Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) is the preferred procedure for biliary and pancreatic drainage. While ERCP is successful in about 95% of cases, a small subset of cases are unsuccessful due to altered anatomy, peri-ampullary pathology, or malignant obstruction. Endoscopic ultrasound-guided drainage is a promising technique for biliary, pancreatic and recently gallbladder decompression, which provides multiple advantages over percutaneous or surgical biliary drainage. Multiple retrospective and some prospective studies have shown endoscopic ultrasound-guided drainage to be safe and effective. Based on the currently reported literature, regardless of the approach, the cumulative success rate is 84%-93% with an overall complication rate of 16%-35%. endoscopic ultrasound-guided drainage seems a viable therapeutic modality for failed conventional drainage when performed by highly skilled advanced endoscopists at tertiary centers with expertise in both echo-endoscopy and therapeutic endoscopy
Keywords: Endoscopic ultrasound, Endoscopic ultrasound-guided biliary drainage, Consortium, Biliary drainage, Pancreatic drainage, Endoscopic ultrasound-guided
Core tip: This summary of the endoscopic ultrasound (EUS)-guided biliary drainage consortium held in 2012 focuses on technical improvements in both EUS-Guided biliary and pancreatic drainage techniques. This summary also provides a detailed overview of EUS-guided choledochoduodenostomy compared to percutaneous transhepatic cholangiography and surgical drainage. Other EUS Guided techniques such as endoscopic ultrasound-guided pancreatico-gastrostomy and endoscopic ultrasonography-guided cholecystoduodenostomy and cholecystogastrostomy have been discussed. Lastly, an extensive review of therapeutic endoscopic interventions in surgically altered anatomy has been provided as well.
INTRODUCTION
Endoscopic ultrasound (EUS) has evolved from a simple diagnostic procedure in the last two decades. This is in part due to the landmark study published by Wiersema[1], whom first used the EUS to guide a cholangiography to define the anatomy of the biliary tree. This shifting paradigm paper paved the ground for the subsequent evolution of the EUS as a powerful therapeutic tool. Giovannini et al[2] was the first article describing the use of EUS guiding biliary drainage, follow by Kahaleh et al[3] characterizing the “rendezvous” techniques.
Endoscopic transpapillary biliary drainage is the procedure of choice for biliary decompression in patients with unresectable pancreatic cancer associated to obstructive jaundice[3-6]. However, endoscopic retrograde cholangio-pancreatography (ERCP) failure can occur in 3%-10% of cases[3-5]. This failure can be due to operator inexperience, anatomic variation, tumor extension, prior surgery or incomplete drainage[3-9].
The indications for EUS-Guided biliary drainage include (1) failed conventional ERCP; (2) altered anatomy; (3) tumor preventing access into the biliary tree; and (4) contraindication to percutaneous access (i.e., ascites, etc.). The Consensus guidelines for the management of biliary obstruction using EUS guided biliary drainage were reviewed and updated to reflect practice patterns, techniques and new indications among the field.
TECHNIQUES APPROACHES IN EUS-GUIDED BILIARY DRAINAGE
Access
Biliary drainage guided by EUS can be performed at two locations, depending on the level of access to the biliary system.
Intrahepatic: The intrahepatic biliary system can be reached either transesophageal, transgastric or transjejunal (in altered anatomy), being the biliary segment III of the left hepatic lobe the most frequent and best visualized duct, especially when the EUS probe is placed at the stomach cardia and lesser curvature[9-11].
Extrahepatic: This technique can be performed having the needle accessing the common bile duct (CBD) directly, either using the transmural access from the antral part of the stomach or duodenum. In one hand, this approach provide not only a better visualization of the CBD, that some endoscopist considered advantageous at the time to select the route of access to the biliary system, but also due to the anatomical position of the CBD (located in the retroperitoneal space) might be safer in patients with ascites[10]. On the other hand, the major limitation for this technique is the difficulty inherent in the anterograde placement of the stent because of the angle of the needle entering the biliary duct.
Drainage
Rendezvous: Modality that allows advancing a guided wire through the papilla in antegrade fashion in which the bile duct is located and cannulated using the EUS rather than by retrograde cannulation with a duodenoscope. Technique especially used in those cases where biliary drainage is needed, ERCP failed and the duodenal anatomy allows the placement of the scope at the ampulla and the wire is identified traversing the papilla. Therefore, duodenal anatomy will determine the feasibility of the procedure, and that represent the main limitation[9].
Antegrade: In those cases where the luminal obstruction can not be overcome and the papilla is not visualized; but, the transpapillary wire access was obtained with EUS-guidance, then the alternative for biliary drainage is the antegrade placement of a biliary stent across the obstruction.
Transmural: Biliary drainage is obtained by creating a fistula in those cases where the wire cannot be positioned across the papilla due to either anomaly in the anatomy (biliary obstruction by a tumor) or technical complication (difficult position)[9].
ENDOSCOPIC ULTRASOUND-GUIDED BILIARY DRAINAGE
There is no consensus among the experts in the field of advance endoscopy regarding when to use EUS-BD, however, most of them can agree that failure in the use of conventional ERCP might be used as main explanation to justify the procedure, however, the indications for EUS-BD are not being established yet[12]. Therefore, EUS-BD has been rapidly being accepted as an alternative and reasonable option in those cases where bile duct cannulation cannot be achieved[11]. Either surgically altered anatomy (bariatric surgery or intestinal diversion for pancreatic cancer or other diseases) or obstruction of the gastrointestinal (GI) tract or bile duct (must often due to malignant causes) can be considered the principal causes for unsuccessful ERCP[13,14].
Most recent data reports that the accumulative success rate for extrahepatic EUS-BD (Table 1) is approximately 93% over the last 12 years[3,4,6,13-56], and in case of intrahepatic EUS-BD (Table 2) the cumulative success rate published is 84%[3,6,13,14,34,36,40,43,47,50-52,57]. However, data from two large multicenter retrospective trials failed to report advantages of any of these techniques[58,59].
Table 1.
Studies on extrahepatic endoscopic ultrasound-guided biliary drainage
| Ref. | No./total sample | Method | Disease | Approach | Initial stent | Percent success rate | Complications |
| Giovannini et al[27] (2001) | 1/1 | Direct (1) | Malignant (1) | Duodenum | PS (1) | 100 | None |
| Burmester et al[6] (2003) | 3/4 | Direct (4) | Malignant (4) | Duodenum (2), stomach (1), jejunum (1) | PS (3) | 75 | Bile leak (1) |
| Mallery et al[28] (2004) | 2/2 | Rendez-vous (2) | Malignant (2) | Duodenum (2) | MS (2) | 100 | None |
| Lai and Freeman[29] (2005) | 1/1 | Rendez-vous (1) | Malignant (1) | Duodenum (1) | MS (1) | 100 | None |
| Püspök et al[13] (2005) | 5/5 | Direct (5) | Malignant (4), benign (1) | Duodenum (5) | PS (5) | 80 | Subacute phlegmonous cholecystitis (1) |
| Kahaleh et al[3] (2006) | 10/23 | Direct (2), rendez-vous (7) | Malignant (8), benign (2) | Duodenum (5), jejunum (5) | PS (4), MS (5) | 90 | Bile leak (1), pneumoperitoneum (2) |
| Yamao et al[20] (2006) | 2/2 | Direct (2) | Malignant (2) | Duodenum (2) | PS (2) | 100 | None |
| Ang et al[4] (2007) | 2/2 | Direct (2) | Malignant (2) | Duodenum (2) | PS (2) | 100 | None |
| Fujita et al[30] (2007) | 1/1 | Direct (1) | Malignant (1) | Duodenum (1) | PS (1) | 100 | None |
| Will et al[31] (2007) | 8/8 | Direct (8) | Malignant (7), benign (1) | Stomach (4), jejunum (3), esophagus (1) | PS (2), MS (5) | 88 | Slight pain (2), cholangitis (1) |
| Yamao et al[21] (2008) | 3/3 | Direct (3) | Malignant (3) | Duodenum (3) | PS (3) | 100 | Pneumoperitoneum (1) |
| Tarantino et al[19] (2008) | 8/8 | Direct (4), Rendez-vous (4) | Malignant (7), benign (1) | Duodenum (8) | PS (8) | 100 | None |
| Itoi et al[32] (2008) | 4/4 | direct (4) | Malignant (4) | Duodenum (4) | PS (3), NBD (1) | 100 | Focal peritonitis (1), bleeding (1) |
| Brauer et al[33] (2009) | 12/12 | Direct (4), Rendez-vous (7) | Malignant (8), benign (4) | NA | PS (5), SEMS (5) | 92 | Pneumoperitoneum (1), respiratory failure (1) |
| Horaguchi et al[14] (2009) | 9/16 | NA | Malignant (9) | Duodenum (8), stomach (1) | PS (14), plastic PT (1), NBT (1) | 100 | Peritonitis (1) |
| Hanada et al[17] (2009) | 4/4 | Direct (4) | Malignant (4) | Duodenum (4) | PS (4) | 100 | None |
| Maranki et al[34] (2009) | 14/49 | Direct (6), Rendez-vous (8) | Malignant (9), benign (5) | NA | NA | 86 | Biliary peritonitis (1), abdominal pain (1), pneumoperitoneum (1) |
| Kim et al[35] (2010) | 15/15 | Rendez-vous (15) | Malignant (10), benign (5) | Duodenum (15) | PS (4), MS (8) | 80 | Pancreatitis (1) |
| Nguyen-Tang et al[36] (2010) | 1/5 | Rendez-vous (1) | Malignant (1) | NA | MS (1) | 100 | None |
| Iwamuro et al[37] (2010) | 7/7 | Direct (7) | Malignant (7) | Duodenum (5), stomach (2) | PS (7) | 100 | Bile peritonitis (2) |
| Artifon et al[38] (2010) | 3/3 | Direct (3) | Malignant (3) | Duodenum (3) | MS (3) | 100 | None |
| Belletrutti et al[39] (2010) | 1/1 | Direct (1) | Malignant (1) | Duodenum (1) | MS (1) | 100 | None |
| Park do et al[40] (2011) | 31/57 | Direct (31) | Malignant (51), benign (6) | Duodenum (31) | PS (6), MS (25) | 87 | Pneumoperitoneum (6), mild bleeding (2) |
| Fabbri et al[41] (2011) | 16/16 | Direct (13), Rendez-vous (3) | Malignant (16) | Duodenum (15), stomach (1) | PS (4), MS (8) | 80 | Pancreatitis (1) |
| Hara et al[42] (2011) | 18/18 | Direct (18) | Malignant (18 | NA | PS (17) | 94 | Peritonitis (2), bleeding (1) |
| Ramírez-Luna et al[43] (2011) | 9/11 | Direct (9) | Malignant (9) | Duodenum (9) | plastic DPT (9) | 89 | Biloma (1) |
| Siddiqui et al[44] (2011) | 8/8 | Direct (8) | Malignant (8) | Duodenum (80 | MS (8) | 100 | Stent migration (1), duodenal perforation (1) |
| Komaki et al[45] (2011) | 15/15 | Direct (14), Rendez-vous (1) | Malignant (15) | Duodenum (15) | PS (15) | 100 | None |
| Prachayakul et al[46] (2011) | 1/1 | Direct (1) | Malignant (1) | Duodenum (1) | PS (1) | 100 | None |
| Artifon et al[22] (2012) | 13/13 | Direct (13) | Malignant (13) | Duodenum (13) | MS (13) | 100 | Bile leak (1), bleeding (1) |
| Attasaranya et al[47] (2012) | 10/31 | Direct (9), Antegrade (1) | Malignant (23), benign (8) | Duodenum | NA | 60 | 4/10 (40%) |
| Katanuma et al[48] (2012) | 1/1 | Direct (1) | Benign (1) | Duodenum (1) | PS (1) | 100 | None |
| Kawakubo et al[49] (2012) | 2/2 | Direct (2) | Malignant (2) | Duodenum (2) | PS (2) | 100 | None |
| Khashab et al[50] (2012) | 7/9 | Direct (2), antegrade (2), Rendez-vous (3) | Malignant (7) | Duodenum (6), gastric (1) | MS (7) | 100 | Pancreatitis (1), cholecystitis (1), abdominal pain (1) |
| Kim et al[51] (2012) | 9/13 | Direct (9) | Malignant (9) | Duodenum (9) | MS (9) | 100 | Pneumoperitoneum (2), migration (2), peritonitis (1) |
| Song et al[52] (2012) | 15/15 | Direct (15) | Malignant (15) | Duodenum (15) | MS (15) | 100 | Pneumoperitoneum (2), cholangitis (1) |
| Dhir et al[53] (2012) | 58/58 | Rendez-vous (58) | Malignant (43), benign (15) | Duodenum (58) | NA | 98 | Bile leak (2) |
| Hara et al[54] (2013) | 18/18 | Direct (18) | Malignant (18) | Duodenum (18) | MS (18) | 94 | Bile peritonitis (2) |
| Park do et al [55] (2013) | 16/45 | Direct (2), Rendez-Vous (14) | Malignant (39), benign (6) | Duodenum (16) | MS (16) | 88 | Pancreatitis (1), bile peritonitis (1) |
| Itoi et al [56] (2013) | 1/1 | Direct (1) | Malignant (1) | Stomach (1) | MS (1) | 100 | None |
| Total | 365 | 338/365 (93%) | 58/365 (16%) |
Table 2.
Studies on intrahepatic endoscopic ultrasound-guided biliary drainage
| Ref. | No./total sample | Method | Disease | Approach | Initial stent | Percent success rate | Complications |
| Burmester et al[6] (2003) | 1/4 | Direct (1) | Malignant (1) | Stomach (1) | PS (1) | 100 | Bile leak (1) |
| Püspök et al[13] (2005) | 1/1 | Direct (1) | Malignant (1) | jejunum (1) | PS (1), MS (1) | 100 | None |
| Kahaleh et al[3] (2006) | 13/23 | Direct (1), Rendez-vous (12) | Malignant (9), benign (4) | Stomach (13) | PS (6), MS (6) | 92 | Minor bleeding (1) |
| Bories et al[16] (2007) | 11/11 | Direct (9), Antegrade (2) | Malignant (3), benign (8) | Stomach (3), Duodenum (3), stenosis (5) | PS (7), MS (3) | 91 | Transient ileus (1), biloma (1), cholangitis (1) |
| Horaguchi et al[14] (2009) | 7/16 | NA | Malignant (7) | Stomach (5), esophagus (2) | PS (2), MS (5) | 100 | None |
| Maranki et al[34] (2009) | 35/49 | Direct (9), Antegrade (24) | Malignant (26), benign (9) | NA | NA | 83 | Bleeding (1), pneumoperitoneum (3), aspiration pneumonia (1) |
| Nguyen-Tang et al[36] (2010) | 4/5 | Rendez-vous (4) | Malignant (3), benign (1) | Duodenum (1), Stomach (3) | MS (5) | 100 | None |
| Park do et al[40] (2011) | 31/57 | Direct (31) | Malignant (51), benign (6) | Duodenum (31) | PS (6) MS (25) | 87 | Pneumoperitoneum (1), bile peritonitis (2) |
| Ramírez-Luna et al[43] (2011) | 2/11 | Direct (2) | Malignant (2) | Stomach (2) | PS (2) | 100 | Stent migration (1) |
| Attasaranya et al[47] (2012) | 16/31 | Direct (16) | Malignant (23), benign (8) | NA | NA | 81 | 6/16 (38%) |
| Khashab et al[50] (2012) | 2/9 | Antegrade (1), rendez-vous (1) | Malignant (2) | Stomach | MS (2) | 100 | Nausea (1) |
| Kim et al[51] (2012) | 4/13 | Direct (4) | Malignant (4) | Stomach (4) | MS (3) | 75 | Peritonitis (1), stent migration (1) |
| Park do et al[55] (2013) | 29/45 | Direct (9), antegrade (14), rendez-vous (5) | Malignant (39), benign (6) | Stomach (29) | NA | 66 | Biloma (1) |
| Iwashita et al[57] (2013) | 6/6 | Direct (1) | Malignant (1), benign (5) | NA | MS (1) | 100 | Pancreatitis (1), abdominal pain (1) |
| Total | 162 | 136/162 (84%) | 26/162 (16%) |
EUS-guided rendezvous
This procedure is performed inserting a needle under EUS and doppler guidance into either left intrahepatic duct or common bile duct. Here, the FNA needle will permit also the advance of guidewire distally into the duodenum. Upon insertion of the needle into the duct, suspected by EUS imaging, the aspiration of the bile will confirm the intraductal placement of the needle, which is follow by injection of contrast drawing the biliary tree (cholangiogram). Next, the insertion of the guidewire through the FNA needle toward the duodenum and perform the conversion to a conventional ERCP in a retograde fashion. If by any reason the transpapillary passage is not successfully accomplish, then the FNA needle need to be replaced by the sphincterotome or dilating bougie, allowing the manipulation of the wire freely and safely to facilitate the passage through the ampulla. At this point, the EUS scope is removed leaving the wire in the duodenum and the duodenoscope is advanced to the ampulla where the guidewire can be grasped using a snare or forceps and pulled it back through the working channel of the duodenoscope and permit the subsequent over-the-wire cannulation or alternately use the duodenoscope to cannulate the common bile duct next to the prior placed guidewire. Now the procedure can be completed by conventional endoscopic retrograde cholangiography with stent placement in retrograde manner[11].
EUS-guided choledochoduodenostomy vs other techniques (percutaneous transhepatic cholangiography and surgical drainage)
Burmester et al[6] described a one-step method using a new device consisted of a 19 gauge fistulotome with a 0.25-inch guidewire, a pusher tube and an 8.5F plastic stent fixed with a 3.0 nylon-suture. This method of direct puncture of the extrahepatic or an intrahepatic duct could reduce the risk of guidewire dislocation during the instrument change, what must be made with the two-step method[17]. However, more studies with this device are needed.
EUS-guided biliary drainage has many advantages over PTBD[3,4,16,17]. The proximity of the transducer to the bile duct during EUS is the major advantage[3,19]. Even in patients who have undergone total gastrectomy or partial gastrectomy with a Billroth II reconstruction, EUS can reveal the etiology of extrahepatic cholestasis, situations in that ERCP may not be possible[1,3,4,15,16]. Other advantages include puncture of the biliary tree with color-Doppler information to avoid vascular injury, the lack of ascites in the interventional field and the lack of an external tube, improving the quality of life of the patients[4,20].
Choledochoduodenostomy can prevent clogging and tumor ingrowth and/or overgrowth, because it creates a fistula far from the obstructing tumor[20,21]. Many studies described this procedure with high success rates (more than 90%) and low rate of procedure-related complications (around 19%)[18]. The main risk of EUS-guided biliary drainage is bile leakage, especially if stent insertion is unsuccessful[4]. Burmester et al[6] reported the failure of stent placement in 1 of their 4 patients, causing bile peritonitis[17]. They also reported that only local peritonitis developed, which did not contribute to the death of the patient. Some investigators recommend the transhepatic approach to decrease the risk of biliary peritonitis in case of stent failure[17]. Other complications include pneumoperitoneum and minor bleeding[4,5,1].
EUS-guided choledochoduodenostomy for malignant biliary obstruction has been shown to be an effective alternative to PTBD or surgery when ERCP fails. Artifon et al[22] compared EUSCD and PTC in 25 patients with distal biliary malignant obstruction. The 2 groups were similar before intervention in terms of quality of life [EUS-CD (58.3) vs PTBD (57.8), P = 0.78], total bilirubin (16.4 vs 17.2, P = 0.7), alkaline phosphatase (539 vs 518, P = 0.7), and gamma-glutamyl transferase (554.3 vs 743.5, P = 0.56). All procedures were technically and clinically successful in both groups. The study concluded that EUS-CD can be an effective and safe alternative to PTBD with similar success, complication rate, cost, and quality of life.
Another Study conducted by Artifon et al[23] showed results on comparative trial between EUS CD and surgery to patients with biliary distal cancer. There was no significant difference in the technical and clinical outcomes in the two groups. Cost analysis demonstrated a significantly increased cost per patient in the surgical group (P = 0.039). Complications were significantly higher in the surgical group (P = 0.041). There was one case of self-limited bleeding on group EUSCD and one case each of wound abscess, abdominal abscess, internal fistula and pneumonia on group Surgery. All patients in surgical group were managed conservatively.
ENDOSCOPIC ULTRASOUND-GUIDED PANCREATICO-GASTROSTOMY
Main indications are stenosis of pancreatico-jejunal or pancreatico-gastric anstomosis after Whipple resection, which induce recurrent acute pancreatitis, main pancreatic duct stenosis due to chronic pancreatitis, post-acute pancreatitis or post pancreatic trauma after failure of ERCP. EUS guided pancreatico-gastro or bulbostomy offers an alternative to surgery (Figure 1).
Figure 1.
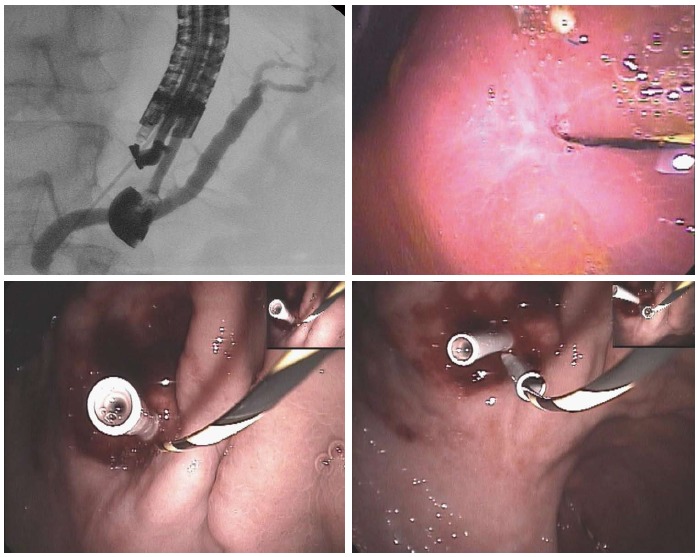
Pancreatico-gastrostomy/stenosis of a wirsung-gastro anastomosis after whipple resection for benign cystic lesion of the head of the pancreas.
By using a linear interventional echoendoscope, the dilated main pancreatic duct (MPD) was well visualized. Endoscopic pancreatico-gastrostomy (EPG) was then performed under combined fluoroscopic and ultrasound guidance, with the tip of the echoendoscope positioned such that the inflated balloon was in the duodenal bulb while the accessory channel remained in the antrum. A needle was inserted transgastrically into the proximal pancreatic duct and contrast medium was injected. Opacification demonstrated a pancreaticogram the needle was exchanged over a guidewire, which was then used to enlarge the channel between the stomach and MPD. The sheath was introduced by using cutting current. After exchange over a guidewire (rigid 0.035 inch diameter), a 7F, 8-cm-long pancreaticogastric stent was positioned. This stent will be exchanged for two 7F or one 8.5F stents one month after the first procedure.
The results of the fours series[3,24-26] of patients published are much too preliminary in nature to recommend wider use of EPG, which in any case should be restricted to tertiary centers specializing in biliopancreatic therapy with a pain relief in 70% of cases. But the complication rate is still high around 15% including bleeding, pancreatic collection and perforation. The largest series was published by Tessier et al[24] on 36 patients. Indications were chronic pancreatitis, with complete obstruction (secondary to a tight stenosis, a stone, or MPD rupture); inaccessible papilla or impossible cannulation (n = 20); anastomotic stenosis after a Whipple procedure (n = 12); complete MPD rupture after acute pancreatitis (AP); or trauma (n = 4). EPG or EPB was unsuccessful in 3 patients; 1 was lost to follow-up. Major complications occurred in 2 patients and included 1 hematoma and 1 severe AP. The median follow-up was 14.5 mo (range: 4-55 mo). Pain relief was complete or partial in 25 patients (69%, intention to treat). Eight patients treated had no improvement of their symptoms (4 were subsequently diagnosed with cancer). Stent dysfunction occurred in 20 patients (55%) and required a total of 29 repeat endoscopies. The last study published by Ergun et al[26] reported the long-term follow-up (37 mo) of 18 patients who underwent a EUS guided pancreatico-gastrostomy. Stent occlusion occurred in 50%, pain relief was always present in 70% and the mean pain score decreased dramatically from 7.5 to 1.6.
It’s very difficult to find today the place of EGD in our experience the best indication is anastomotic stenosis after Whipple procedure for benign pancreatic lesions (cystadenoma, IPMN, NET). EGD offers an alternative to surgery and the best results in the 3 series published (Table 3) were showed in this indication. In another hand, surgery should be considered as the elective treatment of CP after failure of the endoscopic route.
Table 3.
Studies on endoscopic ultrasound-guided pancreatico-gastrostomy
ENDOSCOPIC ULTRASONOGRAPHY-GUIDED CHOLECYSTODUODENOSTOMY AND CHOLECYSTOGASTROSTOMY
Revised Tokyo Guideline (TG13) proposes that early or emergency cholecystectomy should be conducted as the gold standard of treatment for acute cholecystitis[60-63]. In general, cholecystectomy is relatively safe. However, the mortality rate of cholecystectomy in patients at high risk due to comorbid conditions is not lower than in non-critical ill patients[64,65]. Therefore, several literatures advocates that high-risk patients should be tentatively treated by decompress the gallbladder, e.g., percutaneous transhepatic gallbladder drainage (PTGBD), percutaneous transhepatic gallbladder aspiration, endoscopic transpapillary gallbladder drainage[66-69]. Recently, as a novel translumenal access, endoscopic ultrasonography-guided gallbladder drainage (EUS-GBD) has been performed.
Technique of EUS-guided gallbladder drainage
The gallbladder is visualized from the duodenal bulb or the antrum of the stomach using a curved linear array echoendoscope in a long scope position (pushing scope position)[69].
After depiction of neck and body of the gallbladder, a 19-gauge needle is inserted transduodenally or transgastrically into the gallbladder under EUS visualization. After the stylet is removed, bile is aspirated approximately 3 cc and same 3 cc contrast medium is then injected to confirm the tip of a needle is in the gallbladder. Then, a 0.025-inch or 0.035-inch guidewire was inserted through the 19-gauge needle and looped in the gallbladder. A biliary catheter for dilation up to 8-Fr or 4-mm to 6-mm papillary balloon dilator if necessary, are used for dilation of the cholecystoentero fistula. Finally, a 5-8.5-Fr pigtail type naso-gallbladder drainage catheter, double pigtail type plastic stent, or fully-covered self-expandable metal stent are inserted through the gastric or duodenal fistula into the gallbladder.
Ten reports describe the outcome of EUS-guided gallbladder drainage (Table 4)[70-81]. In total, the technical success and clinical success rate were 98.7% (74/74) and 100% (74/74), respectively. As a first puncture, a 19-gauge needle was mainly used. A 5- to 8.5-F plastic stents or naso-gallbladder catheters were used for drainage. In 2 institutions, dedicated fully-covered metal stents, which had double flanges were used.
Table 4.
Summary of published data on endoscopic ultrasound-guided gallbladder drainage
| Ref. | No. of cases | Device for puncture | Approach route | Technical success | Clinical success | Stent | Complication (No. of cases) |
| Baron et al[70] (2007) | 1 | 19 G FN | TD | 100% | 100% | 7F PS | None |
| Kwan et al[71] (2007) | 3 | 19 G FN/FT/CT | TD | 100% | 100% | 8.5F NBD | Bile leakage (1) |
| Lee et al[72] (2007) | 9 | 19 G FN | TD | 100% | 100% | 5F NBD | Pneumoperitoneum (1)1 |
| Kamata et al[74] (2009) | 1 | 19 G FN | TG | 100% | 100% | 7F PS | None |
| Takasawa et al[73] (2009) | 1 | NK | TG | 100% | 100% | 7F PS | None |
| Song et al[75] (2010) | 8 | 19 G FN +/- NK | 1TG/7TD | 100% | 100% | 7F PS | Bile Leakage (1), pneumoperitoneum (1), stent migration (1) |
| Súbtil et al[81] (2010) | 4 | CT/NK | TG | 100% | 100% | 8.5F PS | None |
| Jang et al[77] (2011) | 15 | 19 G FN/NK | 10TG/5TD | 100% | 100% | New SEMS | Pneumoperitoneum (2) |
| Jang et al[79] (2012) | 30 | 19 G FN/NK | TG/TD | 97% | 100% | 5F NBD | Pneumoperitoneum (2) |
| Itoi et al[78] (2012) | 5 | 19 G FN/1 CT | 1TG/4TD | 100% | 100% | New SEMS | Mild bleeding (1) |
| de la Serna-Higuera et al[80] (2013) | 13 | CT | 12TG/1TD | 84.6% | 100% | New SEMS | Hematochezia (1/11), pain (1/11) |
Minor bile leakage without serious bile peritonitis. G: Gauge; NK: Needle knife; FT: Fistulotome; CT: Cystotome; PS: Plastic stent; NBD: Naso-Gallbladder Drain; TD: TransDuodenal; Approach TG: TransGastric approach; Partially covered SEMS: Partially cover self expandable metallic stent.
As an adverse events, 6 of self-limited pneumoperitoneum, 2 of bile leakage, and 1 distal stent migration without bile leakage 3 wk after stent insertion. Interestingly, Jang et al[79] conducted randomized control study, PTGBD vs EUS-guided naso-gallbladder drainage (EUS-NGBD). EUS-NGBD and PTGBD showed similar technical [97% (29/30) vs 97% (28/29); 95%] and clinical [100% (29/29) vs 96% (27/28)] success rates, and similar rates of complications [7% (2/30) vs 3% (1/29)]. The median post-procedure pain score was significantly lower after EUS- NGBD than after PTGBD (1 vs 5, P < 0.001). On the basis of the results, they suggested that EUS-NGBD is comparable with PTGBD in terms of the technical feasibility and efficacy; there were no statistical differences in the safety. EUS-GBD is a good alternative for high-risk patients with acute cholecystitis who cannot undergo an emergency cholecystectomy.
EUS-guided gallbladder drainage has been developed as an alternative drainage method for acute cholecystitis. However, there are several limitations of this procedure. One of the big issues is that basically there is no adhesion and relatively distance between the GI tract including gastric and duodenal wall and the gallbladder wall. In addition, tubular biliary plastic and metal stents for biliary decompression have significant shortcomings when used for transenteric drainage. Plastic stents have the disadvantage of a small lumen diameter, which can limit drainage and may necessitate reintervention. Currently available SEMSs have a larger lumen diameter but may show inward and outward migration. Furthermore, abutment of the end of a tubular SEMS against the lumen wall may cause tissue injury and bleeding. Although serious adverse events have never been reported, we should consider the possibility of such unexpected events.
Recently, Itoi et al[78] de la Serna-Higuera et al[80] and Jang et al[79] reported a newly designed fully-covered metallic stent with anchor for EUS-guided gallbladder drainage. These dedicated devices may suggest that this procedure is becoming safer and more reliable. That causes reducing the risk of serious complications like bile leakage and stent migration.
SURGICALLY ALTERED ANATOMY
Surgery of the upper GI tract involving change in the configuration of the antro-duodenal C-loop is commonly referred to as postoperative or surgically altered anatomy (SAA). Obesity surgery, peptic ulcer disease, iatrogenic bile-duct injury and chronic pancreatitis are common benign causes of SAA. Gastric, biliary and pancreatic malignancies also lead to radical resections with curative intent or to palliative bypass procedures resulting in SAA. Interventions performed for both benign and malignant disease include Billroth II, variations on the Whipple operation, and Roux-en-Y reconstructions, either with intact papillae[82] or with bilio-enteric anastomoses[83]. Most of these interventions result in long afferent limbs of small bowel (jejunum) impairing endoscopic access to the papilla.
Indications for biliary drainage in SAA patients
More insidious biliary obstructive symptoms caused by recurrent malignancy are seen in afferent limb syndrome, a condition difficult to diagnose and challenging to treat[84]. Obesity and weight loss are associated with common bile duct stones. Gallstones are almost always present in patients undergoing cholecystectomy, which is the major cause of bile-duct injury. Chronic reflux of GI contents across widely patent bilio-enteric anastomoses causes sump syndrome and stone formation. Leakage or anastomotic strictures may develop acutely after bilio-digestive bypass surgery. Anastomotic strictures more commonly cause chronic cholestasis and/or recurrent cholangitis over the long-term, with a reported incidence up to 30% after a mean follow-up of six years[85].
Dominant ERCP strategies in SAA
Access to the papilla and ease of en-face view are nearly normal in some cases with past SAA for benign conditions, such as Billroth I for peptic ulcer or choledochoduodenostomy for common bile duct stones. Nowadays, experienced operators report similarly high success rates for duodenoscope-based ERCP in patients with Billroth II. Whipple and Roux-en-Y reconstructions, particularly Roux-en-Y gastric by-pass (RYGB) for obesity, have ultra-long afferent limbs. Special endoscopes are usually required to access the proximal afferent limb for ERCP in these patients. Balloon or spiral enteroscopes are currently used at expert centers[86]. Enteroscopy-based ERCP is nonetheless a labor-intensive procedure with limited overall success rates of 60%. Seventy-five percent of failures occur because the papilla or bilio-enteric anastomosis cannot be identified, cannulated or drained, even despite successful limb intubation[86,87]. These figures underscore the limitations to ERCP using long, narrow channel, forward viewing enteroscopes.
An alternative approach to enteroscopy-based ERCP in long limb SAA patients is percutaneous transenteric access under open[6] or laparoscopic[88] surgical assistance. Typically, laparoscopic surgeons access the distal gastric remnant of RYGB, allowing intraoperative transgastric insertion of a duodenoscope and antegrade ERCP[87-89]. Bariatric procedures involving distal gastrectomy are less readily amenable to laparoscopically-assisted ERCP, often requiring open surgery for duodenoscope passage[87]. The complex logistics of intraoperative ERCP help understand its limited dissemination. It should be noted nonetheless that higher success rates have been reported with laparoscopy-assisted transgastric ERCP compared to enteroscopy ERCP in RYGB patients, especially when Roux limbs are longer than 150 cm from the ligament of Treitz to the jejuno-jejunal anastomosis[90].
Non-standard approaches to ERCP in SAA
Prior indwelling transenteric feeding tubes may allow percutaneous ERCP through mature tracks in the occasional SAA patient. A standard duodenoscope was passed percutaneously into the jejunum after jejunostomy track dilation and ERCP with sphincterotomy was performed uneventfully in a patient with Roux-en-Y gastrectomy[91]. However, a two-step approach is usually required in RYGB. Gastrostomy to the distal gastric remnant is first performed for the purpose of providing a conduit for duodenoscope passage, so that elective ERCP can be carried out through the mature gastrostomy track 2-4 wk later. In this two-step approach, percutaneous gastrostomy to the remnant stomach can be performed by surgery[92-94], interventional radiology[95] or endoscopy[96]. In keeping with data from the intraoperative transgastric approach, ERCP success rates using this two-step percutaneous transgastric approach are significantly higher than success rates for enteroscopy-based ERCP[94]. Complications related to the gastrostomy are however not negligible[93,94]. Successful therapeutic ERCP through gastrostomy to the remnant stomach placed by interventional radiology has been reported in a single RYGB case[95]. However, radiographically guided percutaneous access to the non-distended remnant stomach entails risk and difficulty. EUS-guided puncture and distension of the remnant stomach from the proximal gastric pouch by injecting contrast and air appears to facilitate percutaneous radiographic gastrostomy[97]. Double-balloon enteroscopy retrograde access to the remnant stomach for PEG placement prior to transgastric antegrade ERCP appears a reasonable option, given the high failure rate of enteroscopy-based ERCP even after successful limb intubation. Retrograde enteroscopy PEG placement has been reported as the initially chosen approach for ERCP in RYGB[96], and it might become a convenient same-session salvage procedure in cases of failed enteroscopy ERCP despite successful limb intubation.
Antegrade endoscopic access to the gastric remnant through spontaneous gastro-gastric communications across the staple line has successfully used for ERCP in two RYGB patients. The defect was large enough to allow passage of a duodenoscope for sphincterotomy and stone removal in one case[98]. Another patient, however, required balloon dilation. A covered self-expandable esophageal stent was placed across the staple line to provide a conduit for iterative ERCP. Once final resolution of a benign biliary stricture was achieved, the transgastric covered stent was removed[99]. The concept of temporary covered metal stents serving as conduits for endoscope passage and therapeutic ERCP has also been applied to the percutaneous[96,100,101] or transluminal EUS-guided routes[102].
Afferent loop syndrome resulting in chronic biliary obstruction caused by recurrent malignancy is an increasingly frequent clinical problem. Palliation often involves permanent percutaneous drains, which adversely impact quality of life[84]. A novel EUS-based approach was used at two different institutions in three patients. The distended afferent loop was punctured from the distal antrum or duodenum, and a double plastic pigtail placed transmurally. Cholangitis or cholestasis resolved in all three case[103,104].
EUS-guided access and drainage routes in SAA
EUS-guided biliary drainage (EUSBD) is carried out by any of three possible routes, transmural, transpapillary antegrade or transpapillary retrograde (rendezvous) encompassed under a procedure hybrid between EUS and ERCP known as endosonography-guided cholangiopancreatography (ESCP)[105]. Patients with SAA represent around a fourth of cases in both current[55,58,106] and early[2,6,13] ESCP series inclusive of both intrahepatic and extrahepatic EUS-guided access (Table 5). Patients with SAA are not present however in ESCP series reporting predominantly on choledocho-duodenostomy or extrahepatic rendezvous, which represent two thirds of the total ESCP cases reported[105]. Direct drainage routes (transmural and antegrade transpapillary) are typically chosen for EUSBD in patients with SAA instead of rendezvous. The ability to endoscopically reach the papilla is a requisite for rendezvous, and in most patients with SAA the reason for ERCP failure dictating the need for ESCP is precisely failed access to the papilla. EUS rendezvous has nonetheless been attempted in patients with past Billroth II from an extrahepatic[107] or an intrahepatic[108] approach to extract CBD stones or to relieve malignant obstruction.
Table 5.
Outcomes of biliary drainage in endosonographic cholangiopancreatography series including surgically altered anatomy patients n (%)
| Ref. | n | Patients with SAA | Overall technical success |
Type of ESCP drainage |
Complications | |||
|
Transpapillary |
Transmural |
|||||||
| RV | AG | HG | CD | |||||
| Will et al[31] (2007) | 8 | 7 (87) | 7 (87) | 1 | - | 6 | - | 2 (25) |
| Bories et al[16] (2007) | 11 | 4 (36) | 10 (91) | - | - | 10 | - | 4 (36) |
| Horaguchi et al[14] (2009) | 16 | 4 (25) | 16 (100) | - | - | 8 | 8 | 2 (12) |
| Maranki et al[34] (2009) | 49 | 7 (14) | 41 (84) | 201 | 141 | 3 | 4 | 8 (16) |
| Shah et al[106] (2012) | 68 | 19 (28) | 58 (85) | 392 | 102 | 8 | 1 | 6 (9) |
| Iwashita et al[118] (2012) | 40 | 9 (40) | 29 (73) | 29 | - | - | - | 5 (13) |
| Park et al[55] (2013) | 45 | 14 (31) | 41 (95) | 16 | 9 | 14 | 2 | 5 (11) |
| Total | 237 | 64 (27) | 202 (85) | 105 | 33 | 49 | 15 | 32 (13) |
| 138 (68) | 64 (32) | |||||||
Not specified in the original report, data provided by the author;
Not specified in the original report. Data estimated from prior detailed series[48]. SAA: Surgically altered anatomy; ESCP: Endosonographic cholangiopancreatography; RV: Rendezvous; AG: Antegrade; HG: Hepatico-gastrostomy; CD: Choledochoduodenostomy.
The dominant EUS-guided access route in SAA patients is intrahepatic into the left hepatic duct branches from the cardia region: either transgastric from the proximal stomach, transesophageal from the abdominal esophagus, or transjejunal in patients with esophago-jejunostomy[31]. Even if left intrahepatic duct branches are less obvious target for EUS-guided puncture than the CBD, the rationale for it in SAA patients is strong. SAA frequently involves distal gastrectomy, as in Roux-en-Y gastrectomy for gastric cancer, or an excluded distal stomach, as in RYGB for obesity. As the CBD is typically imaged under EUS from the distal antrum or the duodenal bulb, extrahepatic EUS-guided access is usually not possible in SAA. An exception to this rule would be the occasional patient with distal biliary obstruction in whom the common hepatic duct can be imaged and accessed under EUS from the mid stomach. This frequently overlooked transgastric access site into the proximal extrahepatic bile duct may be useful in those SAA patients without dilated intrahepatics. Kahaleh et al[107] reported this option in 2 out of their first 5 cases. Another potentially useful secondary EUS-guided access route in highly selected SAA patients with intact stomach and proximal biliary obstruction but no left intrahepatic dilation, would be transduodenal from the bulb into the right hepatic duct or its branches. This recently reported access site was chosen in 6 cases with selective right intrahepatic dilation not amenable to standard left intrahepatic or extrahepatic access. Three of these 6 patients had Roux-en-Y hepaticojejunostomy. Hepaticoduodenostomy and antegrade transanastomotic intervention (stenting and balloon dilation) were used to carry out drainage[109]. EUSBD in patients with SAA usually involves transmural or antegrade transpapillary (or transanastomotic) stenting after left intrahepatic duct puncture. Exceptions to these general rules may nonetheless allow salvage of specific patient subsets, such as secondary access sites for those without left intrahepatic dilation[109] or retrograde rendezvous drainage for those requiring cross-over after failed antegrade or transmural ESCP approaches[55,106]. EUSBD has been reported to date in around a hundred patients with SAA. Only two small series focus specifically on EUSBD in SAA[57,110], patient specifics can be traced to procedural approach and outcomes in just 47 cases, 22 malignant (Table 6) and 25 benign (Table 7).
Table 6.
Patients with malignant biliary obstruction and surgically altered anatomy drained by endosonographic cholangiopancreatography
| Ref. | No. of patients1 |
Type of SAA |
Etiology |
Type of ESCP drainage2 |
Success/complex4 |
|||||||||
|
Transpapillary |
Transmural |
|||||||||||||
| RYG | RYHJ | PD | Other | Gast | Panc | Other | RV | AG | HG | Other3 | Sn | Cn | ||
| Burmester et al[6] (2003) | 2/4 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 (0) | 1 (0) | 2/2 | 0 |
| Giovannini et al[2] (2003) | 1/1 | 1 | - | - | - | 1 | - | - | - | - | 1 (1)5 | - | 1/1 | 0 |
| Püspök et al[13] (2005) | 1/6 | 1 | - | - | - | 1 | - | - | - | 1 (1) | - | 1 (0)6 | 1/1 | 0 |
| Kahaleh et al[3] (2006) | 2/23 | - | - | - | 2 | - | 1 | 1 | 2 (1) | - | - | - | 2/2 | 0 |
| Will et al[31] (2007) | 6/8 | 4 | 1 | 1 | - | 4 | - | 2 | - | - | 3 (3) | 3 (1) | 5/6 | 2 |
| Bories et al[16] (2007) | 1/11 | - | - | 1 | - | - | 1 | - | - | - | 1 (0) | - | 1/1 | 0 |
| Horaguchi et al[14] (2009) | 4/16 | - | 2 | - | 2 | 1 | 1 | 2 | - | - | 2 (2)5 | 2 (1)5 | 4/4 | 1 |
| Chopin-Laly et al[115] (2009) | 1/1 | - | - | - | 1 | - | 1 | - | - | - | 1 (1) | - | 1/1 | 0 |
| Nguyen-Tang et al[36] (2010) | 1/5 | - | 1 | - | - | - | - | 1 | - | 1 (1) | - | - | 1/1 | 0 |
| Ma et al[117] (2011) | 1/1 | - | - | 1 | - | - | - | 1 | - | - | 1 (0) | - | 1/1 | 0 |
| Henry et al[116] (2011) | 1/1 | - | - | - | 1 | - | - | 1 | - | - | - | 1 (0)7 | 1/1 | 1 |
| Iwashita et al[118] (2012) | 1/7 | 1 | - | - | - | 1 | - | - | - | 1 (1) | - | - | 1/1 | 1 |
| Total | 22 | 8 | 4 | 3 | 7 | 5 | 8 | 2 (1) | 3 (3) | 10 (7) | 7 (1) | |||
| 22 | 9 (40%) | 13 (60%) | 5 (23%) | 17 (77%) | 21 (95%) | 5 (23%) | ||||||||
Number of surgically altered anatomy (SAA) patients with malignant biliary obstruction per total number of patients in the series;
Number of patients with attempted stent placement (ITT), followed by number of metal stents in parentheses;
Transmural variants of HG (hepatico-esophagostomy/jejunostomy) following intrahepatic access, except when stated otherwise;
Number of successful cases or complications per number of SAA patients with malignant biliary obstruction;
Initial plastic stent replaced at follow-up. Only final stent tallied in the total count;
Additional hepato-esophageal plastic stent combined with AG metal stent in recurrent gastric cancer after RYG. Not tallied in the total count;
Choledochoduodenostomy after palliative gastrojejunostomy. SAA: Surgically altered anatomy; ESCP: Endosonographic cholangiopancreatography; RYG: Roux-en-Y gastrectomy; RYHJ: Roux-en-Y hepatico-jejunostomy; PD: Pancreatico-duodenectomy; Gast: Gastric; Panc: Pancreatic; RV: Rendezvous; AG: Antegrade; HG: Hepatico-gastrostomy; ITT: Intention-to-treat; Sn: Successful cases; Cn: Complications.
Table 7.
Patients with benign biliary obstruction and surgically altered anatomy drained by endosonographic cholangiopancreatography
| Ref. | No. of patients1 |
Type of SAA |
Etiology |
Type of ESCP drainage |
Success/complex5 |
|||||||||
|
Transpapillary2 |
Transmural3 |
|||||||||||||
| RYG | RYGB | RYHJ | Other | Stone | Strx | RV | AGS | AGB | HG | Other4 | Sn | Cn | ||
| Püspök et al[13] (2005) | 1/6 | - | - | - | 1 | 1 | - | - | - | - | - | 1 (0)6 | 1/1 | 0 |
| Kahaleh et al[3] (2006) | 2/23 | - | - | 1 | 1 | 1 | 1 | 2 | - | - | - | - | 1/2 | 1 |
| Will et al[31] (2007) | 1/8 | - | - | 1 | - | - | 1 | 17 | - | - | - | 1 (0) | 1/1 | 0 |
| Bories et al[16] (2007) | 3/11 | - | - | - | 3 | - | 3 | 17 | - | - | 3 (1) | - | 2/3 | 1 |
| Weilert et al[110] (2011) | 6/6 | - | 6 | - | - | 6 | - | 2 | - | 4 | - | - | 6/6 | 1 |
| Artifon et al[112] (2011) | 1/1 | - | - | 1 | - | - | 1 | - | 1 | 19 | - | - | 1/1 | 0 |
| Park et al[113] (2012) | 1/1 | - | - | 1 | - | - | 1 | - | - | 1 | - | - | 1/1 | 0 |
| Bapaye et al[114] (2012) | 1/1 | - | - | 1 | - | - | 1 | - | - | 1 | - | - | 1/1 | 0 |
| Iwashita et al[57] (2013) | 6/7 | 4 | - | - | 2 | 5 | 1 | - | - | 59 | - | - | 6/6 | 1 |
| Park et al[109] (2013) | 3/6 | - | - | 3 | - | - | 3 | - | 1 | 38 | - | - | 2/3 | 0 |
| Total | 25 | 4 | 6 | 8 | 7 | 6 | 2 | 13 | 3 (1) | 2 (0) | ||||
| 25 | 13 (52%) | 12 (48%) | 21 (84%) | 5 (20%) | 22 (88%) | 4 (16%) | ||||||||
Number of surgically altered anatomy (SAA) patients with benign biliary obstruction per total number of patients in the series; 2Number of patients with attempted drainage by stent insertion and/or balloon dilation with/without stone removal (ITT); 3Number of patients with attempted stent placement (ITT), followed by number of metal stents in parentheses;
Transmural variants of HG (hepatico-esophagostomy/jejunostomy) following intrahepatic access, except when stated otherwise;
Number of successful cases or complications per number of SAA patients with benign biliary obstruction;
Choledochoduodenostomy with plastic stents used for transmural stone extraction in Billroth I;
Transpapillary AG metal stent placed at follow-up of prior transmural intrahepatic stenting in anastomotic Strx. Tallied in the total count;
AGB prior to AGS in a single case. AGS tallied as main intervention and AGB not tallied in the total count;
AGB with stone removal plus temporary NBD placement in four patients. AGB of anastomotic Strx and normal cholangiogram in one each. SAA: Surgically altered anatomy; ESCP: Endosonographic cholangiopancreatography; RYG: Roux-en-Y gastrectomy; RYGB: Roux-en-Y gastric bypass; RYHJ: Roux-en-Y hepatico-jejunostomy; RV: Rendezvous; AGS: Antegrade stent insertion; AGB: Antegrade balloon dilation; HG: Hepatico-gastrostomy; ITT: Intention-to-treat; Sn: Successful cases; Cn: Complications.
ESCP interventions for malignant biliary obstruction in SAA
Recurrent gastric, pancreatic and biliary malignancy after surgical resection usually present as obstructive jaundice. Roux-en-Y gastrectomy or hepatico-jejunostomy, pancreatico-duodenectomy and palliative bypass gastro-jejunostomy are the most commonly SAA encountered. PTBD has been classically used in this setting, as it is much simpler and more readily available than the combinations of surgical, percutaneous or enteroscopy-based ERCP described earlier. Two important limitations of PTBD in these patients are failure to provide internal biliary drainage occasionally[16] and the management of relapsing stent dysfunction[2].
As in other settings, EUSBD may overcome those limitations, improving management in patients with SAA and malignant biliary obstruction over that afforded by PTBD. Internal biliary drainage has been provided by hepaticogastrostomy (n = 10) or any of its variants (n = 7), with either plastic or metal stents placed in about half of the cases each (Table 6). Transmural metal stent placement carries the risk of severe bile leakage into the peritoneum caused by stent foreshortening[16]. This risk can be minimized by initial placement of transmural plastic stents followed by elective stent exchange after track maturation[2,14,16]. The recommended technique for plastic stent replacement after transmural EUSBD is over-the-wire snare removal after guide-wire cannulation of the stent[14,111]. The snare over-the-wire technique prevents track disruption and avoids the need for a repeat transhepatic (PTBD or EUSBD) puncture should stent occlusion lead to dysfunction.
As an alternative to transmural stenting, transductal (transpapillary or transanastomotic) stent placement has been reported in just five patients with SAA and malignant biliary obstruction, by means of rendezvous ERCP after antegrade guide-wire passage in 2 cases, and by direct antegrade stent insertion in a further 3 (Table 6). The final position of the stent in antegrade or rendezvous approaches is the same as in internal drainage by PTBD. Püspök et al[13] associated 7F plastic stent hepatico-jejunostomy in a patient with recurrent gastric cancer and prior Roux-en-Y total gastrectomy to antegrade transpapillary metal stent placement. Although their goal in placing this second transmural stent was to minimize the risk of acute leakage, this dual approach might well serve the purpose of avoiding a repeat transhepatic puncture in case of stent dysfunction, the transmural stent serving as an entry point to the bile-duct. A similar strategy to manage stent dysfunction was reported by Bories et al[16]. After initial transmural stenting, they converted drainage electively to antegrade stent placement at follow-up in two cases and to rendezvous in a further case. These authors highlight the ease of endoscopic re-intervention through a transmural metal stent in case of stent dysfunction.
ESCP interventions for benign biliary obstruction in SAA
Stones and anastomotic strictures are the most common benign causes requiring intervention after SAA, about half each in the 25 patients reported so far in the literature (Table 7). In contrast to SAA patients with malignant biliary obstruction in whom EUSBD is predominantly transmural, transductal intervention is the dominant approach to benign disease, chosen in 84% of SAA cases (Table 7). Antegrade balloon dilation with or without stone removal is the major ESCP intervention performed in this setting[57,110], with rendezvous[3,16,31,110] and antegrade stenting[109,112] having a secondary role.
Only two small series have specifically reported these EUSBD interventions in the setting of SAA. Weilert et al[110] succeeded at antegrade CBD stone removal in four RYGB patients and managed to salvage a further two with rendezvous enteroscopy-ERCP. These authors found rendezvous enteroscopy-ERCP with retrograde balloon dilation and stone removal helpful as a cross-over strategy following failed antegrade puncture track dilation. Iwashita et al[57] replicated their approach in patients with CBD stones following Roux-en-Y gastrectomy. To prevent leakage and to allow serial cholangiography, these authors left a nasobiliary drainage tube in place during 2-4 wk. Serial cholangiograms led to repeat intervention in one out of four patients with CBD stones.
Antegrade ESCP balloon dilation of anastomotic strictures without additional stenting has been reported as an effective measure to relieve biliary obstruction by several authors[34,109,113,114]. As effective remodeling of benign biliary strictures usually requires serial endotherapy[85,99], the long-term outcomes of single session balloon dilation remain unproven. A seemingly more effective two-step stricture remodeling strategy was used by Artifon et al[112], who via ESCP placed a temporary covered metal stent in an antegrade fashion across an anastomotic stricture. The stent was removed at follow-up using balloon enteroscopy. Authors limiting intervention to antegrade balloon dilation base the potential compromise in efficacy on concerns about transmural stenting when only minimal intrahepatic dilation is present[109], or about stent removability secondary to impaired access caused by SAA[114].
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 21, 2014
First decision: March 13, 2014
Article in press: June 23, 2014
P- Reviewer: Aytac E, Leitman IM, Li XL, Tsai HH, Yan SL S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102–106. doi: 10.1016/s0016-5107(06)80108-2. [DOI] [PubMed] [Google Scholar]
- 2.Giovannini M, Dotti M, Bories E, Moutardier V, Pesenti C, Danisi C, Delpero JR. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003;35:1076–1078. doi: 10.1055/s-2003-44596. [DOI] [PubMed] [Google Scholar]
- 3.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007;8:438–443. [PubMed] [Google Scholar]
- 5.Marinone MG, Rizzoni D, Ferremi P, Rossi G, Izzi T, Brusotti C. Late taste disorders in bone marrow transplantation: clinical evaluation with taste solutions in autologous and allogeneic bone marrow recipients. Haematologica. 1991;76:519–522. [PubMed] [Google Scholar]
- 6.Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246–251. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 7.Giovannini M, Pesenti Ch, Bories E, Caillol F. Interventional EUS: difficult pancreaticobiliary access. Endoscopy. 2006;38 Suppl 1:S93–S95. doi: 10.1055/s-2006-946665. [DOI] [PubMed] [Google Scholar]
- 8.Irisawa A, Hikichi T, Shibukawa G, Takagi T, Wakatsuki T, Takahashi Y, Imamura H, Sato A, Sato M, Ikeda T, et al. Pancreatobiliary drainage using the EUS-FNA technique: EUS-BD and EUS-PD. J Hepatobiliary Pancreat Surg. 2009;16:598–604. doi: 10.1007/s00534-009-0131-5. [DOI] [PubMed] [Google Scholar]
- 9.Kedia P, Gaidhane M, Kahaleh M. Endoscopic guided biliary drainage: how can we achieve efficient biliary drainage? Clin Endosc. 2013;46:543–551. doi: 10.5946/ce.2013.46.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372–1379. doi: 10.3748/wjg.v19.i9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver. 2013;7:129–136. doi: 10.5009/gnl.2013.7.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K, Fujita N, Horaguchi J, Noda Y, Kobayashi G. Current issues regarding endosonography-guided biliary drainage for biliary obstruction. Dig Endosc. 2010;22 Suppl 1:S132–S136. doi: 10.1111/j.1443-1661.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 13.Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743–1747. doi: 10.1111/j.1572-0241.2005.41806.x. [DOI] [PubMed] [Google Scholar]
- 14.Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, Takasawa O, Koshita S, Kanno Y. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009;21:239–244. doi: 10.1111/j.1443-1661.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 15.Ashida R, Chang KJ. Interventional EUS for the treatment of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2009;16:592–597. doi: 10.1007/s00534-009-0129-z. [DOI] [PubMed] [Google Scholar]
- 16.Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287–291. doi: 10.1055/s-2007-966212. [DOI] [PubMed] [Google Scholar]
- 17.Hanada K, Iiboshi T, Ishii Y. Endoscopic ultrasound-guided choledochoduodenostomy for palliative biliary drainage in cases with inoperable pancreas head carcinoma. Dig Endosc. 2009;21 Suppl 1:S75–S78. doi: 10.1111/j.1443-1661.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 18.Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video) Gastrointest Endosc. 2009;69:S8–12. doi: 10.1016/j.gie.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008;40:336–339. doi: 10.1055/s-2007-995455. [DOI] [PubMed] [Google Scholar]
- 20.Yamao K, Sawaki A, Takahashi K, Imaoka H, Ashida R, Mizuno N. EUS-guided choledochoduodenostomy for palliative biliary drainage in case of papillary obstruction: report of 2 cases. Gastrointest Endosc. 2006;64:663–667. doi: 10.1016/j.gie.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340–342. doi: 10.1055/s-2007-995485. [DOI] [PubMed] [Google Scholar]
- 22.Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–774. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 23.Artifon EL, Silva R, Gupta K, Ferreira FC, De Moura EG, Sakai P, Rassian S. Guided Choledochoduodenostomy Versus Surgical Drainage in Patients With Unresectable Distal Malignant Biliary Obstruction: A Randomized Prospective Trial. Gastrointest Endosc. 2012;75:AB162–AB163. [Google Scholar]
- 24.Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Barkay O, Sherman S, McHenry L, Yoo BM, Fogel EL, Watkins JL, DeWitt J, Al-Haddad MA, Lehman GA. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010;71:1166–1173. doi: 10.1016/j.gie.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518–525. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 28.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–107. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 29.Lai R, Freeman ML. Endoscopic ultrasound-guided bile duct access for rendezvous ERCP drainage in the setting of intradiverticular papilla. Endoscopy. 2005;37:487–489. doi: 10.1055/s-2005-861250. [DOI] [PubMed] [Google Scholar]
- 30.Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, Horaguchi J, Takasawa O, Nakahara K. Histological changes at an endosonography-guided biliary drainage site: a case report. World J Gastroenterol. 2007;13:5512–5515. doi: 10.3748/wjg.v13.i41.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292–295. doi: 10.1055/s-2007-966215. [DOI] [PubMed] [Google Scholar]
- 32.Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008;14:6078–6082. doi: 10.3748/wjg.14.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video) Gastrointest Endosc. 2009;70:471–479. doi: 10.1016/j.gie.2008.12.233. [DOI] [PubMed] [Google Scholar]
- 34.Maranki J, Hernandez AJ, Arslan B, Jaffan AA, Angle JF, Shami VM, Kahaleh M. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009;41:532–538. doi: 10.1055/s-0029-1214712. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496–502. doi: 10.1055/s-0029-1244082. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 37.Iwamuro M, Kawamoto H, Harada R, Kato H, Hirao K, Mizuno O, Ishida E, Ogawa T, Okada H, Yamamoto K. Combined duodenal stent placement and endoscopic ultrasonography-guided biliary drainage for malignant duodenal obstruction with biliary stricture. Dig Endosc. 2010;22:236–240. doi: 10.1111/j.1443-1661.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- 38.Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010;11:597–600. [PubMed] [Google Scholar]
- 39.Belletrutti PJ, Gerdes H, Schattner MA. Successful endoscopic ultrasound-guided transduodenal biliary drainage through a pre-existing duodenal stent. JOP. 2010;11:234–236. [PubMed] [Google Scholar]
- 40.Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 41.Fabbri C, Luigiano C, Fuccio L, Polifemo AM, Ferrara F, Ghersi S, Bassi M, Billi P, Maimone A, Cennamo V, et al. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: a case series. Endoscopy. 2011;43:438–441. doi: 10.1055/s-0030-1256097. [DOI] [PubMed] [Google Scholar]
- 42.Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239–1245. doi: 10.1038/ajg.2011.84. [DOI] [PubMed] [Google Scholar]
- 43.Ramírez-Luna MA, Téllez-Ávila FI, Giovannini M, Valdovinos-Andraca F, Guerrero-Hernández I, Herrera-Esquivel J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy. 2011;43:826–830. doi: 10.1055/s-0030-1256406. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui AA, Sreenarasimhaiah J, Lara LF, Harford W, Lee C, Eloubeidi MA. Endoscopic ultrasound-guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surg Endosc. 2011;25:549–555. doi: 10.1007/s00464-010-1216-6. [DOI] [PubMed] [Google Scholar]
- 45.Komaki T, Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasonography-guided biliary drainage: evaluation of a choledochoduodenostomy technique. Pancreatology. 2011;11 Suppl 2:47–51. doi: 10.1159/000323508. [DOI] [PubMed] [Google Scholar]
- 46.Prachayakul V, Aswakul P, Kachintorn U. EUS-guided choledochoduodenostomy for biliary drainage using tapered-tip plastic stent with multiple fangs. Endoscopy. 2011;43 Suppl 2 UCTN:E109–E110. doi: 10.1055/s-0030-1256140. [DOI] [PubMed] [Google Scholar]
- 47.Attasaranya S, Netinasunton N, Jongboonyanuparp T, Sottisuporn J, Witeerungrot T, Pirathvisuth T, Ovartlarnporn B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center’s Experience in 31 Cases. Gastroenterol Res Pract. 2012;2012:680753. doi: 10.1155/2012/680753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katanuma A, Maguchi H, Osanai M, Takahashi K. Endoscopic ultrasound-guided biliary drainage performed for refractory bile duct stenosis due to chronic pancreatitis: a case report. Dig Endosc. 2012;24 Suppl 1:34–37. doi: 10.1111/j.1443-1661.2012.01256.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawakubo K, Isayama H, Nakai Y, Sasahira N, Kogure H, Sasaki T, Hirano K, Tada M, Koike K. Simultaneous Duodenal Metal Stent Placement and EUS-Guided Choledochoduodenostomy for Unresectable Pancreatic Cancer. Gut Liver. 2012;6:399–402. doi: 10.5009/gnl.2012.6.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khashab MA, Fujii LL, Baron TH, Canto MI, Gostout CJ, Petersen BT, Okolo PI, Topazian MD, Levy MJ. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos) Gastrointest Endosc. 2012;76:209–213. doi: 10.1016/j.gie.2012.03.170. [DOI] [PubMed] [Google Scholar]
- 51.Kim TH, Kim SH, Oh HJ, Sohn YW, Lee SO. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J Gastroenterol. 2012;18:2526–2532. doi: 10.3748/wjg.v18.i20.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song TJ, Hyun YS, Lee SS, Park do H, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012;18:4435–4440. doi: 10.3748/wjg.v18.i32.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75:354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 54.Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–396. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 55.Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 56.Itoi T, Itokawa F, Tsuchiya T, Tsuji S, Tonozuka R. Endoscopic ultrasound-guided choledochoantrostomy as an alternative extrahepatic bile duct drainage method in pancreatic cancer with duodenal invasion. Dig Endosc. 2013;25 Suppl 2:142–145. doi: 10.1111/den.12065. [DOI] [PubMed] [Google Scholar]
- 57.Iwashita T, Yasuda I, Doi S, Uemura S, Mabuchi M, Okuno M, Mukai T, Itoi T, Moriwaki H. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig Dis Sci. 2013;58:2417–2422. doi: 10.1007/s10620-013-2645-6. [DOI] [PubMed] [Google Scholar]
- 58.Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80–87. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 59.Kahaleh M, Perez-Miranda M, Artifon EL, Gupta K, Park DH, Moon JH, Choi HJ, De La Serna C, De La Mora-Levy JG, Alonso-Larraga JO, et al. 140 Endoscopic ultrasound (EUS) guided biliary drainage: what have we learned? Gastrointest Endosc. 2013;77(5 Suppl):AB127–AB128. [Google Scholar]
- 60.Miura F, Takada T, Strasberg SM, Solomkin JS, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yoshida M, Mayumi T, et al. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:47–54. doi: 10.1007/s00534-012-0563-1. [DOI] [PubMed] [Google Scholar]
- 61.Takada T, Strasberg SM, Solomkin JS, Pitt HA, Gomi H, Yoshida M, Mayumi T, Miura F, Gouma DJ, Garden OJ, et al. TG13: Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:1–7. doi: 10.1007/s00534-012-0566-y. [DOI] [PubMed] [Google Scholar]
- 62.Tsuyuguchi T, Itoi T, Takada T, Strasberg SM, Pitt HA, Kim MH, Supe AN, Mayumi T, Yoshida M, Miura F, et al. TG13 indications and techniques for gallbladder drainage in acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:81–88. doi: 10.1007/s00534-012-0570-2. [DOI] [PubMed] [Google Scholar]
- 63.Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, et al. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:578–585. doi: 10.1007/s00534-012-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Houghton PW, Jenkinson LR, Donaldson LA. Cholecystectomy in the elderly: a prospective study. Br J Surg. 1985;72:220–222. doi: 10.1002/bjs.1800720327. [DOI] [PubMed] [Google Scholar]
- 65.Frazee RC, Nagorney DM, Mucha P. Acute acalculous cholecystitis. Mayo Clin Proc. 1989;64:163–167. doi: 10.1016/s0025-6196(12)65670-5. [DOI] [PubMed] [Google Scholar]
- 66.Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Tsukamoto S, Takeuchi M, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video) Gastrointest Endosc. 2008;68:455–460. doi: 10.1016/j.gie.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 68.Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71:1038–1045. doi: 10.1016/j.gie.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 69.Nahrwold DL. Acute cholecystitis. In: Sabiston D Jr., editor. Textbook of surgery. 15th ed. Philadelphia: Saunders; 1997. pp. 1126–1131. [Google Scholar]
- 70.Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007;65:735–737. doi: 10.1016/j.gie.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 71.Kwan V, Eisendrath P, Antaki F, Le Moine O, Devière J. EUS-guided cholecystenterostomy: a new technique (with videos) Gastrointest Endosc. 2007;66:582–586. doi: 10.1016/j.gie.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 72.Lee SS, Park do H, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008–1012. doi: 10.1016/j.gie.2007.03.1080. [DOI] [PubMed] [Google Scholar]
- 73.Takasawa O, Fujita N, Noda Y, Kobayashi G, Ito K, Horaguchi J, Obana T. Endosonography-guided gallbladder drainage for acute cholecystitis following covered metal stent deployment. Dig Endosc. 2009;21:43–47. doi: 10.1111/j.1443-1661.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 74.Kamata K, Kitano M, Komaki T, Sakamoto H, Kudo M. Transgastric endoscopic ultrasound (EUS)-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2009;41 Suppl 2:E315–E316. doi: 10.1055/s-0029-1215258. [DOI] [PubMed] [Google Scholar]
- 75.Song TJ, Park do H, Eum JB, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: a pilot study (with video) Gastrointest Endosc. 2010;71:634–640. doi: 10.1016/j.gie.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 76.Itoi T, Itokawa F, Kurihara T. Endoscopic ultrasonography-guided gallbladder drainage: actual technical presentations and review of the literature (with videos) J Hepatobiliary Pancreat Sci. 2011;18:282–286. doi: 10.1007/s00534-010-0310-4. [DOI] [PubMed] [Google Scholar]
- 77.Jang JW, Lee SS, Park do H, Seo DW, Lee SK, Kim MH. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176–181. doi: 10.1016/j.gie.2011.03.1120. [DOI] [PubMed] [Google Scholar]
- 78.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 79.Jang JW, Lee SS, Song TJ, Hyun YS, Park do H, Seo DW, Lee SK, Kim MH, Yun SC. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805–811. doi: 10.1053/j.gastro.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 80.de la Serna-Higuera C, Pérez-Miranda M, Gil-Simón P, Ruiz-Zorrilla R, Diez-Redondo P, Alcaide N, Sancho-del Val L, Nuñez-Rodriguez H. EUS-guided transenteric gallbladder drainage with a new fistula-forming, lumen-apposing metal stent. Gastrointest Endosc. 2013;77:303–308. doi: 10.1016/j.gie.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 81.Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010;2:203–209. doi: 10.4253/wjge.v2.i6.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part I: reconstruction without alteration of pancreaticobiliary anatomy. Gastrointest Endosc. 2001;54:743–749. doi: 10.1067/mge.2001.120169. [DOI] [PubMed] [Google Scholar]
- 83.Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part II: postsurgical anatomy with alteration of the pancreaticobiliary tree. Gastrointest Endosc. 2002;55:75–79. doi: 10.1067/mge.2002.120385. [DOI] [PubMed] [Google Scholar]
- 84.Pannala R, Brandabur JJ, Gan SI, Gluck M, Irani S, Patterson DJ, Ross AS, Dorer R, Traverso LW, Picozzi VJ, et al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc. 2011;74:295–302. doi: 10.1016/j.gie.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 85.Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, Galasso D. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551–557. doi: 10.1016/j.gie.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 86.Shah RJ, Smolkin M, Yen R, Ross A, Kozarek RA, Howell DA, Bakis G, Jonnalagadda SS, Al-Lehibi AA, Hardy A, et al. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video) Gastrointest Endosc. 2013;77:593–600. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Mergener K, Kozarek RA, Traverso LW. Intraoperative transjejunal ERCP: case reports. Gastrointest Endosc. 2003;58:461–463. [PubMed] [Google Scholar]
- 88.Pimentel RR, Mehran A, Szomstein S, Rosenthal R. Laparoscopy-assisted transgastrostomy ERCP after bariatric surgery: case report of a novel approach. Gastrointest Endosc. 2004;59:325–328. doi: 10.1016/s0016-5107(03)02549-5. [DOI] [PubMed] [Google Scholar]
- 89.Bertin PM, Singh K, Arregui ME. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography (ERCP) after gastric bypass: case series and a description of technique. Surg Endosc. 2011;25:2592–2596. doi: 10.1007/s00464-011-1593-5. [DOI] [PubMed] [Google Scholar]
- 90.Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–756. doi: 10.1016/j.gie.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 91.Baron TH, Chahal P, Ferreira LE. ERCP via mature feeding jejunostomy tube tract in a patient with Roux-en-Y anatomy (with video) Gastrointest Endosc. 2008;68:189–191. doi: 10.1016/j.gie.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 92.Baron TH, Vickers SM. Surgical gastrostomy placement as access for diagnostic and therapeutic ERCP. Gastrointest Endosc. 1998;48:640–641. doi: 10.1016/s0016-5107(98)70052-5. [DOI] [PubMed] [Google Scholar]
- 93.Tekola B, Wang AY, Ramanath M, Burnette B, Ellen K, Schirmer BD, Hallowell PT, Sauer BG, Kahaleh M. Percutaneous gastrostomy tube placement to perform transgastrostomy endoscopic retrograde cholangiopancreaticography in patients with Roux-en-Y anatomy. Dig Dis Sci. 2011;56:3364–3369. doi: 10.1007/s10620-011-1743-6. [DOI] [PubMed] [Google Scholar]
- 94.Choi EK, Chiorean MV, Coté GA, El Hajj II, Ballard D, Fogel EL, Watkins JL, McHenry L, Sherman S, Lehman GA. ERCP via gastrostomy vs. double balloon enteroscopy in patients with prior bariatric Roux-en-Y gastric bypass surgery. Surg Endosc. 2013;27:2894–2899. doi: 10.1007/s00464-013-2850-6. [DOI] [PubMed] [Google Scholar]
- 95.Martinez J, Guerrero L, Byers P, Lopez P, Scagnelli T, Azuaje R, Dunkin B. Endoscopic retrograde cholangiopancreatography and gastroduodenoscopy after Roux-en-Y gastric bypass. Surg Endosc. 2006;20:1548–1550. doi: 10.1007/s00464-005-0267-6. [DOI] [PubMed] [Google Scholar]
- 96.Baron TH, Song LM, Ferreira LE, Smyrk TC. Novel approach to therapeutic ERCP after long-limb Roux-en-Y gastric bypass surgery using transgastric self-expandable metal stents: experimental outcomes and first human case study (with videos) Gastrointest Endosc. 2012;75:1258–1263. doi: 10.1016/j.gie.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 97.Attam R, Leslie D, Freeman M, Ikramuddin S, Andrade R. EUS-assisted, fluoroscopically guided gastrostomy tube placement in patients with Roux-en-Y gastric bypass: a novel technique for access to the gastric remnant. Gastrointest Endosc. 2011;74:677–682. doi: 10.1016/j.gie.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 98.Madan A, Urayama S. Successful ERCP in a Roux-en-Y gastric bypass patient, performed via a small remnant of gastrogastric communication. Endoscopy. 2011;43 Suppl 2 UCTN:E73–E74. doi: 10.1055/s-0030-1256038. [DOI] [PubMed] [Google Scholar]
- 99.Shear W, Gaidhane M, Kahaleh M. ERCP in Roux-en-Y gastric bypass: creation of an antegrade gastrogastric conduit using a fully covered metal esophageal stent. Endoscopy. 2012;44 Suppl 2 UCTN:E58–E59. doi: 10.1055/s-0031-1291562. [DOI] [PubMed] [Google Scholar]
- 100.Perez-Miranda M, Mata L, Saracibar E, Sanchez-Antolin G, Barrio J, Caro-Paton A. Temporary access fistulas (TAFs) using covered self-expandable metal stents (cSEMS): a feasible tool for interventional pancreaticobiliary endoscopy [abstract] Gastrointest Endosc. 2007;65:AB123. [Google Scholar]
- 101.Baron TH, Song LM. Percutaneous assisted transprosthetic endoscopic therapy (PATENT): expanding gut access to infinity and beyond! (with video) Gastrointest Endosc. 2012;76:641–644. doi: 10.1016/j.gie.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 102.Perez-Miranda M, Sancho-Del VL, Lorenzo Pelayo S, Ruiz-Zorrilla R, Alcaide N, Herranz MT, de-Serna C, Caro-Paton A. Transmural Access and therapy of bile-duct disease in patients with complex anatomy using temporary covered self-expandable metal stents (CSEMS) [abstract] Gastrointest Endosc. 2011;73:AB126. [Google Scholar]
- 103.Jürgensen C, Wentrup R, Zeitz M. Endoscopic ultrasound (EUS)-guided transduodenal drainage of an obstructed jejunal loop after hepaticojejunostomy as treatment for recurrent biliary sepsis. Endoscopy. 2013;45 Suppl 2 UCTN:E40–E41. doi: 10.1055/s-0032-1325891. [DOI] [PubMed] [Google Scholar]
- 104.Matsumoto K, Kato H, Tomoda T, Sakakihara I, Yamamoto N, Noma Y, Sonoyama T, Tsutsumi K, Okada H, Yamamoto K, et al. A case of acute afferent loop syndrome treated by transgastric drainage with EUS. Gastrointest Endosc. 2013;77:132–133. doi: 10.1016/j.gie.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 105.Perez-Miranda M, Barclay RL, Kahaleh M. Endoscopic ultrasonography-guided endoscopic retrograde cholangiopancreatography: endosonographic cholangiopancreatography. Gastrointest Endosc Clin N Am. 2012;22:491–509. doi: 10.1016/j.giec.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56–64. [Google Scholar]
- 107.Kahaleh M, Yoshida C, Kane L, Yeaton P. Interventional EUS cholangiography: A report of five cases. Gastrointest Endosc. 2004;60:138–142. doi: 10.1016/s0016-5107(04)01528-7. [DOI] [PubMed] [Google Scholar]
- 108.Kahaleh M, Wang P, Shami VM, Tokar J, Yeaton P. EUS-guided transhepatic cholangiography: report of 6 cases. Gastrointest Endosc. 2005;61:307–313. doi: 10.1016/s0016-5107(04)02585-4. [DOI] [PubMed] [Google Scholar]
- 109.Park SJ, Choi JH, Park do H, Choi JH, Lee SS, Seo DW, Lee SK, Kim MH. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video) Gastrointest Endosc. 2013;78:374–380. doi: 10.1016/j.gie.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 110.Weilert F, Binmoeller KF, Marson F, Bhat Y, Shah JN. Endoscopic ultrasound-guided anterograde treatment of biliary stones following gastric bypass. Endoscopy. 2011;43:1105–1108. doi: 10.1055/s-0030-1256961. [DOI] [PubMed] [Google Scholar]
- 111.Fujita N, Sugawara T, Noda Y, Kobayashi G, Ito K, Obana T, Horaguchi J, Takasawa O. Snare-over-the-wire technique for safe exchange of a stent following endosonography-guided biliary drainage. Dig Endosc. 2009;21:48–52. doi: 10.1111/j.1443-1661.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- 112.Artifon EL, Safatle-Ribeiro AV, Ferreira FC, Poli-de-Figueiredo L, Rasslan S, Carnevale F, Otoch JP, Sakai P, Kahaleh M. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610–613. [PubMed] [Google Scholar]
- 113.Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided transhepatic antegrade balloon dilation for benign bilioenteric anastomotic strictures in a patient with hepaticojejunostomy. Gastrointest Endosc. 2012;75:692–693. doi: 10.1016/j.gie.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 114.Bapaye A, Dubale N. Endoscopic ultrasound-guided antegrade dilation of a stenosed hepaticojejunostomy. Endoscopy. 2012;44 Suppl 2 UCTN:E435–E436. doi: 10.1055/s-0032-1325894. [DOI] [PubMed] [Google Scholar]
- 115.Chopin-Laly X, Ponchon T, Guibal A, Adham M. Endoscopic biliogastric stenting: a salvage procedure. Surgery. 2009;145:123. doi: 10.1016/j.surg.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 116.Henry WA, Singh VK, Kalloo AN, Khashab MA. Simultaneous EUS-guided transbulbar pancreaticobiliary drainage (with video) Gastrointest Endosc. 2012;76:1065–1067, 1067.e1-e2. doi: 10.1016/j.gie.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 117.Ma J, Liu Y, Li Z, Jin Z. Endoscopic ultrasound-guided transgastric biliary drainage after partial gastrectomy. Endoscopy. 2011;43 Suppl 2 UCTN:E102. doi: 10.1055/s-0030-1256104. [DOI] [PubMed] [Google Scholar]
- 118.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]


