Abstract
AIM: To investigate the prognostic factors after resection for hepatitis B virus (HBV)-associated intrahepatic cholangiocarcinoma (ICC) and to assess the impact of different extents of lymphadenectomy on patient survival.
METHODS: A total of 85 patients with HBV-associated ICC who underwent curative resection from January 2005 to December 2006 were analyzed. The patients were classified into groups according to the extent of lymphadenectomy (no lymph node dissection, sampling lymph node dissection and regional lymph node dissection). Clinicopathological characteristics and survival were reviewed retrospectively.
RESULTS: The cumulative 1-, 3-, and 5-year survival rates were found to be 60%, 18%, and 13%, respectively. Multivariate analysis revealed that liver cirrhosis (HR = 1.875, 95%CI: 1.197-3.278, P = 0.008) and multiple tumors (HR = 2.653, 95%CI: 1.562-4.508, P < 0.001) were independent prognostic factors for survival. Recurrence occurred in 70 patients. The 1-, 3-, and 5-year disease-free survival rates were 36%, 3% and 0%, respectively. Liver cirrhosis (HR = 1.919, P = 0.012), advanced TNM stage (stage III/IV) (HR = 2.027, P < 0.001), and vascular invasion (HR = 3.779, P = 0.02) were independent prognostic factors for disease-free survival. Patients with regional lymph node dissection demonstrated a similar survival rate to patients with sampling lymph node dissection. Lymphadenectomy did not significantly improve the survival rate of patients with negative lymph node status.
CONCLUSION: The extent of lymphadenectomy does not seem to have influence on the survival of patients with HBV-associated ICC, and routine lymph node dissection is not recommended, particularly for those without lymph node metastasis.
Keywords: Intrahepatic cholangiocarcinoma, Hepatitis B virus, Lymph node metastases, Postoperative survival, Lymph node dissection
Core tip: Some recently published studies show a relation between chronic hepatitis B infection and the development of intrahepatic cholangiocarcinoma. Hepatitis B-associated patients with cholangiocarcinoma appear to have different clinicopathological characteristics compared with seronegative patients. In this context, the authors analyzed the data of patients with hepatitis B virus-associated intrahepatic cholangiocarcinoma who underwent curative resection retrospectively. They found in multivariate analysis that liver cirrhosis and multiple tumors were independent prognostic factors for overall survival. Independent prognostic factors for disease-free survival were liver cirrhosis, vascular invasion and advanced TNM stage. The patients were divided into three groups depending on the extent of lymph node dissection (no lymph node dissection, sampling lymph node dissection and regional lymph node dissection). The outcomes were not statistically different between the three groups.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC), which arises from the more peripheral branches of the intrahepatic bile duct, is the second most common primary liver cancer after hepatocellular carcinoma (HCC), accounting for 5%-10% of primary liver malignancies[1,2]. It demonstrates a relatively high prevalence in parts of East Asia[3].
Recently, an increasing number of studies have shown that viral hepatitis B and C are statistically related to ICC[4-8]. The mechanism for the development of ICC in patients with HBV infection is still uncertain. Hepatitis B and C virus infections are the most common risk factors for HCC. Hepatitis-associated HCC contains elements of cholangiocarcinoma[9,10], suggesting that the two malignancies share a common origin from bipotential stem cells and pathogenic mechanisms[9,11]. Clinically, some studies have also found that both viral hepatitis-associated ICC and HCC have similar age profiles and different age distributions between patients with chronic hepatitis B and those with chronic hepatitis C[7,8,12]. In addition, in our previous study, we have found that HBV-associated ICC have specific clinicopathological characteristics[13]. Compared with seronegative ICC patients, HBV-associated ICC patients tend to be male and younger, with higher α-fetoprotein levels and a lower frequency of lymphatic metastasis, which has been proved by other studies[7,12]. It is suggested that HBV-associated ICC and HCC share common disease processes for carcinogenesis, and should be distinguished from those without HBV infection, because they have different clinicopathological characteristics and surgical outcomes[14].
The prognosis of ICC is generally poor as compared with that of HCC[15,16], because it is frequently associated with lymph node involvement, intrahepatic metastasis, and peritoneal dissemination[17,18]. In consideration of similar pathogenesis and clinicopathological characteristics, studies should be conducted to ascertain whether HBV-associated ICC can be successfully treated by the methods used to treat HCC. Curative resection is still known to be the only effective therapeutic measure. However, there is no established consensus on the prognostic significance of lymph node dissection (LND), or even the extent of LND[19]. Prognostic factors after resection for HBV-associated ICC have not been confirmed due to its rarity and low resectability. Therefore, this study was conducted to investigate the outcomes of HBV-associated ICC patients after hepatic resection, and to analyze the prognostic factors affecting the survival and recurrence of the patients. The study also investigated the impact of the extent of LND on survival.
MATERIALS AND METHODS
Patients
Primary curative resection was performed in 127 out of 146 patients diagnosed with ICC at Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Shanghai, China) from January 2005 to December 2006. Only 114 patients were available for data analysis, and the rest of the patients could not be followed after operation.
With respect to a case of hepatitis B surface antigen being positive [HBsAg (+)], it is impossible to determine whether it is a chronic infection, acute infection, or false positive result. Generally speaking, the case of anti-hepatitis B core (HBc) being positive [anti-HBc (+)] only indicates that there may be past infection or that it may be present after the disappearance of HBsAg and the appearance of anti-HBs antibody, thus indicating recent infection. It can also be considered a sentinel marker for detection of occult HBV infection (HBsAg carrier)[20]. Therefore, the present study defined chronic HBV infection as either HBsAg (+) or anti-HBc (+)[21].
Data analysis was conducted on 85 patients (74.6%) with chronic HBV infection. The diagnosis of ICC was confirmed by pathological examination. Patients with hilar cholangiocarcinoma, gallbladder cancer, or combined type carcinoma consisting of HCC and ICC were excluded from this study. Curative resection was defined as negative surgical margins on microscopic examination, absence of macroscopic intrahepatic metastasis in the residual liver at the time of surgery, and absence of abdominal dissemination. The study protocol was approved by the clinical research ethics committee of the hospital. Written consent was obtained from all patients according to the policies of the committee.
Preoperative investigations
Preoperative evaluation was conducted before the decision for surgery was made. We evaluated resectability of ICC by ultrasonography, computed tomography (CT), and magnetic resonance image (MRI) examinations. Liver function was evaluated via Child-Pugh classification. Before the operation, patients at or over the age of 60 were routinely evaluated for formal cardiopulmonary and general conditions. Resection criteria were constant during the study period, including the number of resectable tumors, presence or absence of tumor thrombus and gross metastatic focus, and adequate liver function reserve, as reported in our previous study[22,23].
Surgical procedures and parameter definitions
Liver resection was carried out using finger fracture and clamp crushing with intermittent Pringle’s maneuver under room temperature. Lymphadenectomy was not conducted uniformly for every patient. 35 out of 85 patients did not undergo lymphadenectomy, neither did they present lymph node metastasis in the preoperative evaluation and intraoperative assessment. We classified the extent of lymphadenectomy into lymph node sampling and regional LND. Lymph node sampling was performed in 22 patients, and the lymph nodes along the hepatoduodenal ligament were removed. Regional LND was performed in 28 patients who underwent complete excision of soft tissue and lymph nodes at the hepatic hilum, common hepatic artery stations, hepatoduodenal ligament, and posterior to the upper portion of the pancreatic head. The extent of LND was similar for right- and left-sided tumors, with the exception of dissection of lymph nodes along the lesser curvature of the stomach for tumors located in the left lobe of the liver. The patients were divided into three groups according to the extent of LND: (1) LND (-) group: with no lymphadenectomy; (2) lymph node sampling group: with lymph node sampling; and (3) regional LND group: with regional lymphadenectomy.
ICC was classified on the basis of gross appearance, as raised by the Liver Cancer Study Group of Japan (LCSGJ), including types of mass-forming (MF), periductal infiltrating (PI), and intraductal growth (IG). Tumor-node-metastasis (TNM) staging of tumors complied with the guidelines of the 7th edition of the American Joint Committee on Cancer/International Union against Cancer (AJCC/UICC). In this study, multiple tumors referred to more than one tumorous node (including micrometastases that could be discovered only on pathological examination); tumor size referred to the maximum tumor diameter; major liver resection was defined as resection of three or more hepatic segments; minor liver resection was defined as resection of one or two hepatic segments.
Follow-up
The clinical data from all patients were recorded prospectively. Follow-up after resection consisted of routine blood tests, physical examination, and abdominal ultrasonography every 3 mo postoperatively for the first 2 years and twice a year thereafter at our hospital. Suspected recurrences were confirmed by CT or MRI. If unavailable to undergo this procedure, the patient was followed by telephone or letter every year, as reported in our previous study[22,23].
Statistical analysis
Overall survival (OS) and disease-free survival (DFS) rates and curves were analyzed by the Kaplan-Meier method, and differences were compared by the log-rank test. Comparisons between groups were made using the Chi-square test or Fisher’s exact test. After univariate analysis, only significant variables with P < 0.05 were adopted in the multivariate analysis. Multivariate analysis was conducted by means of the Cox proportional hazards model to identify prognostic factors. All statistical analyses were carried out with software package SPSS 18.0 (SPSS Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
RESULTS
Clinicopathological characteristics of the patients
The follow-up duration was calculated from the day of operation to either the day of death or the date of December 31, 2012. There were a total of 66 males and 19 females with a median age of 54 years (range: 28-79 years). Out of the 85 patients, 40 underwent major liver resection while 45 underwent minor resection. Additional procedures were carried out in 10 patients (Table 1). Eight patients had surgical complications, including biliary leakage in 3 cases, subphrenic infection in 2 cases, liver abscess in 1 case, bowel obstruction in 1 case, and bleeding in 1 case.
Table 1.
Operative procedures for intrahepatic cholangiocarcinoma
| Operative modality | Number |
| Hepatic resection (n = 85) | |
| Major resection (n = 40) | |
| Partial hepatectomy | 24 |
| Right trisectionectomy | 1 |
| Left trisectionectomy | 4 |
| Right hemihepatectomy | 1 |
| Left hemihepatectomy | 8 |
| Central bisectionectomy | 2 |
| Minor resection (n = 45) | |
| Partial hepatectomy | 33 |
| Right anterior sectionectomy | 2 |
| Right posterior sectionectomy | 3 |
| Left lateral sectionectomy | 4 |
| Bisegmentectomy | 3 |
| Combined resection (n = 10) | |
| Spleen | 2 |
| Gallbladder | 8 |
Survival and recurrence
The cumulative 1-, 3-, and 5-year survival rates were 60%, 18%, and 13%, respectively (Figure 1). The median survival time was 25 mo. Univariate analysis revealed that the following prognostic factors were predictive of worse survival: liver cirrhosis (P = 0.002), tumor number (P < 0.001), tumor size (P = 0.006), lymph node metastasis (P = 0.005), vascular invasion (P = 0.013), and TNM classification (P < 0.001; Table 2). Age, gender, and other tumor-related factors were not significantly related with survival. Through multivariate analysis by means of Cox’s proportional hazards model, liver cirrhosis (HR = 1.875, 95%CI: 1.197-3.278, P = 0.008) and multiple tumors (HR = 2.653, 95%CI: 1.562-4.508, P < 0.001) were identified as independent prognostic factors (Table 2).
Figure 1.
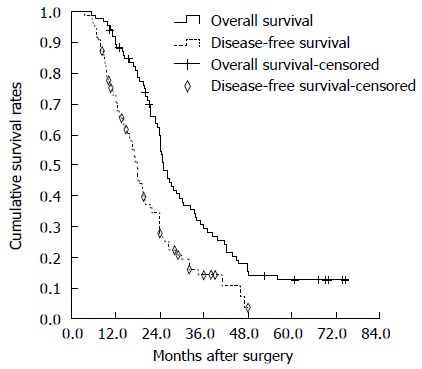
Overall survival and disease-free survival curves of the 85 hepatitis B virus-associated intrahepatic cholangiocarcinoma patients after curative resection. The median overall survival was 25 mo. The median disease-free survival was 17.9 mo.
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival of hepatitis B virus-associated intrahepatic cholangiocarcinoma patients included in this study
| Variable | n |
Univariate analysis |
Multivariateanalyses |
||||
| 1-yr | 5-yr | P value | HR | 95%CI | P value | ||
| Gender | 0.205 | NA | NA | NA | |||
| Female | 19 | 66% | 23% | ||||
| Male | 66 | 58% | 10% | ||||
| Age | 0.769 | NA | NA | NA | |||
| ≤ 60 | 59 | 64% | 13% | ||||
| > 60 | 26 | 50% | 12% | ||||
| Capsule formation | 0.522 | NA | NA | NA | |||
| Yes | 32 | 55% | 10% | ||||
| No | 53 | 63% | 14% | ||||
| Cirrhosis | 0.002 | 1.981 | 1.197-3.278 | 0.008 | |||
| Yes | 48 | 56% | 0% | ||||
| No | 37 | 65% | 27% | ||||
| Child-Pugh class | 0.257 | NA | NA | NA | |||
| A | 76 | 61% | 14% | ||||
| B | 9 | 18% | 0% | ||||
| AFP (ng/mL) | 0.342 | NA | NA | NA | |||
| ≤ 20 | 48 | 51% | 11% | ||||
| > 20 | 37 | 72% | 15% | ||||
| CA19-9 (U/mL) | 0.342 | NA | NA | NA | |||
| ≤ 37 | 55 | 66% | 14% | ||||
| > 37 | 30 | 50% | 10% | ||||
| Gross type | 0.402 | NA | NA | NA | |||
| MF | 63 | 61% | 13% | ||||
| PI + IG | 22 | 56% | 11% | ||||
| Differentiation | 0.119 | NA | NA | NA | |||
| Well or Moderate | 63 | 64% | 16% | ||||
| Poor | 22 | 50% | 0% | ||||
| Tumor number | < 0.001 | 2.653 | 1.562-4.508 | < 0.001 | |||
| Single | 60 | 69% | 17% | ||||
| Multiple | 25 | 38% | 0% | ||||
| Tumor size | 0.006 | NA | NA | NA | |||
| < 5 cm | 43 | 70% | 21% | ||||
| ≥ 5 cm | 42 | 49% | 5% | ||||
| LN metastasis | 0.005 | NA | NA | NA | |||
| Yes | 11 | 31% | 0% | ||||
| No | 74 | 64% | 14% | ||||
| Vascular invasion | 0.013 | NA | NA | NA | |||
| Yes | 11 | 24% | 0% | ||||
| No | 74 | 64% | 14% | ||||
| TNM classification | < 0.001 | NA | NA | NA | |||
| Stage I or II | 61 | 66% | 17% | ||||
| Stage III or IV | 24 | 43% | 0% | ||||
| Width of resection margin | 0.274 | NA | NA | NA | |||
| < 1 cm | 37 | 69% | 17% | ||||
| ≥ 1 cm | 48 | 53% | 9% | ||||
| Surgical procedure | 0.962 | NA | NA | NA | |||
| Major hepatectomy | 40 | 61% | 13% | ||||
| Minor hepatectomy | 45 | 59% | 12% | ||||
AFP: Alpha-fetoprotein; CA19-9: Carbohydrate antigen 19-9; MF: Mass-forming; PI: Periductal infiltrating; IG: Intraductal growth; TNM: Tumor-node-metastasis; NA: Not available; HR: Hazard ratio; LN: Lymph node.
Seventy patients had recurrence (82.4%). The 1-, 3-, and 5-year DFS rates were 36%, 3% and 0%, respectively. (Figure 1). The sites of recurrence are shown in Table 3. The most common recurrence pattern was intrahepatic. Two patients (2.9%) with recurrence underwent further liver resection, while 48 patients (68.6%) underwent post-recurrence TACE. Other measures taken against recurrence included radiotherapy (n = 11), systemic chemotherapy (n = 2), and supportive therapy or Chinese herbal therapy (n = 7) in patients with liver dysfunction. Univariate analysis revealed that liver cirrhosis (P = 0.003), tumor number (P < 0.001), lymph node metastasis (P < 0.001), vascular invasion (P < 0.001), and TNM classification (P < 0.001) were significant risk factors for recurrence (Table 4). Multivariate analysis revealed that liver cirrhosis (HR = 1.919, P = 0.012), advanced TNM stage (stage III or IV) (HR = 2.027, P < 0.001), and vascular invasion (HR = 3.779, P = 0.02) were independent prognostic factors affecting DFS (Table 4).
Table 3.
Sites of initial recurrences in hepatitis B virus-associated intrahepatic cholangiocarcinoma patients after curative resection
| Site of initial recurrence | No. of patients |
| Liver, lymph nodes | 6 |
| Liver, lung | 1 |
| Liver | 46 |
| Lymph nodes | 8 |
| Peritoneum | 5 |
| Wound site | 1 |
| Bone | 1 |
| Lung | 2 |
Table 4.
Univariate and multivariate analyses of prognostic factors for disease-free survival in hepatitis B virus-associated intrahepatic cholangiocarcinoma patients included in this study
| Variable | n |
Univariate analysis |
Multivariateanalyses |
||||
| 1-yr | 5-yr | P value | HR | 95%CI | P value | ||
| Gender | 0.312 | NA | NA | NA | |||
| Female | 19 | 50% | 0% | ||||
| Male | 66 | 32% | 4% | ||||
| Age | 0.853 | NA | NA | NA | |||
| ≤ 60 | 59 | 33% | 1% | ||||
| > 60 | 26 | 42% | 5% | ||||
| Capsule formation | 0.970 | NA | NA | NA | |||
| Yes | 32 | 40% | 7% | ||||
| No | 53 | 33% | 0% | ||||
| Cirrhosis | 0.003 | 1.919 | 1.153-3.192 | 0.012 | |||
| Yes | 48 | 22% | 0% | ||||
| No | 37 | 52% | 5% | ||||
| Child-Pugh class | 0.237 | NA | NA | NA | |||
| A | 76 | 50% | 20% | ||||
| B | 9 | 27% | 0% | ||||
| AFP (ng/mL) | 0.648 | NA | NA | NA | |||
| ≤ 20 | 48 | 32% | 3% | ||||
| > 20 | 37 | 40% | 2% | ||||
| CA19-9 (U/mL) | 0.189 | NA | NA | NA | |||
| ≤ 37 | 55 | 39% | 3% | ||||
| > 37 | 30 | 29% | 2% | ||||
| Gross type | 0.917 | NA | NA | NA | |||
| MF | 63 | 37% | 3% | ||||
| PI + IG | 22 | 30% | 0% | ||||
| Differentiation | 0.076 | NA | NA | NA | |||
| Well or Moderate | 63 | 42% | 3% | ||||
| Poor | 22 | 18% | 0% | ||||
| Tumor number | < 0.001 | NA | NA | NA | |||
| Single | 60 | 48% | 3% | ||||
| Multiple | 25 | 5% | 0% | ||||
| Tumor size | 0.091 | NA | NA | NA | |||
| < 5 cm | 43 | 44% | 1% | ||||
| ≥ 5 cm | 42 | 27% | 5% | ||||
| LN metastasis | < 0.001 | NA | NA | NA | |||
| Yes | 11 | 0% | 0% | ||||
| No | 74 | 40% | 3% | ||||
| Vascular invasion | 3.779 | 1.601-8.923 | 0.020 | ||||
| Yes | 11 | ||||||
| No | 74 | ||||||
| TNM classification | < 0.001 | NA | NA | NA | |||
| Stage I or II | 61 | 0% | 0% | ||||
| Stage III or IV | 24 | 51% | 20% | ||||
| Width of resection margin | < 0.001 | NA | NA | NA | |||
| < 1 cm | 37 | 49% | 4% | ||||
| ≥ 1 cm | 48 | 0% | 0% | ||||
| Surgical procedure | 0.391 | NA | NA | NA | |||
| Major hepatectomy | 40 | 41% | 2% | ||||
| Minor hepatectomy | 45 | 31% | 3% | ||||
AFP: Alpha-fetoprotein; CA19-9: Carbohydrate antigen 19-9; MF: Mass-forming; PI: Periductal infiltrating; IG: Intraductal growth; TNM: Tumor-node-metastasis; NA: Not available; HR: Hazard ratio; LN: Lymph node.
Influence of LND on survival
The pathological characteristics associated with the extent of lymphadenectomy are as shown in Table 5. The LND (-) group did not demonstrate lymph node metastases as revealed by preoperative imaging (CT and MRI) and intraoperative assessment. No survival difference was recorded between the LND (-) group and the group of patients without lymph node metastasis [LN (-) group] (P =0.729). Among patients who underwent lymph node sampling or dissection, the LN (-) group showed better survival than the group of patients with lymph node metastasis [LN (+) group] (P = 0.033) (Figure 2). No survival difference was recorded between the lymph node sampling and regional LND groups (P = 0.089) (Figure 3). Among the patients with negative lymph node status, a survival difference was not present between the lymph node sampling group and regional LND group (P = 0.182). Therefore, lymphadenectomy did not significantly improve the survival rate in node negative patients (Figure 4).
Table 5.
Comparison of the pathological factors according to the extent of lymph node dissection
| Variable | LND (-) | LN sampling | Regional LND | P value |
| Cirrhosis | 0.439 | |||
| Yes | 17 | 13 | 18 | |
| No | 18 | 9 | 10 | |
| Gross type | 0.617 | |||
| MF | 24 | 17 | 22 | |
| PI + IG | 11 | 5 | 6 | |
| Differentiation | 0.478 | |||
| Well or Moderate | 25 | 15 | 23 | |
| Poor | 10 | 7 | 5 | |
| Capsule formation | 0.931 | |||
| Yes | 14 | 8 | 10 | |
| No | 21 | 14 | 18 | |
| Tumor number | 0.512 | |||
| Single | 27 | 14 | 18 | |
| Multiple | 8 | 8 | 10 | |
| Tumor size | 0.145 | |||
| < 5 cm | 21 | 12 | 10 | |
| ≥ 5 cm | 14 | 10 | 18 | |
| Pathologica LN metastasis | 0.085 | |||
| Yes | NA | 2 | 9 | |
| No | NA | 20 | 19 | |
| Vascular invasion | 0.181 | |||
| Yes | 2 | 3 | 6 | |
| No | 33 | 19 | 22 |
MF: Mass-forming; PI: Periductal infiltrating; IG: Intraductal growth; LN: Lymph node; LND: Lymph node dissection; NA: Not available.
Figure 2.
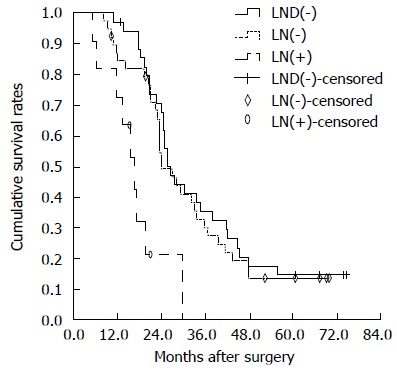
Overall survival curves of patients in lymph node dissection (-), lymph node (-) and lymph node (+) groups. There was no statistically significant difference in OS between the lymph node dissection (LND) (-) and LN (-) groups (P = 0.729). There were significant differences between LN (-) and LN (+) groups (P = 0.033). A statistically significant difference between LND (-) and LN (+) groups was observed (P = 0.004). LND: Lymph node dissection; LN: Lymph node.
Figure 3.
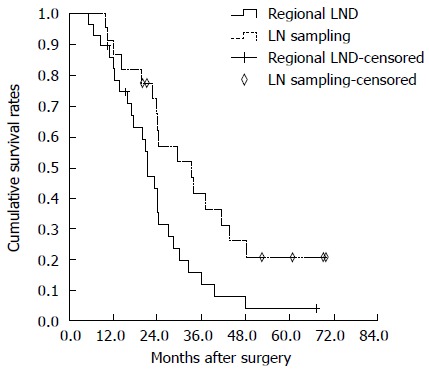
Overall survival curves of hepatitis B virus-associated intrahepatic cholangiocarcinoma patients undergoing sampling lymph node sampling and regional lymph node dissection. There were no significant differences between the two groups (P = 0.089). LND: Lymph node dissection; LN: Lymph node.
Figure 4.
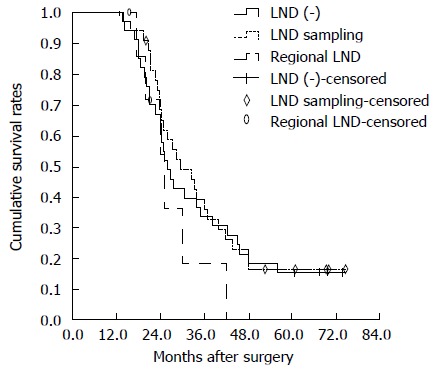
Overall survival curves of hepatitis B virus-associated intrahepatic cholangiocarcinoma patients without lymph node involvement. There was no survival difference between lymph node sampling and regional LND groups (P = 0.182). There was no statistically significant difference in overall survival between LND (-) and lymph node sampling groups (P = 0.709). There were also no significant differences between LND (-) and regional LND groups (P = 0.309). LND: Lymph node dissection.
DISCUSSION
We analyzed a group of 85 patients who underwent potentially curative surgery for HBV-associated ICC. The cumulative 1-, 3-, and 5-year survival rates were 60%, 18%, and 13%, respectively. Several clinicopathological factors that were suspected to significantly influence survival and recurrence were examined. In some studies, vascular invasion, lymph node metastasis, microscopic presence of tumor cells on the resection margin, multiple tumors, and tumor size were found to be significant prognostic factors for survival[24-30]. In this study, univariate analysis showed that liver cirrhosis, tumor number, tumor size, lymph node metastasis, vascular invasion, and TNM classification were significant prognostic factors for survival. Multivariate analysis showed that liver cirrhosis and multiple tumors were independent prognostic factors for survival.
The recurrence rate was reported to be 61%-65%[24,25,28,29]. The most common recurrence site was the remnant liver, as reported in other studies [24,25,28]. In this study, liver cirrhosis, vascular invasion, and advanced TNM stage (stage III or IV) were independent prognostic factors for DFS.
The presence of liver cirrhosis was obviously more common in HBV-associated ICC. In this study, there were 48 patients (56.5%) with liver cirrhosis. Cirrhosis was an independent prognostic factor for HCC. However, there is little documentation showing that this is associated with ICC. We have demonstrated that cirrhosis not only is an independent prognostic factor for DFS, but also is significantly related to poor survival of the patients with HBV-associated ICC after surgery. It may be due to the fact that HBV-associated ICC shares common disease processes for carcinogenesis with HCC.
Lymph node metastasis was found to be an important prognostic factor in previous studies[27,30]. It was associated with OS and DFS on univariate analysis, but not on multivariate analysis in this study, probably because the sample size was relatively small.
It remains unclear whether the clearance of the route of lymph node metastasis is beneficial to survival[24]. There are no clear guidelines on LND. It is reported that extended lymphadenectomy in ICC patients did not seem to show any advantage without control of liver metastases, because most recurrences occurred in patients with known liver metastases[17,24]. However, other reports indicated that extensive hepatectomy with extended LND produced a better outcome[31,32]. Defining the role of lymphadenectomy is important, because lymphadenectomy is a factor controllable by a surgeon out of numerous prognostic factors. The present study has demonstrated that regional LND group has a survival rate similar to that of the lymph node sampling group. The extent of lymphadenectomy (lymph node sampling vs regional LND) does not affect the survival. The patients without LND have similar survival to patients with LND or negative lymph node status. It is revealed that lymphadenectomy does not significantly improve the survival of patients with negative lymph node status.
Nevertheless, this study was limited by its retrospective design. More than five surgeons were involved in treating ICC in this study. It was difficult to determine the exact reasons and extent of LND performed for each patient.
In conclusion, liver cirrhosis and multiple tumors are independent prognostic factors for survival in patients with HBV-associated ICC. The extent of LND does not seem to have influence on the survival of patients with HBV-associated ICC. LND cannot significantly improve the survival of patients with negative lymph node status. We recommend intraoperative lymph node exploration, as opposed to routine regional LND. Regional LND should be performed when lymph node involvement is clinically recognized or it is positive as demonstrated by a frozen section examination.
COMMENTS
Background
Some recently published studies show a relation between chronic hepatitis B infection and the development of intrahepatic cholangiocarcinoma. Patients with hepatitis B-associated cholangiocarcinoma appear to have different clinicopathological characteristics compared with seronegative patients.
Research frontiers
Hepatitis B virus (HBV)-associated intrahepatic cholangiocarcinoma (ICC) have different clinicopathological characteristics and surgical outcomes, and should be distinguished from those without HBV infection. Because of the rarity and low resectability of HBV-associated ICC, prognostic factors after resection for HBV-associated ICC have not been clearly established. The influences of the extent of lymph node dissection (LND) on survival were also not been clearly established.
Innovations and breakthroughs
This study was conducted to investigate the outcomes of HBV-associated ICC patients after hepatic resection, and analyzed the prognostic factors affecting the survival and recurrence. The impact of the extent of LND on survival was also investigated.
Applications
Liver cirrhosis and multiple tumors are independent prognostic factors for survival in patients with HBV-associated ICC. The extent of lymph node dissection does not seem to have influence on the survival of patients with HBV-associated ICC. Lymphadenectomy cannot significantly improve the survival of lymph node negative patients.
Peer review
The reported data are interesting. Some recently published studies show a relation between chronic hepatitis B infection and the development of intrahepatic cholangiocarcinoma. Patients with hepatitis B-associated cholangiocarcinoma appear to have different clinicopathological characteristics compared with seronegative patients.
Footnotes
Supported by National Natural Science Foundation of China, No. 81402523.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 14, 2014
First decision: June 18, 2014
Article in press: September 19, 2014
P- Reviewer: Bo J, Chiang TA, Shaikh S S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Casavilla FA, Marsh JW, Iwatsuki S, Todo S, Lee RG, Madariaga JR, Pinna A, Dvorchik I, Fung JJ, Starzl TE. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429–436. doi: 10.1016/s1072-7515(97)00088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 3.Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059–1067. doi: 10.1097/PAS.0b013e31802b34b6. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 6.Hai S, Kubo S, Yamamoto S, Uenishi T, Tanaka H, Shuto T, Takemura S, Yamazaki O, Hirohashi K. Clinicopathologic characteristics of hepatitis C virus-associated intrahepatic cholangiocarcinoma. Dig Surg. 2005;22:432–439. doi: 10.1159/000091446. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765–1770. doi: 10.1038/sj.bjc.6605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Wang H, Zhou D, Wang H, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Bhagat V, Javle M, Yu J, Agrawal A, Gibbs JF, Kuvshinoff B, Nava E, Iyer R. Combined hepatocholangiocarcinoma: case-series and review of literature. Int J Gastrointest Cancer. 2006;37:27–34. doi: 10.1385/IJGC:37:1:27. [DOI] [PubMed] [Google Scholar]
- 10.Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–125. doi: 10.1016/j.amjsurg.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 12.Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao KY, Chen XG, Yang YF, Su ZX, Chen B, et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol. 2011;18:1258–1266. doi: 10.1245/s10434-010-1458-5. [DOI] [PubMed] [Google Scholar]
- 13.Wu ZF, Yang N, Li DY, Zhang HB, Yang GS. Characteristics of intrahepatic cholangiocarcinoma in patients with hepatitis B virus infection: clinicopathologic study of resected tumours. J Viral Hepat. 2013;20:306–310. doi: 10.1111/jvh.12005. [DOI] [PubMed] [Google Scholar]
- 14.Zhou HB, Wang H, Li YQ, Li SX, Wang H, Zhou DX, Tu QQ, Wang Q, Zou SS, Wu MC, et al. Hepatitis B virus infection: a favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World J Gastroenterol. 2011;17:1292–1303. doi: 10.3748/wjg.v17.i10.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou XD, Tang ZY, Fan J, Zhou J, Wu ZQ, Qin LX, Ma ZC, Sun HC, Qiu SJ, Yu Y, et al. Intrahepatic cholangiocarcinoma: report of 272 patients compared with 5,829 patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1073–1080. doi: 10.1007/s00432-009-0547-y. [DOI] [PubMed] [Google Scholar]
- 16.Tang D, Nagano H, Nakamura M, Wada H, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Dono K, Monden M. Clinical and pathological features of Allen’s type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg. 2006;10:987–998. doi: 10.1016/j.gassur.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Takasaki K, Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999;29:147–150. doi: 10.1093/jjco/29.3.147. [DOI] [PubMed] [Google Scholar]
- 19.Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Hamba H, Tanaka H, Kinoshita H. Histologic factors affecting prognosis following hepatectomy for intrahepatic cholangiocarcinoma. World J Surg. 2001;25:865–869. doi: 10.1007/s00268-001-0042-3. [DOI] [PubMed] [Google Scholar]
- 20.Vitale F, Tramuto F, Orlando A, Vizzini G, Meli V, Cerame G, Mazzucco W, Virdone R, Palazzo U, Villafrate MR, et al. Can the serological status of anti-HBc alone be considered a sentinel marker for detection of occult HBV infection? J Med Virol. 2008;80:577–582. doi: 10.1002/jmv.21121. [DOI] [PubMed] [Google Scholar]
- 21.Shiota G, Oyama K, Udagawa A, Tanaka K, Nomi T, Kitamura A, Tsutsumi A, Noguchi N, Takano Y, Yashima K, et al. Occult hepatitis B virus infection in HBs antigen-negative hepatocellular carcinoma in a Japanese population: involvement of HBx and p53. J Med Virol. 2000;62:151–158. doi: 10.1002/1096-9071(200010)62:2<151::aid-jmv5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Yang T, Zhang J, Lu JH, Yang LQ, Yang GS, Wu MC, Yu WF. A new staging system for resectable hepatocellular carcinoma: comparison with six existing staging systems in a large Chinese cohort. J Cancer Res Clin Oncol. 2011;137:739–750. doi: 10.1007/s00432-010-0935-3. [DOI] [PubMed] [Google Scholar]
- 23.Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y, Yang GS. Postoperative adjuvant transcatheter arterial chemoembolisation improves survival of intrahepatic cholangiocarcinoma patients with poor prognostic factors: results of a large monocentric series. Eur J Surg Oncol. 2012;38:602–610. doi: 10.1016/j.ejso.2012.02.185. [DOI] [PubMed] [Google Scholar]
- 24.Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, Chung JB. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–1531. doi: 10.1046/j.1365-2168.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–733. doi: 10.1007/s00268-005-7761-9. [DOI] [PubMed] [Google Scholar]
- 27.Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol. 2007;96:160–165. doi: 10.1002/jso.20792. [DOI] [PubMed] [Google Scholar]
- 28.Nakagohri T, Asano T, Kinoshita H, Kenmochi T, Urashima T, Miura F, Ochiai T. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–293. doi: 10.1007/s00268-002-6696-7. [DOI] [PubMed] [Google Scholar]
- 29.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 30.Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004;91:99–104. doi: 10.1002/bjs.4366. [DOI] [PubMed] [Google Scholar]
- 31.Asakura H, Ohtsuka M, Ito H, Kimura F, Ambiru S, Shimizu H, Togawa A, Yoshidome H, Kato A, Miyazaki M. Long-term survival after extended surgical resection of intrahepatic cholangiocarcinoma with extensive lymph node metastasis. Hepatogastroenterology. 2005;52:722–724. [PubMed] [Google Scholar]
- 32.Uenishi T, Yamazaki O, Horii K, Yamamoto T, Kubo S. A long-term survivor of intrahepatic cholangiocarcinoma with paraaortic lymph node metastasis. J Gastroenterol. 2006;41:391–392. doi: 10.1007/s00535-006-1757-6. [DOI] [PubMed] [Google Scholar]


