Abstract
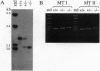
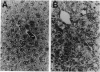
We inactivated the mouse metallothionein (MT)-I and MT-II genes in embryonic stem cells and generated mice homozygous for these mutant alleles. These mice were viable and reproduced normally when reared under normal laboratory conditions. They were, however, more susceptible to hepatic poisoning by cadmium. This proves that these widely expressed MTs are not essential for development but that they do protect against cadmium toxicity. These mice provide a means for testing other proposed functions of MT in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Huet Y. M., Lehman L. D., Dey S. K. Metallothionein gene regulation in the preimplantation rabbit blastocyst. Development. 1987 Jul;100(3):463–469. doi: 10.1242/dev.100.3.463. [DOI] [PubMed] [Google Scholar]
- Bauman J. W., Liu J., Liu Y. P., Klaassen C. D. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S. K., McMaster M. T., Andrews G. K. Endotoxin induction of murine metallothionein gene expression. J Biol Chem. 1990 Sep 5;265(25):15267–15274. [PubMed] [Google Scholar]
- De S. K., McMaster M. T., Dey S. K., Andrews G. K. Cell-specific metallothionein gene expression in mouse decidua and placentae. Development. 1989 Nov;107(3):611–621. doi: 10.1242/dev.107.3.611. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Analysis of the detoxification of heavy metal ions by mouse metallothionein. Experientia Suppl. 1987;52:457–463. doi: 10.1007/978-3-0348-6784-9_45. [DOI] [PubMed] [Google Scholar]
- Harris E. D. Copper transport: an overview. Proc Soc Exp Biol Med. 1991 Feb;196(2):130–140. doi: 10.3181/00379727-196-43171b. [DOI] [PubMed] [Google Scholar]
- Holt D., Webb M. Intestinal and hepatic binding of cadmium in the neonatal rat. Arch Toxicol. 1983 Apr;52(4):291–301. doi: 10.1007/BF00316497. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Kojima Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987;52:25–61. doi: 10.1007/978-3-0348-6784-9_3. [DOI] [PubMed] [Google Scholar]
- Mason R. Metabolism of cadmium in the neonate: effect of hepatic zinc, copper and metallothionein concentrations on the uptake of cadmium in the rat liver. Biochem Pharmacol. 1982 May 1;31(9):1761–1764. doi: 10.1016/0006-2952(82)90681-5. [DOI] [PubMed] [Google Scholar]
- McKim J. M., Jr, Liu J., Liu Y. P., Klaassen C. D. Distribution of cadmium chloride and cadmium-metallothionein to liver parenchymal, Kupffer, and endothelial cells: their relative ability to express metallothionein. Toxicol Appl Pharmacol. 1992 Feb;112(2):324–330. doi: 10.1016/0041-008x(92)90203-5. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Michalska A. E., Choo K. H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C. V., Shaikh Z. A. The vascular endothelium as a target tissue in acute cadmium toxicity. Life Sci. 1986 Oct 20;39(16):1403–1409. doi: 10.1016/0024-3205(86)90543-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Sandgren E. P., Koeller D. M., Brinster R. L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993 Sep;13(9):5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife C., Durnam D., Mottet N. K. Cadmium hypersusceptibility in the C3H mouse liver: cell specificity and possible role of metallothionein. Toxicol Appl Pharmacol. 1984 Oct;76(1):9–17. doi: 10.1016/0041-008x(84)90024-3. [DOI] [PubMed] [Google Scholar]
- Quaife C., Hammer R. E., Mottet N. K., Palmiter R. D. Glucocorticoid regulation of metallothionein during murine development. Dev Biol. 1986 Dec;118(2):549–555. doi: 10.1016/0012-1606(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Tamai K. T., Gralla E. B., Ellerby L. M., Valentine J. S., Thiele D. J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. A., Heiniger H. J., Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc Soc Exp Biol Med. 1973 Jul;143(3):629–633. doi: 10.3181/00379727-143-37380. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Veness-Meehan K. A., Cheng E. R., Mercier C. E., Blixt S. L., Johnston C. J., Watkins R. H., Horowitz S. Cell-specific alterations in expression of hyperoxia-induced mRNAs of lung. Am J Respir Cell Mol Biol. 1991 Dec;5(6):516–521. doi: 10.1165/ajrcmb/5.6.516. [DOI] [PubMed] [Google Scholar]