origin, these structures differ drastically from the epidermis, and, importantly, completely different genes govern the development of these structures. Given this degree of similarity between genes expressed in Merkel cells and inner ear hair cells (Liu et al., 2014), it is fair to speculate that the developmental process leading to their formation might be similar.
Our work also uncovers a complex regulatory network of transcription factors in place to control Merkel cell development. We show that Atoh1, one of the earliest expressed transcription factor in Merkel cells, is essential for Merkel cell specification, as loss of Atoh1 leads to complete loss of Merkel cells at all developmental stages. Intriguingly, we show that the transcription factors Sox2 and Isl1 physically interact and cooperate to control Merkel cell maturation by promoting Atoh1 transcription. Our data also reinforce the importance of Sox2-interacting proteins in controlling gene expression and cell fate determination. Indeed, in embryonic stem cells it was shown that Sox2 requires the interacting partner Nanog to promote stem cell maintenance, and a mutant form of Sox2 that lacks interaction with Nanog is unable to maintain embryonic stem self-renewal (Gagliardi et al., 2013). Similarly, in cochlea cells, Sox2 cooperates with Eya1/Six1 to promote cochlea development (Ahmed et al., 2012). Our data identify a novel Sox2-interacting partner, Isl1, and show the crucial role of Sox2/Isl1 in controlling Atoh1 expression and promoting Merkel cell differentiation. This genetic, protein and transcriptional interaction is intriguing, as currently there are no other examples of Sox2 and Isl1 cooperation in other developmental systems, in which both of these proteins are co-expressed.
Our studies were focused on the embryonic development of Merkel cells. It remains to be determined whether a similar developmental program and transcriptional regulation exists to maintain Merkel cells in the adult organism during normal homeostasis and upon skin wounding. In fact, adult and embryonic Merkel cell differentiation processes might be different, based on recent findings that Krt17+ Merkel cell progenitors, located in the epidermal layer above the Merkel cells, replenish Merkel cells in adult mouse skin (Doucet et al., 2013).
Further understanding of the transcriptional regulators of Merkel cell development will be important not only for general understanding of Merkel cell biology, but also for uncovering processes leading to human pathologies, such as reduced tactile sensitivity in patients with diabetes and inflammation or with Merkel cell carcinoma, a highly aggressive form of cancer with no current treatment. Importantly, as both Sox2 and Isl1 have been shown to be upregulated in Merkel cell carcinoma cells, it will be important in the future to explore the contribution of these transcription factors to tumorigenesis (Agaimy et al., 2013; Tilling and Moll, 2012).
MATERIALS AND METHODS
Mice
All mice were housed and cared for according to ISMMS and IACUC approved protocols. At least two animals from independent litters were used for each analysis. Sox2 and Isl1flox mice were generously provided by Silvia Nicolis (University of Milan, Italy) and Chenleng Cai (Icahn School of Medicine at Mount Sinai, NY, USA), respectively. Krt14-Cre, Atoh1-EGFP and Atoh1flox mice were obtained from Jackson Laboratories. Mice were genotyped by PCR using DNA extracted from tail skin. Back skin, whisker regions and paws were embedded in OCT immediately following sacrifice.
Whole-mount IF
Back skins were collected from newborn mice and placed in dispase for 1 h at 37°C, after which the epidermal part of the skin was peeled from the dermis and fixed in 4% PFA for 2 h. Skins were blocked overnight in PBS-Triton with bovine serum albumin/normal goat serum/normal donkey serum (BSA/NGS/NDS). Primary antibodies were diluted in blocking solution and incubations were carried out for 4 h at room temperature, followed by incubation in secondary antibodies for 4 h at room temperature. Skins were then counterstained with DAPI and mounted in antifade mounting media for imaging.
IF
Tissues were collected from mice and either embedded fresh into OCT or PFA fixed for 2 h and equilibrated in sucrose before embedding, and subsequently cut into 10 μm-sections using a Leica Cryostat. Slides were then fixed for 10 min in 4% PFA and blocked for 1 h in PBS-Triton with BSA/NGS/NDS. Primary antibodies were diluted in blocking solution, and incubations were carried out for 1 h at room temperature, followed by incubation in secondary antibody for 1 h at room temperature. Slides were then counterstained with DAPI and mounted using antifade mounting media.
Antibodies for IF
Antibodies were used as follows: anti-Keratin 14 (generous gift of Julie Segre, National Human Genome Research Institute, MD, USA.; 1:20,000); anti-Keratin 5(generous gift of Elaine Fuchs, The Rockefeller University, NY, USA; 1:500); anti-K8 (DSHB, TROMA-I; 1:500); anti-K18 (Abcam, ab668; 1:100); anti-Isl1 (Abcam, ab109517; 1:400); anti-Isl1/2 (DSHB, 39.4D5; 1:100); anti-Isl2 (DSHB, 67.4E12; 1:500); anti-Rab3C (Abcam, ab3336; 1:250); anti-NF200 (Abcam, ab93340; 1:250); anti-Sox2 (Stemgent, 09-0024; 1:150); anti-K20 (Dako, M7019; 1:70); anti-AcCasp3 (R&D Systems, AF835; 1:250); anti-GFP (Abcam, ab13970; 1:1000); anti-K10 (Covance, PRB-145P; 1:500); anti-Loricrin (Covance, PRB-159P; 1:250).
Microscopy and quantification
Slides were imaged using a Leica DM6000 inverted slide microscope and either 10×, 20× or 40× objectives. Fluorescence intensity was calculated from at least three raw, single-channel grayscale images per condition using Leica LAS AF software. Fluorescence intensity was normalized to non-nuclear background. The quantification of Merkel cells per mm of skin was performed as described in Bardot et al. (2013).
Statistics
In all column bar graphs, means±s.d. are shown. Box-and-whisker plots show first-to-third quartiles around the median, with whiskers showing the 5%-95% range and outliers presented as individual data points. All quantifications were performed on multiple cell populations from different animals. To determine the significance between two groups (as indicated in the figures by parentheses), comparisons were made using Student's t-test (GraphPad Prism 5). For all statistical tests, the 0.05 level of confidence was accepted for statistical significance and actual P-values (to four decimal places) are provided in the figure legends.
Co-immunoprecipitation and western blot
For co-immunoprecipitation, HEK293T cells were transiently co-transfected with plasmids expressing Isl1, Sox2 and GFP, which was used as a negative control. 72 h after transfection, nuclear extracts were prepared and disrupted by sonication. Co-IP was performed overnight with anti-Sox2 (Stemgent, 09-0024; 1:50) or anti-IgG (Abcam, ab27478; 1:50) and Dynal Protein G magnetic beads (Invitrogen) in lysis buffer (50 mM HEPES pH 7.6, 250 mM NaCl, 0.1% NP-40, 0.2 mM EDTA and protease inhibitors). The following day, unbound material was washed with lysis buffer, bound material was eluted by boiling in Laemmli buffer and subjected to western blot analysis. Western blot analysis was performed using anti-Isl1 (Abcam, ab109517; 1:1000), anti-Sox2 (Stemgent, 09-0024; 1:1000) and anti-Histone H3 (Abcam, ab1791; 1:10,000).
Supplementary Material
Acknowledgements
For help, advice and critical suggestions on our work, we would like to thank Rob Krauss and Jose Silva. We are grateful for the assistance and reagents provided by Julie Segre, Michael Rendl, Chenleng Cai and Silvia Nicolis.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
C.N.P., E.S.B., V.J.V. and E.E. designed the study. C.N.P., E.S.B. and V.J.V. performed the experiments. F.J.S. assisted with data analysis. C.N.P., E.S.B., V.J.V. and E.E. analyzed the data. E.E. wrote the manuscript with input from all other authors.
Funding
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) [R01 AR063724]. C.N.P. was supported by a European Molecular Biology Organization (EMBO) fellowship [ALTF 552-2012]. V.J.V. is a Pew Latin American Fellow in the Biomedical Sciences, supported by The Pew Charitable Trusts. E.E. was a Basil O'Connor Scholar with the March of Dimes Foundation. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.112169/-/DC1
References
- Agaimy A., Erlenbach-Wünsch K., Konukiewitz B., Schmitt A. M., Rieker R. J., Vieth M., Kiesewetter F., Hartmann A., Zamboni G., Perren A. et al. (2013). ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod. Pathol. 26, 995-1003 10.1038/modpathol.2013.40 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Wong E. Y. M., Sun J., Xu J., Wang F. and Xu P.-X. (2012). Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 22, 377-390 10.1016/j.devcel.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot E. S., Valdes V. J., Zhang J., Perdigoto C. N., Nicolis S., Hearn S. A., Silva J. M. and Ezhkova E. (2013). Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. EMBO J. 32, 1990-2000 10.1038/emboj.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C. and Fuchs E. (2009). Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207-217 10.1038/nrm2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W. E., Pasolli H. A. and Fuchs E. (2006). Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20, 3022-3035 10.1101/gad.1477606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M. (2009). Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 10, 44-52 10.1038/nrm2595 [DOI] [PubMed] [Google Scholar]
- Doucet Y. S., Woo S.-H., Ruiz M. E. and Owens D. M. (2013). The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 3, 1759-1765 10.1016/j.celrep.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi A., Mullin N. P., Ying Tan Z., Colby D., Kousa A. I., Halbritter F., Weiss J. T., Felker A., Bezstarosti K., Favaro R. et al. (2013). A direct physical interaction between Nanog and Sox2 regulates embryonic stem cell self-renewal. EMBO J. 32, 2231-2247 10.1038/emboj.2013.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Kemp D. M. and Thomas M. K. (2005). Minireview: transcriptional regulation in pancreatic development. Endocrinology 146, 1025-1034 10.1210/en.2004-1576 [DOI] [PubMed] [Google Scholar]
- Haeberle H., Fujiwara M., Chuang J., Medina M. M., Panditrao M. V., Bechstedt S., Howard J. and Lumpkin E. A. (2004). Molecular profiling reveals synaptic release machinery in Merkel cells. Proc. Natl. Acad. Sci. USA 101, 14503-14508 10.1073/pnas.0406308101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R., Cha M., Ling J., Jia Z., Coyle D. and Gu J. G. (2014). Merkel cells transduce and encode tactile stimuli to drive abeta-afferent impulses. Cell 157, 664-675 10.1016/j.cell.2014.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz K.-L., Moretti A., Caron L., Nakano A. and Chien K. R. (2008). Islet1 cardiovascular progenitors: a single source for heart lineages? Development 135, 193-205 10.1242/dev.001883 [DOI] [PubMed] [Google Scholar]
- Lesko M., Driskell R., Kretzschmar K., Goldie S. and Watt F. (2013). Sox2 modulates the function of two distinct cell lineages in mouse skin. Dev. Biol. 382, 15-26 10.1016/j.ydbio.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Chen P. and Wang J. (2014). Molecular mechanisms and potentials for differentiating inner ear stem cells into sensory hair cells. Dev. Biol. 390, 93-101 10.1016/j.ydbio.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Lumpkin E. A., Marshall K. L. and Nelson A. M. (2010). The cell biology of touch. J. Cell Biol. 191, 237-248 10.1083/jcb.201006074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S., Baba Y. and Lumpkin E. A. (2013). Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann. N. Y. Acad. Sci. 1279, 13-21 10.1111/nyas.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S., Nakatani M., Baba Y., Nelson A. M., Marshall K. L., Wellnitz S. A., Firozi P., Woo S.-H., Ranade S., Patapoutian A. et al. (2014). Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617-621 10.1038/nature13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich S. M., Wellnitz S. A., Nelson A. M., Lesniak D. R., Gerling G. J., Lumpkin E. A. and Zoghbi H. Y. (2009). Merkel cells are essential for light-touch responses. Science 324, 1580-1582 10.1126/science.1172890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich S. M., Morrison K. M., Mathes E. L. and Brewer B. M. (2012). Rodents rely on Merkel cells for texture discrimination tasks. J. Neurosci. 32, 3296-3300 10.1523/JNEUROSCI.5307-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison K. M., Miesegaes G. R., Lumpkin E. A. and Maricich S. M. (2009). Mammalian Merkel cells are descended from the epidermal lineage. Dev. Biol. 336, 76-83 10.1016/j.ydbio.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilling T. and Moll I. (2012). Which are the cells of origin in Merkel cell carcinoma? J. Skin Cancer 2012, 680410 10.1155/2012/680410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Mascre G., Youseff K. K., Harel I., Michaux C., De Geest N., Szpalski C., Achouri Y., Bloch W., Hassan B. A. et al. (2009). Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 187, 91-100 10.1083/jcb.200907080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.-H., Stumpfova M., Jensen U. B., Lumpkin E. A. and Owens D. M. (2010). Identification of epidermal progenitors for the Merkel cell lineage. Development 137, 3965-3971 10.1242/dev.055970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.-H., Ranade S., Weyer A. D., Dubin A. E., Baba Y., Qiu Z., Petrus M., Miyamoto T., Reddy K., Lumpkin E. A. et al. (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622-626 10.1038/nature13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.