Abstract
Objectives
This study aimed to compare pancreaticojejunostomy (PJ) with pancreaticogastrostomy (PG) after pancreaticoduodenectomy (PD).
Methods
A literature search of PubMed and the Cochrane Central Register of Controlled Trials for studies comparing PJ with PG after PD was conducted. The primary outcome for meta-analysis was pancreatic fistula. Secondary outcomes were morbidity, mortality, biliary fistula, intra-abdominal fluid collection, hospital length of stay (LoS), postoperative haemorrhage and reoperation. Outcome measures were odds ratios (ORs) and mean differences with 95% confidence intervals (CIs).
Results
Seven recent RCTs encompassing 1121 patients (559 PJ and 562 PG cases) were involved in this meta-analysis. Incidences of pancreatic fistula (10.6% versus 18.5%; OR 0.52, 95% CI 0.37–0.74; P = 0.0002), biliary fistula (2.3% versus 5.7%; OR 0.42, 95% CI 0.03–3.15; P = 0.03) and intra-abdominal fluid collection (8.0% versus 14.7%; OR 0.50, 95% CI 0.34–0.74; P = 0.0005) were significantly lower in the PG than the PJ group, as was hospital LoS (weighted mean difference: −1.85, 95% CI −3.23 to −0.47; P = 0.008). Subgroup analysis indicated that severe pancreatic fistula (grades B or C) occurred less frequently in the PG than the PJ group (8.3% versus 20.5%; OR 0.37, 95% CI 0.23–0.59; P < 0.00001). However, there was no significant difference in morbidity (48.9% versus 51.0%; OR 0.90, 95% CI 0.70–1.16; P = 0.41), mortality (3.2% versus 3.5%; OR 0.82, 95% CI 0.43–1.58; P = 0.56), delayed gastric emptying (16.6% versus 14.7%; relative risk: 1.02, 95% CI 0.62–1.68; P = 0.94), postoperative haemorrhage (9.6% versus 11.1%; OR 0.82, 95% CI 0.54–1.24; P = 0.35) or reoperation (9.9% versus 9.8%; OR 0.93, 95% CI 0.60–1.43; P = 0.73).
Conclusions
Pancreaticogastrostomy provides benefits over PJ after PD, including in the incidences of pancreatic fistula, biliary fistula and intra-abdominal fluid collection and in hospital LoS. Therefore, PG is recommended as a safer and more reasonable alternative to PJ reconstruction after PD.
Introduction
Pancreaticoduodenectomy (PD) is a standard surgical treatment for patients with malignant or benign disease of the pancreatic head and the periampullary region. There has been an obvious decline in perimortality rates (to <5%) after PD carried out at high-volume institutions and PD may perhaps represent the most striking accomplishment in pancreatic surgery to date.1,2 However, rates of surgical morbidity lie at 40–50% in many large specialized centres.3–5 The development of a postoperative pancreatic fistula remains the main factor influencing morbidity and even mortality.6,7 Therefore, pancreatic anastomotic leak following PD remains an obstacle for surgeons and intensive medicine specialists. In the context of such challenging circumstances, many techniques have been proposed to guarantee continuity between the pancreatic remnant and the digestive tract.8–10 However, the issue of which technique is optimal to restore pancreatic digestive continuity remains controversial.
Four prospective randomized controlled trials (RCTs) and three meta-analyses have demonstrated similar rates of pancreatic fistula in pancreaticojejunostomy (PJ) and pancreaticogastrostomy (PG) after PD.11–17 However, three recent RCTs reported lower rates of pancreatic fistula in association with PG (3.8%, 15.3% and 8.0%, respectively) than PJ (18.2%, 34.5% and 19.8%, respectively).18–20 Almost all retrospective studies comparing PG and PJ have suggested that the rate of pancreatic fistula is reduced in PG. These recent RCTs and retrospective studies may suggest that PG is more efficient than PJ in reducing the incidence of pancreatic fistula after PD.11 Hence, in order to evaluate and compare the results of PG and PJ after PD, a meta-analysis of RCTs published between 1992 and 2013 was performed. The primary outcome of interest was the rate of pancreatic fistula.
Materials and methods
Literature search
A thorough literature search was performed using PubMed and the Cochrane Central Register of Controlled Trials for potentially relevant RCTs comparing outcomes in, respectively, PJ and PG, conducted between January 1992 and December 2013. The search was performed independently by two authors (J-MC and WG) using the keywords: ‘pancreatogastrostomy’; ‘pancreaticogastrostomy’; ‘pancreaticogastric anastomosis’; ‘pancreatojejunostomy’; ‘pancreaticojejunostomy’; ‘pancreatijcojejunal anastomosis’; ‘pancreatoenteric anastomosis’; ‘pancreatoduodenectomy’, and ‘pancreatoduodenal resection’. References within the articles were also analysed for other relevant studies.
Inclusion and exclusion criteria
The goal of the meta-analysis was to evaluate surgical and functional outcomes in cohorts of patients submitted to PJ or PG after PD. Studies were required to meet the following criteria: (i) to be published in English; (ii) to compare PJ with PG; (iii) to use an RCT design; (iv) to report at least one outcome, and (v) to clearly document the surgical procedure. If two studies were found to overlap, the higher-quality publication was selected. Articles were excluded if they failed to fulfil any of these criteria.
Data extraction and quality assessment
Once studies had been identified as qualifying for inclusion in the meta-analysis, data extraction and critical appraisal were undertaken independently by two authors (F-BL and WG). Any disagreements were discussed and resolved by consensus.
The primary outcome measure was the occurrence of pancreatic fistula. Secondary outcomes were mortality, morbidity, occurrence of biliary fistula, occurrence of delayed gastric emptying, occurrence of intra-abdominal collection, reoperation rate, occurrence of postoperative haemorrhage and hospital length of stay (LoS).
Statistical analysis
All statistical analyses were carried out using RevMan Version 5.1 (Cochrane Collaboration, Oxford, UK). Continuous data (e.g. hospital LoS) were analysed using the weighted mean difference (WMD) or standard mean difference (SMD) with 95% confidence intervals (CIs). Dichotomous data were analysed using the odds ratio (OR) and a fixed-effects model.21 When significant heterogeneity existed, data were analysed using a random-effects model.22 Heterogeneity was evaluated using the chi-squared test, in which a P-value of <0.1 was considered significant. Differences resulting in a P-value of <0.05 were considered statistically significant. Risk for bias was evaluated using a funnel plot. The quality of all studies was evaluated using the scoring systems of Jadad et al.23 and Chalmers et al.24
Results
Results of the search
The literature search strategy and trial selection are shown in Fig. 1. In total, seven RCTs14–20 were selected for inclusion in this meta-analysis. A total of 1121 patients had been randomized to either PJ (559 patients) or PG (562 patients). The sample sizes of trials ranged from 116 to 329. The main characteristics of the seven RCTs are shown in Tables 1 and 2. Patient characteristics and preoperative parameters were similar in the two groups.
Figure 1.
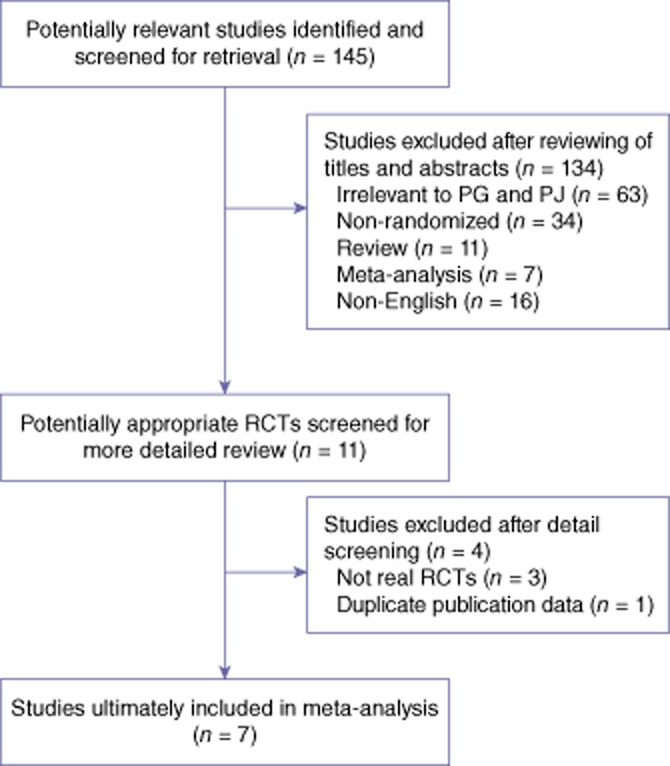
Flowchart of literature search. RCT, randomized controlled trial
Table 1.
Characteristics of seven randomized controlled trials comparing outcomes of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) after pancreaticoduodenectomy
| Study | Sample size | Male patients, n | Age, years, mean ± SD | Soft parenchyma, % | PPPD, % | Setting |
|---|---|---|---|---|---|---|
| Bassi et al. (2005)14 | PG 69 | 44 | 59.3 | 100% | 95.7% | Single centre |
| PJ 82 | 51 | 55.5 | 100% | 85.4% | ||
| Duffas et al. (2005)15 | PG 81 | 51 | 58.2 ± 11 | 60.5% | 22% | Multicentre |
| PJ 68 | 35 | 58.6 ± 12 | 60.3% | 26% | ||
| Fernandez-Cruz et al. (2008)17 | PG 53 | 29 | 63 ± 13 | 45.3% | 100% | Single centre |
| PJ 55 | 38 | 63 ± 14 | 45.5% | 100% | ||
| Figueras et al. (2013)18 | PG 65 | 44 | 67 | 52.3% | 46.2% | Two centres |
| PJ 58 | 37 | 65.5 | 56.9% | 48.3% | ||
| Topal et al. (2013)19 | PG 162 | 100 | 67.0 | – | 60.5% | Multicentre |
| PJ 167 | 91 | 66.1 | – | 61.1% | ||
| Wellner et al. (2012)20 | PG 59 | 27 | 67 | 59.3% | 88.1% | Single centre |
| PJ 57 | 29 | 64 | 50.8% | 94.5% | ||
| Yeo et al. (1995)16 | PG 73 | 33 | 61.5 ± 1.7 | 21.9% | 82.2% | Single centre |
| PJ 72 | 40 | 62.4 ± 1.4 | 23.6% | 81.9% |
SD, standard deviation; PPPD, pylorus-preserving pancreaticoduodenectomy.
Table 2.
Characteristics of seven randomized controlled trials comparing outcomes of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) after pancreaticoduodenectomy
| Study | Sample size, n | Patients with diseases, n |
Surgical techniques | ||||
|---|---|---|---|---|---|---|---|
| PDAC | DD | Amp | DBD | Others | |||
| Bassi et al. (2005)14 | PG 69 | 32 | 1 | 13 | 1 | 22 | Single-layer non-absorbable interrupted stitches |
| PJ 82 | 28 | 1 | 11 | 2 | 40 | Single-layer duct to mucosa or side-to-side | |
| Duffas et al. (2005)15 | PG 81 | 34 | 3 | 17 | 8 | 19 | Not described |
| PJ 68 | 25 | 3 | 19 | 11 | 10 | End-to-end or end to side | |
| Fernandez-Cruz et al. (2008)17 | PG 53 | 26 | 1 | 12 | 8 | 6 | Duct-to-mucosa with gastric partition |
| PJ 55 | 28 | 1 | 10 | 7 | 9 | End-to-side duct mucosa | |
| Figueras et al. (2013)18 | PG 65 | 33 | 6 | 8 | 8 | 10 | Two-layer invagination |
| PJ 58 | 29 | 10 | 7 | 3 | 9 | Duct-to-mucosa | |
| Topal et al. (2013)19 | PG 162 | 98 | 11 | 23 | 28 | 2 | End-to-side telescope |
| PJ 167 | 107 | 14 | 28 | 15 | 3 | End-to-side telescope | |
| Wellner et al. (2012)20 | PG 59 | 26 | 3 | 9 | 2 | 19 | Invagination |
| PJ 57 | 30 | 2 | 7 | 2 | 16 | Duct-to-mucosa | |
| Yeo et al. (1995)16 | PG 73 | 40 | 4 | 7 | 6 | 16 | Two-layer, end-to-end |
| PJ 72 | 40 | 5 | 11 | 7 | 9 | Two-layer, end-to-end or end-to-side | |
PDAC, pancreatic ductal adenocarcinoma; DD, duodenal carcinoma; Amp, ampullary carcinoma; DBD, distal bile duct cancer; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy.
According to Jadad et al.23 and Chalmers et al.,24 two of the trials included were of low quality as a result of their use of inadequate randomization methods and an absence of blinding, power calculations and intention-to-treat analysis. Five trials were scored as being of moderate strength as a result of their use of relatively good randomization techniques and the presence of blinding, power calculations and intention-to-treat analysis.
Definitions of pancreatic fistula and its severity were extracted from the International Study Group on Pancreatic Fistula (ISGPF) in four RCTs.17–20 The other three RCTs applied centre-specific definitions of pancreatic fistula.14–16 Based on ISGPF criteria, pancreatic fistula was defined according to an increased level of amylase in the effluent drain three times higher than the normal serum amylase level. These data were further classified to indicate fistula of grade A (transient, without clinical impact), grade B (abnormal laboratory parameters with a non-invasive change in therapeutic management), and grade C (abnormal laboratory parameters that require invasive treatment and are associated with sepsis or death), respectively.25
Biliary fistula was diagnosed according to bilirubin containing discharge in a distinctive colour or fistulography.14,15,18
Risk for publication bias
A funnel plot analysis (Fig. 2) was applied to assess the possibility of publication bias; findings showed a non-significant likelihood.
Figure 2.
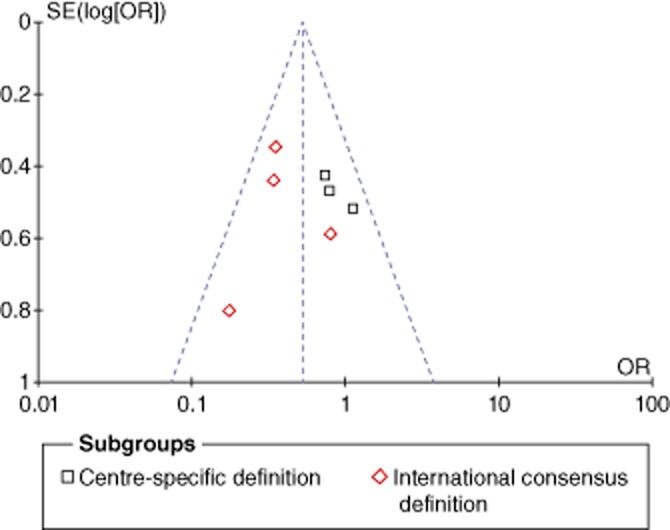
Funnel plot analysis showing no publication bias for the occurrence of pancreatic fistula. SE, standard error; OR, odds ratio
Primary outcome
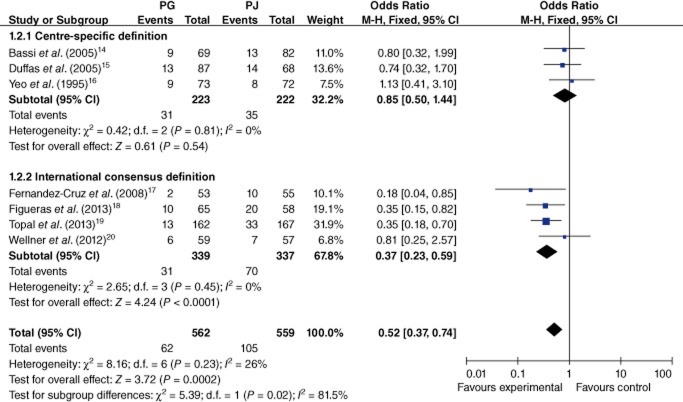
The meta-analysis of the primary outcome investigated the occurrence of pancreatic fistula in all of the included RCTs (14.5%). The raw incidence of pancreatic fistula was significantly lower in the PG than the PJ group (10.6% versus 18.5%; OR 0.52, 95% CI 0.37–0.74; P = 0.0002) (Fig. 3). Subgroup analysis indicated that severe pancreatic fistula defined according to the international consensus definition (grades B or C) was less likely to occur in association with PG than PJ (8.3% versus 20.5%; OR 0.37, 95% CI 0.23–0.59; P < 0.00001) (Fig. 3).
Figure 3.
Comparison of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) in terms of pancreatic fistula. 95% CI, 95% confidence interval; M-H, Mantel–Haenszel method
Secondary outcomes
Morbidity
Six trials provided specific information about total complications.14–19 There was no significant heterogeneity among these trials (I2 = 30%; P = 0.21). Meta-analysis showed that the level of risk for the development of any postoperative complication was statistically similar in both groups (48.9% versus 51.0%; OR 0.90, 95% CI 0.70–1.16; P = 0.41) (Fig. 4).
Figure 4.
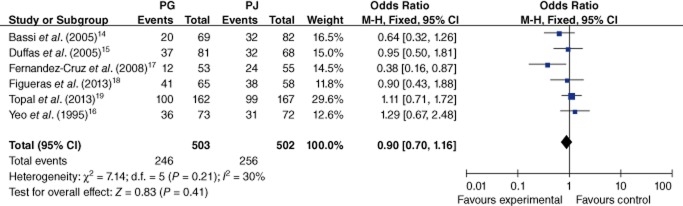
Comparison of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) in terms of morbidity. 95% CI, 95% confidence interval; M-H, Mantel–Haenszel method
Mortality
Mortality was compared across all studies.14–20 Mortality rates in the PG and PJ groups were 3.2% (18 of 562 patients) and 3.6% (20 of 559 patients), respectively. There was no significant difference in mortality between the two groups (OR 0.82, 95% CI 0.43–1.58; P = 0.56). There was no evidence of heterogeneity among studies (I2 = 0%; P = 0.84).
Biliary fistula
Five articles covering a total of 676 patients reported data on the occurrence of biliary fistula (4.0%).14–18 The occurrence of biliary fistula was significantly reduced in the PG group in comparison with the PJ group (2.3% versus 5.7%; OR 0.42, 95% CI 0.03–3.15; P = 0.03) (Fig. 5). There was no statistical heterogeneity among these studies (I2 = 46%; P = 0.11).
Figure 5.
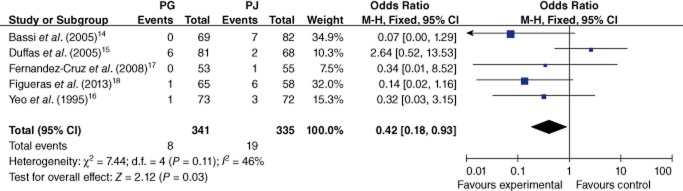
Comparison of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) in terms of biliary fistula. 95% CI, 95% confidence interval; M-H, Mantel–Haenszel method
Intra-abdominal fluid collection
All trials provided specific information on the occurrence of intra-abdominal fluid collection.14–20 There was no heterogeneity among the trials (I2 = 38%; P = 0.14). In the fixed-effects model (OR 0.50, 95% CI 0.34–0.74; P = 0.0005) (Fig. 6), the risk for intra-abdominal fluid collection was lower in the PG than the PJ group (8.0% versus 14.7%).
Figure 6.
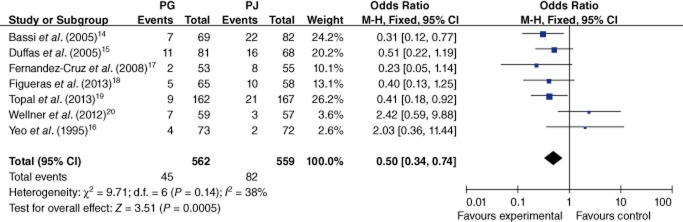
Comparison of pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) in terms of intra-abdominal fluid collection. 95% CI, 95% confidence interval; M-H, Mantel–Haenszel method
Delayed gastric emptying
Six studies reported delayed gastric emptying.14,16–20 Meta-analysis indicated there was no significant difference in delayed gastric emptying between the PG and PJ groups (random-effects model, 16.6% versus 14.7%; relative risk 1.02, 95% CI 0.62–1.68; P = 0.94), but there was evidence of significant heterogeneity (I2 = 58%; P = 0.03).
Postoperative haemorrhage
Six studies compared PG with PJ for the occurrence of postoperative haemorrhage.14,15,17–20 Using a fixed-effects model, the pooled data showed there was no significant difference in postoperative bleeding between the two groups (9.6% versus 11.1%; OR 0.82, 95% CI 0.54–1.24; P = 0.35), and no significant heterogeneity (I2 = 0%; P = 0.60).
Reoperation
Reoperation was analysed in five studies.14,15,17,19,20 Rates of reoperation in the PG and PJ groups were 9.9% and 9.8%, respectively, and thus showed no significant difference (OR 0.93, 95% CI 0.60–1.43; P = 0.73). No heterogeneity was found (I2 = 0%; P = 0.82).
Length of hospital stay
All studies reported the hospital LoS. However, data from four studies were excluded because information on the standard deviation was lacking.15,18–20 The hospital LoS was significantly shorter in the PG than the PJ group (random-effects model, WMD −1.85, 95% CI −3.23 to −0.47; P = 0.008). However, significant heterogeneity was apparent among these studies (I2 = 95%; P < 0.00001).
Discussion
Since the first successful PD was performed by Kausch in 1909 and its description published in 1912,26 the best technique for pancreatic anastomosis has remained controversial.27 This meta-analysis of seven RCTs allowed for an analysis of pooled data for PG and PJ, respectively, after PD. The pooled results showed that PG represents a better option than PJ after PD by comparing the occurrences of pancreatic fistula, biliary fistula and intra-abdominal fluid collection, and hospital LoS. However, no differences between the groups emerged in morbidity, mortality, delayed gastric emptying, postoperative haemorrhage and reoperation.
Pancreatic fistula is the most important postoperative complication and is at times fatal; it may also play a central role in the development of other intra-abdominal complications, such as haemorrhage and leak.4,7,28 Pancreatic surgeons are generally agreed that pancreatic fistula represents the ‘Achilles heel’ of PD.29 Unlike previous meta-analyses of RCTs,11–13,30 the present pooled analysis of data pertaining to the occurrence of pancreatic fistula demonstrated that it is significantly lower in PG than in PJ (10.6% versus 18.5%; OR 0.52, 95% CI 0.37–0.74; P = 0.0002) after PD. Definitions of pancreatic fistula varied until 2005, when the ISGPF presented a unified definition and system of grading severity. The unified definitions have enabled the consistent comparison of treatment outcomes after pancreatic surgery.25 All of the previous three RCTs and meta-analyses employed different definitions of pancreatic fistula, which has limited their comparability.11–16,30,31 The Verona centre-specific definition of pancreatic fistula required confirmation by fistulography, but fistulography is neither mandatory nor recommended in the current International Study Group of Pancreatic Surgery (ISGPS) criteria.14 The Baltimore centre definition of pancreatic fistula referred to drainage of >50 ml of amylase-rich fluid after postoperative day 10 or to the disruption of pancreatic anastomosis demonstrated radiographically, which may underestimate the overall occurrence of postoperative pancreatic fistula in comparison with the ISGPF definition.16 A relatively high rate of mortality (11%) calls into question experiences with both forms of pancreatic reconstruction in an RCT conducted in France.15
Four RCTs used the international consensus definition.17–20 The present subgroup analysis indicated that in the later four RCTs, severe pancreatic fistula (of grade B or C) according to the international consensus occurred less often in the context of PG than PJ (8.3% versus 20.5%; OR 0.37, 95% CI 0.23–0.59; P < 0.00001). Occurrences of pancreatic fistula according to centre-specific definitions in the other three RCTs showed no significant difference between the two groups (14.0% versus 15.8%; OR 0.85, 95% CI 0.50–1.44; P = 0.54). A recent nationwide, large-scale, multicentre randomized superiority trial showed that PG resulted in a lower incidence of clinically relevant pancreatic fistula than PJ (8.0% versus 19.8%), irrespective of the diameter of the pancreatic duct.19
Many other factors can lead to pancreatic fistula, including disease factors (pancreatic texture, pancreatic pathology, pancreatic duct size, pancreatic juice output), patient-related factors (age, sex, jaundice, comorbid illness, previous gastric surgery), surgeon-related factors (familiarity) and operative factors (operation time, type of anastomosis, stenting of pancreatic duct).7,32–34 The method of anastomosis after PD should be selected according to all of these factors. In the discussion of PG versus PJ, it would be preferable to know which method of construction of anastomosis will be better among the end-to-side, dunking, invagination of the pancreas and duct-to-mucosa techniques. However, the meta-analysis of these seven RCTs did not support any conclusions on which technique is optimal in terms of preventing the occurrence of complications.14–20 Details of anastomosis methods used in the seven RCTs are shown in Table 2. Berger et al. reported an RCT in which the authors found that end-to-side invagination PJ in the soft pancreas was associated with a significantly lower rate of pancreatic fistula than duct-to-mucosa PJ in hard pancreas.8 By contrast, Bassi et al. found no statistically significant difference between duct-to-mucosa and one-layer end-to-side invagination anastomosis in pancreatic fistula after PD.35 Therefore, further adequately powered and well-designed RCTs are necessary to establish whether anastomosis to the stomach or the jejunum is preferable in reducing pancreatic anastomotic failure, and to ascertain which specific type of anastomosis is superior.
Previous meta-analyses of observational clinical studies have demonstrated a significantly lower incidence of biliary fistula in PG in comparison with PJ. Similarly, the present meta-analysis showed that biliary fistula was significantly higher in PJ than PG. Although the difference was significant, the present findings should be interpreted with caution. Bassi et al. concluded that a significantly lower incidence of biliary fistula was associated with PG.35 The difference may be mainly associated with the presence in a nearby area of a double anastomosis (jejunal-pancreatic and biliary) in PJ rather than a single biliary anastomosis in PG, which was confirmed by the observation that 42.9% of patients with biliary fistula also had concomitant pancreatic fistula. However, the other four RCTs showed there to be no statistically significant difference between PG and PJ in the occurrence of biliary fistula. Moreover, the average incidence of biliary fistula was only 4.0% and thus biliary fistula was rare with any reconstruction type across all RCTs. Therefore, it seems that further randomized trials are needed to assess the true impact of PG on biliary fistula.
The technique of PG has several theoretical physiological and technical advantages over PJ. Firstly, a physiological advantage is believed to derive from the fact that exocrine pancreatic secretions are easily activated in the presence of intestinal enterokinase and bile, but not in the acidic gastric environment. Secondly, the excellent blood supply to the stomach wall is favourable to anastomotic healing and the thickness of the stomach wall holds sutures well. Thirdly, PG avoids the long jejunal loop, which may impose tension on the anastomosis as a result of the accumulation of pancreaticobiliary secretions and the weight of the loop itself. Fourthly, routine nasogastric decompression facilitates the continuous removal of pancreatic and gastric secretions, which may decrease any tension on the anastomosis. The present meta-analysis has demonstrated the superiority of PG over PJ in terms of pancreatic fistula, biliary fistula, intra-abdominal fluid collection and hospital LoS, the last of which may derive from the aforementioned theoretical advantages.
However, there are several limitations to the present review. Firstly, although no detectable publication bias was found on funnel plotting, all trials had a high risk for bias caused by lack of blinding. The definitions of pancreatic fistula varied among RCTs, three of which used centre-specific definitions. Moreover, the methods of pancreatic anastomosis were themselves heterogeneous and involved different techniques (duct-to-mucosa, invagination, single-layer and two-layer methods) and different types of suture. Different methods of anastomosis may lead to different complications. Additionally, because it was not possible to perform subgroup analyses according to pancreatic duct size, pancreatic texture or pancreatic pathology, it is unclear whether the potential advantages of PG are applicable to all subgroups of patients. The heterogeneity among studies in terms of surgeon experience is unavoidable. Finally, none of the studies described longterm postoperative mortality and morbidity, which are crucial to any assessment of the curative effects of PG and PJ.
In summary, the results of this meta-analysis showed that PG is more efficient than PJ in reducing incidences of pancreatic fistula, biliary fistula and intra-abdominal fluid collection, and hospital LoS. Rates of morbidity, mortality, delayed gastric emptying, postoperative haemorrhage and reoperation were comparable between the two groups. Further adequately powered and well-designed RCTs are required to verify these results and to confirm whether these results apply to an equal extent in all patient subgroups.
Conflicts of interest
None declared.
References
- Venkat R, Puhan MA, Schulick RD, Cameron JL, Eckhauser FE, Choti MA. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg. 2011;146:1277–1284. doi: 10.1001/archsurg.2011.294. [DOI] [PubMed] [Google Scholar]
- Kim CG, Jo S, Kim JS. Impact of surgical volume on nationwide hospital mortality after pancreaticoduodenectomy. World J Gastroenterol. 2012;18:4175–4181. doi: 10.3748/wjg.v18.i31.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145:634–640. doi: 10.1001/archsurg.2010.118. [DOI] [PubMed] [Google Scholar]
- Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber RR, Sauvanet A. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French surgical association. HPB. 2014;16:46–55. doi: 10.1111/hpb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe JC, Parks K, Kumar P, McNeal SF, Morgan DE, Christein JD. Computed tomography attenuation and patient characteristics as predictors of complications after pancreaticoduodenectomy. HPB. 2013;15:709–715. doi: 10.1111/hpb.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BC, Christein JD, Behrman SW, Callery MP, Drebin JA, Kent TS. Assessing the impact of a fistula after a pancreaticoduodenectomy using the Postoperative Morbidity Index. HPB. 2013;15:781–788. doi: 10.1111/hpb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EI Nakeeb A, Salah T, Sultan A, EI Hemaly M, Askr W, Ezzat H. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single centre experience) World J Surg. 2013;37:1405–1418. doi: 10.1007/s00268-013-1998-5. [DOI] [PubMed] [Google Scholar]
- Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208:738–747. doi: 10.1016/j.jamcollsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Yang SH, Dou KF, Sharma N, Song WJ. The methods of reconstruction of pancreatic digestive continuity after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. World J Surg. 2011;35:2290–2297. doi: 10.1007/s00268-011-1159-7. [DOI] [PubMed] [Google Scholar]
- Lai EC, Lau SH, Lau WY. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. Arch Surg. 2009;144:1074–1080. doi: 10.1001/archsurg.2009.193. [DOI] [PubMed] [Google Scholar]
- He T, Zhao Y, Chen Q, Wang X, Lin H, Han W. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a systematic review and meta-analysis. Dig Surg. 2013;30:56–69. doi: 10.1159/000350901. [DOI] [PubMed] [Google Scholar]
- Ma JP, Peng L, Qin T, Lin JW, Chen CQ, Cai SR. Meta-analysis of pancreaticoduodenectomy prospective controlled trials: pancreaticogastrostomy versus pancreaticojejunostomy reconstruction. Chin Med J. 2012;125:3891–3897. [PubMed] [Google Scholar]
- Wente MN, Shrikhande SV, Muller MW, Diener MK, Seiler CM, Friess H. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. doi: 10.1016/j.amjsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy. Ann Surg. 2005;242:767–771. doi: 10.1097/01.sla.0000189124.47589.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM. A controlled randomized multicentre trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720–729. doi: 10.1016/j.amjsurg.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cruz L, Cosa R, Blanco L, Lopez-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248:930–938. doi: 10.1097/SLA.0b013e31818fefc7. [DOI] [PubMed] [Google Scholar]
- Figueras J, Sabater L, Planellas P, Muñoz-Foner E, Lopez-Ben S, Falgueras L. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100:1597–1605. doi: 10.1002/bjs.9252. [DOI] [PubMed] [Google Scholar]
- Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomized trial. Lancet Oncol. 2013;14:655–662. doi: 10.1016/S1470-2045(13)70126-8. [DOI] [PubMed] [Google Scholar]
- Wellner HF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. Randomized controlled single-centre trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16:1686–1695. doi: 10.1007/s11605-012-1940-4. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJ, Gavaghan DJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Chalmers TC, Smith H, Jr, Blackburn B, Silverman B, Schroeder B, Reitman D. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kausch W. Das Carcinoma der Papilla Duodeni und seine radikale Entfernung. Beitr Klin Chir. 1912;78:439–486. [Google Scholar]
- Kleespies A, Albertsmeier M, Obeidat F, Seeliger H, Jauch KW, Bruns CJ. The challenge of pancreatic anastomosis. Langenbecks Arch Surg. 2008;393:459–471. doi: 10.1007/s00423-008-0324-4. [DOI] [PubMed] [Google Scholar]
- Haddad LB, Scatton O, Randone B, Andraus W, Massault PP, Dousset B. Pancreatic fistula after pancreaticoduodenectomy: the conservative treatment of choice. HPB. 2009;11:203–209. doi: 10.1111/j.1477-2574.2009.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1507 patients: a report from the pancreatic anastomotic leak study group. J Gastrointest Surg. 2007;11:1451–1458. doi: 10.1007/s11605-007-0270-4. [DOI] [PubMed] [Google Scholar]
- McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J. Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg. 2006;93:929–936. doi: 10.1002/bjs.5407. [DOI] [PubMed] [Google Scholar]
- Ma JP, Lin JW, Wang Z, Wang L, Chen JH, Chen CQ. Reconstruction of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis of prospectively controlled trials. Zhonghua Yi Xue Za Zhi. 2011;91:2990–2994. [PubMed] [Google Scholar]
- Ramacciato G, Mercantini P, Petrucciani N, Nigri GR, Kazemi A, Muroni M. Risk factors of pancreatic fistula after pancreatioduodenectomy: a collective review. Am Surg. 2011;77:257–269. [PubMed] [Google Scholar]
- Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:1744–1751. doi: 10.1007/s11605-013-2320-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, He XR, Tian JH, Yang KH. Pancreatic duct stents at pancreaticoduodenectomy: a meta-analysis. Dis Surg. 2013;30:415–424. doi: 10.1159/000355982. [DOI] [PubMed] [Google Scholar]
- Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766–771. doi: 10.1016/s0039-6060(03)00345-3. [DOI] [PubMed] [Google Scholar]