Abstract
AIM: To investigate whether Z:ZCLA Mongolian gerbils are readily susceptible to infection by human hepatitis E virus (HEV).
METHODS: Z:ZCLA Mongolian gerbils were infected with a clinical HEV strain isolated from an acute hepatitis E patient, and virus pathogenesis was assessed in this host. Non-infected gerbils served as the control group. Feces samples from gerbils were collected weekly for reverse transcription-nested polymerase chain reaction. Serum anti-HEV IgG and alanine aminotransferase (ALT) were detected by enzyme linked immunosorbent assay. At sacrifice, each animal’s liver, spleen and kidney were collected for histopathologic examination.
RESULTS: HEV-infected gerbils showed fatigue, with histopathological changes observed in the liver, spleen and kidney. HEV RNA was detected in fecal samples taken at day 7 after inoculation and the detectable levels lasted out to day 42 after inoculation. Interestingly, ALT levels were only moderately increased in the HEV-infected animals compared with the non-infected control group.
CONCLUSION: Z:ZCLA Mongolian gerbils are susceptible to human HEV.
Keywords: Hepatitis E virus, Mongolian gerbils, Infection, Interspecies transmission, Zoonosis
Core tip: Z:ZCLA Mongolian gerbils were infected with human hepatitis E virus (HEV). Feces samples from gerbils were collected weekly for reverse transcription-nested polymerase chain reaction. Serum anti-HEV IgG and alanine aminotransferase (ALT) detection was carried out by enzyme linked immunosorbent assay. At sacrifice, liver, spleen and kidney were collected from infected animals and non-infected controls for histopathologic examination. Detectable HEV RNA in fecal samples appeared at post-inoculation day 7 and persisted through day 42. Interestingly, ALT levels were only moderately increased in infected animals compared with control animals. These findings indicate that Z:ZCLA Mongolian gerbils are susceptible to human HEV.
INTRODUCTION
Hepatitis E virus (HEV) infection is a significant public health problem in many developing countries, causing large outbreaks of acute hepatitis. It is known that HEV transmission occurs primarily by the fecal-oral route, mainly through contaminated drinking water in areas with poor sanitation. However, hepatitis E has recently been diagnosed with increasing frequency in industrialized countries[1], and seroreactivity is observed in 5% to 21% of asymptomatic individuals[2].
Interestingly, antibodies to HEV have been detected in a wide range of domestic and feral mammals, and HEV RNA has been isolated from pig[3-5], deer[6], wild boar[7], rodents[8-10] and chickens[11]. The prevalence of HEV-specific antibodies in wild rodents is well documented[12-15], and HEV was recently isolated from rats trapped in several cities of industrialized countries[8]. Thus, it is important to assess whether rodents constitute a potential source of human HEV infection. Wild gerbils, commonly known as midday jird [Meriones meridianus (M. meridianus)], have been experimentally infected with human HEV, and lesions similar to those found in human hepatitis E patients were observed[16]. However, M. meridianus is not a breeding species and the animal’s biological characteristics are unknown. Other studies have reported the transmission of HEV from swine feces in southern China to the Mongolian gerbil M. unguiculatus, which serves as an animal model for a wide range of diseases[17].
The Z:ZCLA Mongolian gerbil (M. unguiculatus) is a relatively new laboratory stock, domesticated by Zhejiang Experimental Animal Center, a branch of our institution. The animals originated from Inner Mongolia of China and have been artificially propagated for more than 30 years. So far, the gerbils have been bred for 46 generations and systematically characterized for basic biological attributes. The aim of the present study was to assess whether the Z:ZCLA Mongolian gerbils are readily susceptible to infection by human HEV.
MATERIALS AND METHODS
Virus
A clinical strain of HEV was isolated from a fecal sample of an acute hepatitis E patient (Hangzhou, China). A 221-nt product was amplified from the fecal sample by reverse transcription-nested polymerase chain reaction (RT-nPCR) and directly sequenced to yield the consensus sequence. The virus sequence showed 99% homology with the Xinjiang strain D11092 (genotype 1). For virus preparation, 5 g of the patient’s feces were resuspended in phosphate-buffered saline (PBS, pH 7.4) containing 0.01% diethyl pyrocarbonate (DEPC), at a proportion of 10% (w/v). After centrifugation of the suspension at 12000 × g for 20 min, the resulting supernatant was filtered sequentially through 0.45 and 0.22 μm filters before inoculation. The viruses were inoculated into each gerbil at a minimum viral count of 5.5 × 102/mL of feces supernatant, as calculated by viral genomic titer determined by real-time reverse quantitative PCR.
Animals and infection
Twenty-one specific pathogen-free male Z:ZCLA Mongolian gerbils with an average body mass of 40 ± 5 g (approximately 5 wk of age) were provided by the Experimental Animal Center at the Zhejiang Academy of Medical Sciences (China) and maintained in a pathogen-free animal facility. Adequate measures were taken to minimize animal discomfort. All procedures involving animals in this study were approved by the local committee of Animal Use and Protection.
Gerbils were divided into two groups, with 14 in the virus infection group and 7 in the control group. The day before infection, each gerbil was injected with 6 × 104-8 × 104 units of penicillin intraperitoneally. The infection group was inoculated intraperitoneally with 100 μL of the virus suspension described above. Gerbils in the control group were inoculated with 100 μL PBS. All animals were provided with food and water ad libitum during the 7-wk study course. Fecal samples were collected each week post-inoculation and stored at -20 °C until use. Two gerbils from the infection group and 1 from the control group were humanely euthanized each week post-inoculation. Serum was obtained from blood samples collected weekly and stored at -20 °C. Liver, spleen and kidney were fixed in 10% neutral buffered formalin immediately upon sampling for subsequent histopathologic examination.
Serologic tests
Serum specimens were assessed for IgG antibodies to HEV by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Wantai Biological Pharmacy Co., Beijing, China), according to the manufacturer’s instructions. The absorbance was determined at 450 nm (Multiscan MCC microplate reader; Titertek Instruments, Huntsville, AL, United States).
ALT levels were detected in serum samples with an automated biochemistry analyzer (AU2700 chemistry immune-analyzer; Olympus, Tokyo, Japan).
Histopathological studies
For histological studies, fixed tissues (liver, spleen, and kidney) were dehydrated with increasing concentrations of ethanol and embedded in paraffin according to standard laboratory procedures. Tissues were cut into 7 μm sections and stained with hematoxylin and eosin for histological evaluation. Observation was carried out on an Olympus CX41 microscope.
RNA extraction and RT-nPCR
Total RNA was extracted from the 10% fecal supernatants. After centrifugation of the suspensions at 12000 × g for 15 min, the resulting supernatant was mixed with 10% PEG 6000 (w/v) and 2.3% NaCl (w/v), and stored at 4 °C overnight. The solution was centrifuged at 12000 × g for 20 min the following day. Total RNA was extracted from the sediment by the TRIzol reagent (Invitrogen, Carlsbad, CA, United States), according to the manufacturer’s instructions, and dissolved in 20 μL ribonuclease (RNase)-free water. Reverse transcription was performed using a commercially available Primescript first-strand cDNA synthesis kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. The resulting cDNA was amplified by nested PCR using primers based on sequences of the open reading frame 2 (5123-7105 nt) of the Chinese HEV isolate (sites based on L08816.1)[18]. The external forward primer (6272-6294 nt, PCR1) was 5’-CCGACAGAATTGATTTCGTCGGC-3’ and the reverse primer (6579-6557 nt, PCR4) was 5’-CCGTAAGTGGACTGGTCATACTC-3’; the internal forward primer (6323-6345 nt, PCR2) was 5’-GTCGTCTCAGCCAATGGCGAGCC-3’, and the reverse primer (6521-6543 nt, PCR3) was 5’-GAAAGCCAAAGCACATCATTAGC-3’. The RT-nPCR product was expected to be 221 base pairs. The first round PCR was set up at 94 °C for 5 min, followed by 33 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 10 min. The second round PCR protocol was the same as the first one except that the melting temperature of 57 °C was used. The PCR products were assessed by electrophoresis on 1% agarose gel. A negative control (water only) was included, and the PCR products were identified by sequencing to exclude the possibility of contamination and failure of amplification.
RESULTS
Clinical evaluation
The HEV-infected gerbils showed fatigue and loose hair. As expected, no evidence of clinical disease was observed in the control gerbils.
Serological analysis
Serum specimens were examined using an automatic biochemical analyzer to determine serum ALT levels. As shown in Figure 1, ALT levels were moderately increased in gerbils infected with HEV, compared with control animals, at days 14 and 21 post-inoculation. Assays were performed in triplicate and data expressed as mean ± SD. In addition, serum anti-HEV antibodies were assessed in HEV-inoculated and non-inoculated groups by ELISA, and no anti-HEV IgG was detected in either group.
Figure 1.
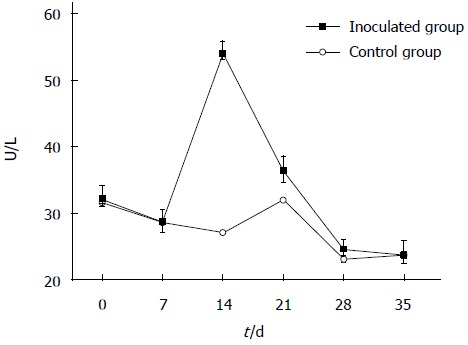
Changes in alanine aminotransferase levels after hepatitis E virus infection. Z:ZCLA Mongolian gerbils showed alanine aminotransferase (ALT) level changes upon hepatitis E virus infection. The ALT levels were moderately increased in the inoculated group compared with the control group at days 14 and 21 post-inoculation. No changes were observed in the control group.
Detection of HEV RNA by RT-nPCR
Viral shedding in feces was detected intermittently by RT-nPCR, beginning at day 7 post-inoculation for all infected gerbils. Indeed, correct sized PCR products were detected by electrophoresis on a 1% agarose gel, which showed a 221 bp DNA fragment (Figure 2). The fecal excretion of HEV lasted for 42 d post-inoculation. All data obtained for the 7-wk experimental period are summarized in Table 1.
Figure 2.
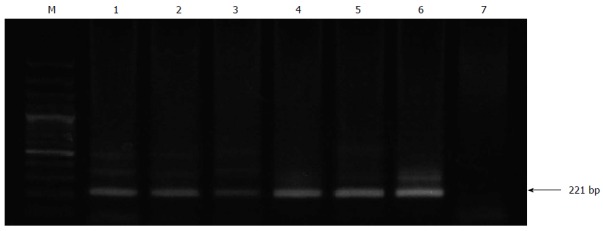
Reverse transcription-nested polymerase chain reaction detection of viral shedding in feces. Hepatitis E virus RNA was detected by reverse transcription-nested polymerase chain reaction (RT-nPCR) in feces from all inoculated gerbils. Lane M: DNA marker; Lanes 1-6 represent experimental time points (in weeks): the second round PCR products of gerbil feces samples on intermittent days; Lane 7: a negative control containing water.
Table 1.
Hepatitis E virus RNA-positive rate in experimental and control Mongolian gerbils
| Post-inoculation day | ||||||||
| Group | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
| HEV-inoculated | 0/14 | 5/14 | 10/12 | 10/10 | 8/8 | 6/6 | 1/4 | 0/2 |
| Control | 0/7 | 0/7 | 0/6 | 0/5 | 0/4 | 0/3 | 0/2 | 0/1 |
The viral shedding in feces was detected by reverse transcription-nested polymerase chain reaction. HEV: Hepatitis E virus.
Histopathologic examination
To determine whether gerbils responded to the HEV-containing inoculum with histopathologic signs of injury, tissues (liver, spleen and kidney) from inoculated and control gerbils were sectioned, stained, and examined by light microscopy. We observed changes attributable to inoculation in the liver, spleen and kidney. Hepatic inflammation and focal hepatocellular necrosis were observed in inoculated gerbils. In addition, mixed infiltration of lymphocytes and eosinophils in the portal tracts was observed (Figure 3A). Enlarged splenocytes and multiple vacuolar degeneration were observed in the spleens of inoculated gerbils (Figure 3B). Moreover, increased lymphocyte and macrophage infiltration was observed in renal tissues after HEV infection (Figure 3C). Of note, no damage was observed in any tissue from control animals (Figure 3D-F).
Figure 3.
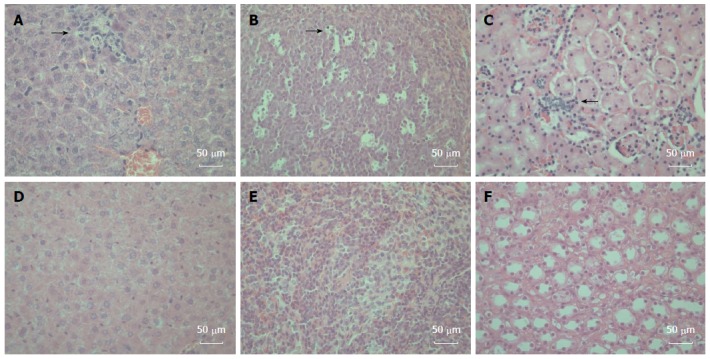
Histopathologic changes in the hepatitis E virus-inoculated Mongolian gerbils. A: A representative liver sample showing slight histiocytic hepatitis and focal accumulation of inflammatory cells surrounding hepatocytes [21 d post-inoculation (DPI)]; B: A representative spleen sample showing ruptured and enlarged splenocytes with multiple vacuolar degeneration (21 DPI); C: A representative kidney showing disarranged kidney cells with increased infiltrating lymphocytes and macrophages (35 DPI); D: A representative negative control liver; E: A representative negative control spleen; F: A representative Negative control kidney. All tissues were stained with hematoxylin and eosin. Original magnifications × 400.
DISCUSSION
Hepatitis E genotype 1 is an infectious agent, which causes a disease considered to be endemic in developing countries, due to poor sanitary conditions. However, recent reports have drawn attention to hepatitis E appearance in developed countries; the most recent survey showed that 21% of United States’ residents are seropositive for anti-HEV IgG[19]. Although some research assays could yield “false-positive” results, with controversy surrounding current enzyme immunoassays used for HEV detection[2], more thorough HEV studies are urgently needed.
HEV infection is described as a disease transmitted primarily via the fecal-oral route through contaminated drinking water[20]. In addition, wild or domestic swine consumption probably plays an important role in the occurrence of hepatitis E genotype 3 or 4[21,22]. However, as evidences began to accumulate, recent investigations suggested the possibility of other modes of transmission. It was reported that HEV can be transmitted from person to person[23], despite the fact that HEV presence in stool is acknowledged as a major source of transmission by contact exposure to other animals[24]. Because rodents and humans often share the environment, especially in inner cities, there is a possibility for rodents to come in contact with human feces. Therefore, rodents are particularly interesting as a potential source of human HEV infections.
Currently, the Mongolian gerbil is a used widely model animal for scientific studies. However, the Z:ZCLA Mongolian gerbil is a relatively new closed colony, of which ancestors were obtained in Inner Mongolia of China by the Zhejiang Experimental Animal Center in 1978. This population has stable biological characteristics, and lives longer than other Mongolian gerbil populations in China. Moreover, these animals have been used in investigations in multiple fields: parasitology, cerebral hemorrhage, virology, bacteriology, lipometabolism, and carbohydrate metabolism. The results presented here suggest that Z:ZCLA Mongolian gerbils are susceptible to human HEV, as evidenced by clinical signs of HEV infection, changes in serum ALT levels, fecal viral shedding, and lesions of organs, including liver, spleen and kidney. As shown above, fecal virus shedding was detected at day 7 post-inoculation and lasted for 5 wk. Similar findings were previously described by Li et al[25], who detected HEV RNA in fecal excretions obtained from gerbils inoculated with swine HEV at 3-4 wk post-inoculation. With the large number of rodents in large cities, HEV can be spread easily and for a long time through rat feces; therefore, the effects of HEV via the fecal-oral route are difficult to predict.
During the experimental infection course of the current study, no anti-HEV antibodies were detected in the inoculated gerbils. Similar to a previous study, in which Wistar rats were infected with human HEV, seroconversion could not be detected out to the experiment-end day 35 of post-inoculation[26]. In another study, gerbils infected with swine HEV produced anti-HEV antibodies at 49 d after inoculation, with noticeably lower optical density values than the positive control[25]. Therefore, the magnitude of rat viremia might be reduced in comparison to that of other inoculated mammals.
The results presented here indicate that Z:ZCLA Mongolian gerbils could be experimentally infected by human HEV; therefore, this rodent should be considered a helpful animal model for HEV studies. Specifically, it might be used to further describe the mechanism of HEV interspecies transmission. In addition, rodent control in inner cities may help prevent HEV transmission.
COMMENTS
Background
Hepatitis E virus (HEV) infection is a significant public health problem in many developing countries, causing large outbreaks of acute hepatitis. However, in industrialized countries, hepatitis E has recently been diagnosed with increasing frequency as a cause of sporadic hepatitis, and seroreactivity has been recorded frequently in asymptomatic persons. Because antibodies to HEV have been detected in a wide range of domestic and feral mammals, and HEV RNA has been recently isolated from rats trapped in several cities in industrialized countries, it is important to understand whether rodents are a potential source of human HEV infection.
Research frontiers
HEV infection is known as a disease transmitted primarily via the fecal-oral route through contaminated drinking water. However, recent investigations have suggested the possibility of other modes of transmission. It was reported that HEV can be transmitted from person to person despite the fact that HEV presence in stool is a major source of transmission by contact exposure to other animals. Because rodents and humans often share the environment, especially in inner cities, rodents could come in contact with human feces easily. Therefore, rodents are particularly interesting as a potential source of human HEV infections.
Innovations and breakthroughs
It has been shown that wild gerbils, commonly known as midday jird [Meriones meridianus (M. meridianus)], can be experimentally infected by human HEV and present with lesions similar to those found in human hepatitis E patients. However, M. meridianus is not a breeding species and their biological characteristics are unknown. Other studies have reported the transmission of HEV from swine feces to the Mongolian gerbil (Meriones unguiculatus), which serves as an animal model for a wide range of diseases. The Z:ZCLA Mongolian gerbil (M. unguiculatus) is a relatively new laboratory stock, domesticated by the Zhejiang Experimental Animal Center, a branch of our institution. The animals originated from Inner Mongolia of China, and have been artificially propagated for more than 30 years. So far, the gerbils have been bred for 46 generations and their basic biological characteristics have been systematically described. The aim of the present study was to investigate whether Z:ZCLA Mongolian gerbils are readily susceptible to infection with human HEV.
Applications
The study results suggest that Z:ZCLA Mongolian gerbils could be experimentally infected by human HEV; therefore, M. unguiculatus should be considered a helpful animal model for HEV studies. Specifically, it can be used to further describe the mechanism of HEV interspecies transmission.
Terminology
HEV is an emerging pathogen and the most common cause of acute viral hepatitis worldwide.
Peer review
This manuscript comprises the experimental infection of a novel laboratory stock of Mongolian gerbils with a clinical strain of human HEV. Apart from the introduction of an animal model for HEV (interspecies) infection, the authors raise the question about the influence of rodents on HEV transmission to humans.
Footnotes
Supported by Science Technology Department of Zhejiang Province No. 2011F20015; and Health and Family Planning Commission of Zhejiang Province, No. XKQ-010001.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 27, 2014
First decision: June 27, 2014
Article in press: September 5, 2014
P- Reviewer: Kim SR S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Liu XM
References
- 1.Purcell RH, Emerson SU. Hepatitis E virus. In: Knipe , DM , Howley PM, eds , editors. Fields virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 3051–3061. [Google Scholar]
- 2.Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51:328–334. doi: 10.1086/653943. [DOI] [PubMed] [Google Scholar]
- 3.van der Poel WH, Verschoor F, van der Heide R, Herrera MI, Vivo A, Kooreman M, de Roda Husman AM. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg Infect Dis. 2001;7:970–976. doi: 10.3201/eid0706.010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang FF, Haqshenas G, Guenette DK, Halbur PG, Schommer SK, Pierson FW, Toth TE, Meng XJ. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol. 2002;40:1326–1332. doi: 10.1128/JCM.40.4.1326-1332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pei Y, Yoo D. Genetic characterization and sequence heterogeneity of a canadian isolate of Swine hepatitis E virus. J Clin Microbiol. 2002;40:4021–4029. doi: 10.1128/JCM.40.11.4021-4029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–505. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Purcell RH, Engle RE, Rood MP, Kabrane-Lazizi Y, Nguyen HT, Govindarajan S, St Claire M, Emerson SU. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis. 2011;17:2216–2222. doi: 10.3201/eid1712.110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol. 2010;91:750–758. doi: 10.1099/vir.0.016584-0. [DOI] [PubMed] [Google Scholar]
- 10.Kanai Y, Miyasaka S, Uyama S, Kawami S, Kato-Mori Y, Tsujikawa M, Yunoki M, Nishiyama S, Ikuta K, Hagiwara K. Hepatitis E virus in Norway rats (Rattus norvegicus) captured around a pig farm. BMC Res Notes. 2012;5:4. doi: 10.1186/1756-0500-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol. 2004;85:1609–1618. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- 12.Arankalle VA, Joshi MV, Kulkarni AM, Gandhe SS, Chobe LP, Rautmare SS, Mishra AC, Padbidri VS. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirano M, Ding X, Li TC, Takeda N, Kawabata H, Koizumi N, Kadosaka T, Goto I, Masuzawa T, Nakamura M, et al. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol Res. 2003;27:1–5. doi: 10.1016/s1386-6346(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 14.Favorov MO, Kosoy MY, Tsarev SA, Childs JE, Margolis HS. Prevalence of antibody to hepatitis E virus among rodents in the United States. J Infect Dis. 2000;181:449–455. doi: 10.1086/315273. [DOI] [PubMed] [Google Scholar]
- 15.Vitral CL, Pinto MA, Lewis-Ximenez LL, Khudyakov YE, dos Santos DR, Gaspar AM. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;100:117–122. doi: 10.1590/s0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 16.Zhao SY, Liao LF, Zou LY, Yu ZY. Study of experiment on HEV infection in Gerbil (Merionesmeridianus) from generation to generation. Zhongguo Meijie Shengwuxue and Kongzhi Zazhi. 2001;12:215–218. [Google Scholar]
- 17.Gaucher D, Chadee K. Molecular cloning and expression of gerbil granulocyte/macrophage colony-stimulating factor. Gene. 2002;294:233–238. doi: 10.1016/s0378-1119(02)00795-3. [DOI] [PubMed] [Google Scholar]
- 18.Bi SL, Purdy MA, McCaustland KA, Margolis HS, Bradley DW. The sequence of hepatitis E virus isolated directly from a single source during an outbreak in China. Virus Res. 1993;28:233–247. doi: 10.1016/0168-1702(93)90024-h. [DOI] [PubMed] [Google Scholar]
- 19.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 21.Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, Kurata Y, Ishida M, Sakamoto S, Takeda N, et al. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis. 2005;11:1958–1960. doi: 10.3201/eid1112.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–349. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 23.Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, Hill VR, Okware S, Hu DJ, Holmberg SD. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis. 2010;50:1006–1010. doi: 10.1086/651077. [DOI] [PubMed] [Google Scholar]
- 24.Huang F, Zhang W, Gong G, Yuan C, Yan Y, Yang S, Cui L, Zhu J, Yang Z, Hua X. Experimental infection of Balb/c nude mice with Hepatitis E virus. BMC Infect Dis. 2009;9:93. doi: 10.1186/1471-2334-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Sun Q, She R, Wang D, Duan X, Yin J, Ding Y. Experimental infection of Mongolian gerbils by a genotype 4 strain of swine hepatitis E virus. J Med Virol. 2009;81:1591–1596. doi: 10.1002/jmv.21573. [DOI] [PubMed] [Google Scholar]
- 26.Maneerat Y, Clayson ET, Myint KS, Young GD, Innis BL. Experimental infection of the laboratory rat with the hepatitis E virus. J Med Virol. 1996;48:121–128. doi: 10.1002/(SICI)1096-9071(199602)48:2<121::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]


