Abstract
Leptomeningeal carcinomatosis occurs very rarely in patients with pancreatic cancer. Leptomeningeal carcinomatosis is characterized by multifocal seeding of the leptomeninges by malignant cells that originate from a solid tumor. To the best of our knowledge, brain metastasis from pancreatic cancer is extremely rare. Leptomeningeal carcinomatosis is estimated to occur in 3% to 8% of cases of solid tumors. The clinical manifestation usually involves neurological symptoms, including dizziness, headache, vomiting, nausea, and hemiparesis, symptoms similar to those of meningitis or brain tumors. Diagnostic methods for leptomeningeal carcinomatosis include brain magnetic resonance imaging and cerebrospinal fluid examination. Here, we describe a case of leptomeningeal carcinomatosis in which the primary tumor was later determined to be pancreatic cancer. Brain magnetic resonance imaging findings showed mild enhancement of the leptomeninges, and cerebrospinal fluid cytology was negative at first. However, after repeated spinal taps, atypical cells were observed on cerebrospinal fluid analysis and levels of tumor markers such as carbohydrate antigen 19-9 in cerebrospinal fluid were elevated. Abdominal computed tomography, performed to determine the presence of extracerebral tumors, revealed pancreatic cancer. Pancreatic cancer was confirmed histopathologically on examination of an endoscopic ultrasound-guided fine needle aspiration specimen.
Keywords: Pancreatic cancer, Radiation therapy, Tumor marker, Leptomeningeal carcinomatosis, Prognosis
Core tip: Leptomeningeal carcinomatosis with pancreatic cancer is a relatively rare finding. To date, only a few cases of brain metastasis originating from pancreatic cancer have been reported. Here, we report on a patient presenting with neurologic symptoms who was found to have pancreatic cancer with leptomeningeal metastasis, and we review the relevant literature.
INTRODUCTION
Pancreatic cancer is the fourth frequent cause of cancer-related deaths in the United States[1]. At the time of diagnosis, most patients have locally advanced or metastasized disease and therefore do not qualify for surgical treatment, resulting in a very poor prognosis with a 5-year survival rate of less than 5%[2]. However, central nervous system (CNS) involvement is rare in pancreatic cancer. Metastatic leptomeningeal carcinomatosis (LC) is estimated to occur in 3% to 8% of cases of solid tumors[3]. Particularly, in patients with pancreatic cancer, the development of metastasis to the CNS is rare (occurring in approximately 0.3% of cases). According to the literature, leptomeningeal metastasis from pancreatic cancer has been reported in only 12 cases.
In this report, we discuss a rare case of pancreatic cancer leading to LC.
CASE REPORT
An 80-year-old man was admitted to the neurology department of our hospital in February 2014 because of episodes of headache and seizure. The headaches began 5 wk before hospital admission and increased in intensity and frequency. Seizure episodes were observed 5 d before admission to the hospital. The duration of each seizure was approximately 10 s, and these were accompanied by upward deviation of the eyeballs. The seizures were generalized tonic-clonic seizures, and the patient was responsive after 3 to 4 min. He had a 40-year history of smoking one pack of cigarettes per a day and no specific family history of other diseases.
The patient had no other complaints except for recent weight loss of 5 kg over 4 mo. On physical examination, he exhibited no focal neurological signs such as abnormal reflexes, sensory deficit, nystagmus, or neck stiffness. His vital signs were as follows: blood pressure, 110/70 mmHg; pulse rate, 72 beats/min; and respiratory rate, 20 breaths/min. Breathing sounds were clear, and heart sounds were regular and without murmur. Findings of the laboratory workup on admission were normal. Magnetic resonance imaging (MRI) of the brain (Figure 1) revealed mild leptomeningeal enhancement in the cerebellar folia and bilateral temporoparietal meninges and solid enhancement in the pineal gland. Laboratory analysis showed relatively normal cerebrospinal fluid (CSF), 4 white blood cells per μL, slightly elevated protein level of 65.3 mg/dL (normal range: 10-45 mg/dL), a slightly decreased glucose level of 31 mg/dL (normal range: 50-75 mg/dL), an adenosine deaminase level of 3.8 IU/L, and negative results on tuberculosis (Tb) polymerase chain reaction and acid-fast bacilli staining. CSF cytology showed no atypical cells. The opening pressure was 12.5 cmH2O (normal range: 8-15 cmH2O). Although the CSF analysis results indicated no specific abnormality, Tb treatment was started as a preventative measure for Tb meningitis, which was prevalent in South Korea. On the basis of these results, the possibility of a pineal gland tumor with leptomeningeal metastasis or an extracerebral tumor with LC was also explored. However, the brain tumor with pineal gland involvement which seeding to meninges is very rare and definitive diagnosis was not made before autopsy. Accordingly, computed tomography (CT) and serum tumor marker measurement were performed to determine the presence of extracerebral tumors. Contrast CT of the chest and abdomen/pelvis showed an infiltrating mass in the body of the pancreas encasing the celiac trunk, with lymph node enlargement in the left paraaortic area, indicative of liver metastases (Figure 2). The serum carbohydrate antigen (CA) 19-9 level was elevated at 8310 IU/mL (normal range, 0-37 IU/mL). Endoscopic ultrasonography showed an ill-defined lesion in the body of the pancreas; the presence of adenocarcinoma cells was confirmed by histopathological examination of a fine-needle aspiration specimen.
Figure 1.
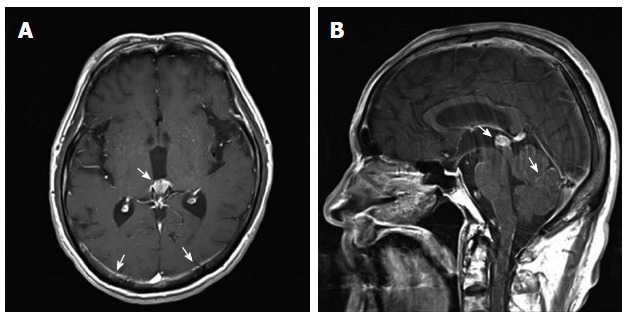
Brain magnetic resonance imaging findings. A: Pineal gland and parietal leptomeningeal enhancement on a T1 axial image (arrows); B: Pineal gland and cerebellar folia enhancement on a T1 sagittal image (arrows).
Figure 2.
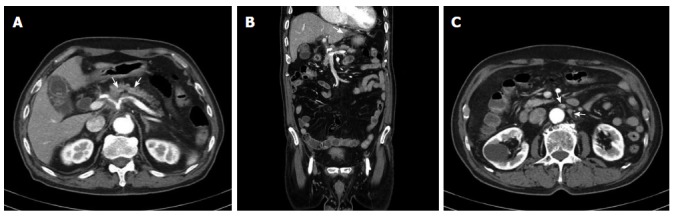
Computed tomography scans of the abdomen and pelvis. A: An infiltrating pancreatic neoplasm involving the body encasing the celiac axis (arrows); B: Low-density lesion at the left lateral segment of the liver (arrow); C: Lymph node enlargement in the left paraaortic area (arrows).
A repeat examination of CSF was performed, including tumor marker measurement. Elevated levels of CA 19-9 (10000 IU/mL; normal range, 0-32 IU/mL) and carcinoembryonic antigen (CEA) (23.2 ng/mL; normal range, 0-5 mg/mL) were detected. At the third spinal tap for CSF, atypical cells were present, which were not detected previously. The pineal gland tumor which metastasis to meninges is mostly germ cell tumor even its prevalence is rare, and usually show elevation of α-Fetoprotein or β-chorionic gonadotropin in CSF. Given the markedly elevated CA 19-9 level in serum and CSF and the clinical and radiologic findings (brain MRI and abdominal CT), a diagnosis of metastatic pancreatic cancer, rather than leptomeningeal seeding from the pineal gland tumor or meningitis, was made.
The patient’s Eastern Cooperative Oncology Group performance status was 3, and he was capable of limited self-care, being confined to a bed or chair for more than 50% of the time for which he was awake. He wished to continue treatment for as long as tolerable. Whole brain radiation with a palliative intent was initiated. Intrathecal chemotherapy was deferred because of the patient’s poor performance status. After four rounds of radiation therapy, the patient was transferred to another, affiliated hospital near house for his wish. Therefore, the patient could not be followed-up thereafter.
DISCUSSION
Pancreatic cancer is a common malignancy and often presents at an advanced stage. Despite advances in early diagnosis and therapy, the survival rate has not changed during recent years. This is because early diagnosis is difficult owing to nonspecific symptoms or the absence of symptoms and because the etiology of pancreatic cancer is unknown.
LC, wherein tumor cells metastasize to the leptomeninges and CSF, is a particularly virulent syndrome with extremely high morbidity and mortality[4-7].
Patients with LC can present with symptoms similar to those of infectious meningitis, such as headache, neck stiffness, nausea, and vomiting[8].
The diagnosis of LC is difficult in most patients because this requires the detection of malignant cells on CSF cytology. As seen in this case, malignant cells are rarely found initially. They are detected in the initial CSF sample in only 50% of patients with LC. Repeat CSF analysis has been found to improve the yield to 90%[9]. At the time of lumbar puncture, an elevated opening pressure of the CSF, an elevated protein level, and a decreased glucose level could aid in the diagnosis[10]. Measurement of levels of specific tumor markers in the CSF, in particular, CEA, CA 125, CA 19-9, and CYFRA 21-1, may also help in early diagnosis of LC[11]. According to the literature, CYFRA 21-1 and neuron-specific enolase levels are usually elevated in CSF in cases of lung cancer and the CEA level is elevated in cases of solid adenocarcinomas, mainly gastrointestinal tumors. These studies suggest that tumor marker levels be considered diagnostic for LC when levels in CSF are greater than those in serum. Because tumor markers are produced directly by tumors or by non-tumor cells in response to the presence of a tumor, elevated tumor marker levels can be detected earlier than radiological radiographic abnormalities[12]. Levels of CSF tumor markers such as CA 19-9 in particular have predictive value for breast cancer and LC[13,14]. However, diagnostic data relating to CSF tumor marker levels are not available for pancreatic cancer with LC, because of the rare occurrence of CNS metastasis from pancreatic cancer. In our case of pancreatic cancer with LC, we found markedly elevated levels of CA 19-9 (10000 IU/mL), and we would like to emphasize that this finding is unique. Further study of CA 19-9 levels in CSF would be meaningful on accumulation of more cases of pancreatic cancer with LC.
Brain MRI with gadolinium-enhanced, T1-weighted images can support the diagnosis in patients with negative cytology findings[15]. On MRI, LC shows thin, diffuse leptomeningeal contrast enhancement and multiple nodular deposits in the subarachnoid space, cerebellar folia, or cortical surface, and can show mass lesions, as in the case presented here.
The treatment goal for LC is to improve or stabilize the patient’s neurologic status and to prolong survival. According to the National Comprehensive Cancer Network, treatment decisions should be based on the patients’ risk group stratification. Patients in the poor risk group (Karnofsky Performance Status score less than 60, multiple serious major neurologic deficits, bulky CNS disease, and encephalopathy) are usually offered supportive care and palliative radiation therapy is considered. Patients in the good risk group (Karnofsky Performance Status score more than 60, no major neurologic deficits, minimal systemic disease) receive radiation therapy and chemotherapy.
To our knowledge, this is the first case of LC from pancreatic cancer in South Korea and the detection of an elevated CA 19-9 level in the CSF in such a patient. When neurological symptoms and signs are present, clinicians should consider the possibility of LC from a solid tumor and perform investigations to exclude neurological involvement.
COMMENTS
Case characteristics
An 80-year-old male presenting with neurologic symptoms who was found to have pancreatic cancer with leptomeningeal metastasis.
Clinical diagnosis
Seizure episodes were observed 5 d before admission.
Differential diagnosis
An extracerebral tumor with leptomeningeal metastasis, Tb meningitis, pineal gland tumor with leptomeningeal metastasis.
Laboratory diagnosis
WBC 10.0 k/uL; HGB 13 g/dL; CA 19-9 (serum) 8310 IU /mL; CA 19-9 (CSF) 10000 IU /mL; CEA (CSF) 23.2 ng/mL; metabolic panel and liver function test were within normal limits.
Imaging diagnosis
Brain magnetic resonance imaging revealed mild leptomeningeal enhancement and abdominal computed tomography showed an infiltrating mass in the body of the pancreas encasing the celiac trunk.
Pathological diagnosis
Histopathological examination of transesophageal ultrasound-guided fine needle aspiration revealed adenocarcinoma on pancreas and at the third spinal tap for cerebrospinal fluid (CSF), atypical cells were present.
Treatment
The patient was treated with whole brain radiation.
Related reports
According to the literature, leptomeningeal metastasis from pancreatic cancer has been reported in only 12 cases.
Term explanation
Leptomeningeal carcinomatosis is characterized by multifocal seeding of the leptomeninges by malignant cells that originate from a solid tumor.
Experiences and lessons
When neurological symptoms and signs are present in patient, clinicians should consider the possibility of leptomeningeal carcinomatosis from a solid tumor and perform investigations to exclude neurological involvement.
Peer review
This article applies repeated CSF taping and checking elevated level of CA 19-9 could help to diagnosis the rare case of pancreatic cancer with leptomeningeal carcinomatosis.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 29, 2014
First decision: June 27, 2014
Article in press: September 5, 2014
P- Reviewer: Fölsch UR, Kapan S S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Lee JL, Kang YK, Kim TW, Chang HM, Lee GW, Ryu MH, Kim E, Oh SJ, Lee JH, Kim SB, et al. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol. 2004;66:167–174. doi: 10.1023/b:neon.0000013462.43156.f4. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain MC. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: a retrospective case series. J Neurooncol. 2012;109:143–148. doi: 10.1007/s11060-012-0880-x. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC, Sandy AD, Press GA. Leptomeningeal metastasis: a comparison of gadolinium-enhanced MR and contrast-enhanced CT of the brain. Neurology. 1990;40:435–438. doi: 10.1212/wnl.40.3_part_1.435. [DOI] [PubMed] [Google Scholar]
- 6.Groves MD, Glantz MJ, Chamberlain MC, Baumgartner KE, Conrad CA, Hsu S, Wefel JS, Gilbert MR, Ictech S, Hunter KU, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10:208–215. doi: 10.1215/15228517-2007-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwińska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol. 2013;30:408. doi: 10.1007/s12032-012-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15 Suppl 4:iv285–iv291. doi: 10.1093/annonc/mdh941. [DOI] [PubMed] [Google Scholar]
- 9.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003;21:25–66. doi: 10.1016/s0733-8619(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 10.Leal T, Chang JE, Mehta M, Robins HI. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Curr Cancer Ther Rev. 2011;7:319–327. doi: 10.2174/157339411797642597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Piao Y, Zhang X, Li W, Hao X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark. 2013;13:123–130. doi: 10.3233/CBM-130338. [DOI] [PubMed] [Google Scholar]
- 12.Shi Q, Pu CQ, Wu WP, Huang XS, Yu SY, Tian CL, Huang DH, Zhang JT. [Value of tumor markers in the cerebrospinal fluid in the diagnosis of meningeal carcinomatosis] Nanfang Yi ke Daxue Xuebao. 2010;30:1192–1194. [PubMed] [Google Scholar]
- 13.Kosmas C, Tsavaris NB, Tsakonas G, Soukouli G, Gassiamis A, Mylonakis N, Karabelis A. Cerebrospinal fluid tumor marker levels in predicting response to treatment and survival of carcinomatous meningitis in patients with advanced breast cancer. Med Sci Monit. 2005;11:CR398–CR401. [PubMed] [Google Scholar]
- 14.Tajima Y, Horiguchi K, Nakano S, Hirono S, Higuchi Y, Oide T, Iwadate Y, Saeki N. [Leptomeningeal carcinomatosis following 27 years remission from breast cancer with epidermoid: a case report] No Shinkei Geka. 2012;40:343–349. [PubMed] [Google Scholar]
- 15.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


