Abstract
Objectives
The optimal locoregional treatment for non-resectable hepatocellular carcinoma (HCC) of ≥3 cm in diameter is unclear. Transarterial chemoembolization (TACE) is the initial intervention most commonly performed, but it rarely eradicates HCC. The purpose of this study was to measure survival in HCC patients treated with adjuvant stereotactic body radiotherapy (SBRT) following TACE.
Methods
A retrospective study of patients with HCC of ≥3 cm was conducted. Outcomes in patients treated with TACE alone (n = 124) were compared with outcomes in those treated with TACE + SBRT (n = 37).
Results
There were no significant baseline differences between the two groups. The pre-TACE mean number of tumours (P = 0.57), largest tumour size (P = 0.09) and total tumour diameter (P = 0.21) did not differ significantly between the groups. Necrosis of the HCC tumour, measured after the first TACE, did not differ between the groups (P = 0.69). Local recurrence was significantly decreased in the TACE + SBRT group (10.8%) in comparison with the TACE-only group (25.8%) (P = 0.04). After censoring for liver transplantation, overall survival was found to be significantly increased in the TACE + SBRT group compared with the TACE-only group (33 months and 20 months, respectively; P = 0.02).
Conclusions
This retrospective study suggests that in patients with HCC tumours of ≥3 cm, treatment with TACE + SBRT provides a survival advantage over treatment with only TACE. Confirmation of this observation requires that the concept be tested in a prospective, randomized clinical trial.
Introduction
The optimal locoregional treatment of hepatocellular carcinoma (HCC) of <3 cm in diameter is tumour ablation.1–4 The optimal locoregional treatment for HCC of ≥3 cm, however, is less clear. According to the Barcelona Clinic Liver Cancer (BCLC) treatment algorithm, transarterial chemoembolization (TACE) is the recommended treatment for intermediate-stage HCC in patients who are not candidates for surgical resection or tumour ablation.3,4 Current guidelines from the American Association for the Study of Liver Diseases (AASLD) and National Comprehensive Cancer Network (NCCN) also recommend TACE as therapy for unresectable HCC.1,2,5 Randomized clinical trials published in the early 2000s comparing TACE with the provision of best supportive care reported 2-year survival rates ranging between 31% and 63%.6,7 However, TACE rarely completely sterilizes the HCC tumour and thus it is considered a palliative, life-prolonging therapy.8
Stereotactic body radiotherapy (SBRT) is a rapidly evolving therapeutic option for inoperable HCC. Improved imaging methods for localizing HCC lesions and precise treatment planning facilitate the delivery of targeted radiation with minimal treatment of uninvolved tissue.9 By contrast with conventional algorithms for the delivery of radiotherapy that stipulate the administration of small doses of radiation spread over several weeks, SBRT involves the precision delivery of a highly focused dose of radiation to the target tumour over a short number of treatments. For example, a common regimen for the delivery of conventional radiotherapy for liver tumours involves the delivery of treatment on 5 days per week over 5 weeks to give a total dose of 50 Gy, whereas a common SBRT regimen would stipulate the delivery of 15 Gy per treatment for a total of three treatments, delivered over 7 days.
Emerging data indicate promising results with the use of SBRT, with 1-year local control rates ranging between 80% and 87% and median survival of 17–19 months.10,11 However, although the NCCN5 recommends SBRT as a possible alternative to TACE for unresectable tumours, this treatment modality is not mentioned in the AASLD1,2 or BCLC3,4 treatment algorithms. The use of SBRT in the treatment of locally advanced HCC has been reported primarily from centres in Asia, where the incidence of HCC is high.10–14 Stereotactic body radiotherapy has been used in various contexts, including as sole therapy, following incomplete TACE and as a salvage treatment for tumour recurrence following other treatments.10–14 The reported outcomes of this treatment vary in these heterogeneous populations and many of the studies were uncontrolled case series.
Stereotactic body radiotherapy was initiated at the University of Alabama at Birmingham (UAB) as a palliative therapy for unresectable HCC, typically in patients for whom no other therapeutic options were available. After surprisingly good clinical outcomes, the centre began to treat select patients in whom HCC persisted after TACE with SBRT as destination therapy and occasionally as bridging therapy for waitlisted liver transplant candidates. There are several potential oncologic advantages of using TACE followed by SBRT. Transarterial chemoembolization is most effective at the centre of the HCC. Failures most commonly occur at the periphery of the HCC tumour, where the ischaemic effects of TACE are least potent because the surrounding uninvolved liver parenchyma is well oxygenated. By contrast, radiation is most effective in the well-oxygenated periphery of the HCC tumour and failures tend to occur in the more hypoxic areas at the centre of the tumour. Large tumours that are not suitable for SBRT alone become more amenable to this therapy following TACE of the tumour centre. In addition, a theoretical radio-sensitization by the cytotoxic agents used in TACE may bring about an improved tumour response.15
The purpose of this study was to measure the outcomes obtained by using TACE alone with those achieved by using a combination of TACE followed by SBRT in patients with unresectable HCC of ≥3 cm in diameter. The study hypothesis assumed that survival would be superior in patients treated with a combination of TACE and SBRT compared with that in those treated with TACE alone.
Materials and methods
Ethics approval for this study was obtained from the UAB Institutional Review Board (protocol no. X100310006). A retrospective chart review was performed for all HCC patients treated with TACE at UAB between January 2008 and August 2013.
Patient population
Patients were diagnosed with HCC according to the AASLD criteria either by biopsy or by the presentation of classic HCC radiologic features.1,2 The decision to offer TACE and SBRT to patients with HCC was made by a multidisciplinary liver tumour board at UAB, which included diagnostic radiology faculty members, pathologists, medical oncologists, radiation oncologists, surgeons, hepatologists and interventional radiologists.
A list of all HCC patients treated with TACE as a first locoregional oncologic therapy was generated from the UAB interventional radiology procedures electronic database (n = 262). Data for patients with an HCC tumour of <3 cm in size (n = 69) and for those in whom an ablation had been performed after the TACE procedure (n = 42) were excluded (Fig. 1). Tumours of HCC that had previously been treated operatively or with another locoregional therapy such as radiofrequency ablation or external beam radiotherapy were excluded from the study. Patients taking chemotherapeutic agents before and/or after the procedure were included in the study.
Figure 1.
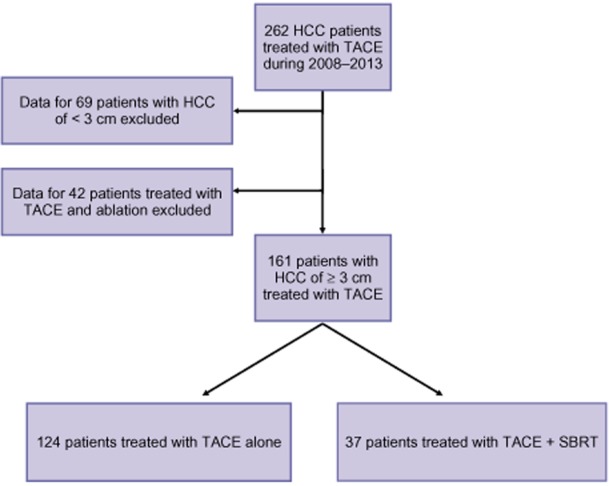
Inclusion and exclusion criteria used to select patients for this retrospective study from among 262 patients with hepatocellular carcinoma (HCC) treated with protocols including transarterial chemoembolization (TACE) and stereotactic body radiotherapy (SBRT)
Transarterial chemoembolization procedures were performed as previously described.16,17
Protocol for SBRT
Patients underwent four-dimensional computed tomography (CT) simulation following immobilization in the supine position. Oral and i.v. contrast materials were administered during the procedure. Computed tomography images were transferred to a computerized treatment planning system. Gross tumour volume (GTV) encompassed all visible disease on contrasted CT simulation. Clinical target volume (CTV) included an additional 4 mm around the tumour. The planned target volume (PTV) was constructed by adding a 5-mm geometric uncertainty margin around the CTV. The dose–volume constraints used during SBRT planning are fairly standardized: care was taken to ensure that at least 700 cm3 of normal liver parenchyma was exposed to <15 Gy over the course of SBRT, consistent with published recommendations.18 Patients were treated on a linear accelerator with respiratory gating. Cone-beam CT, orthogonal imaging and a real-time tracking technique were used for image guidance according to standard practice in the department at the time of treatment. The typical prescription dose was 45 Gy administered in three fractions to the PTV. All patients were premedicated with oral anti-nausea medications prior to SBRT. All patients underwent blood counts and liver function testing, and were evaluated clinically for SBRT-related toxicities during the course of their treatment and at periodic intervals thereafter (Fig. 2).
Figure 2.
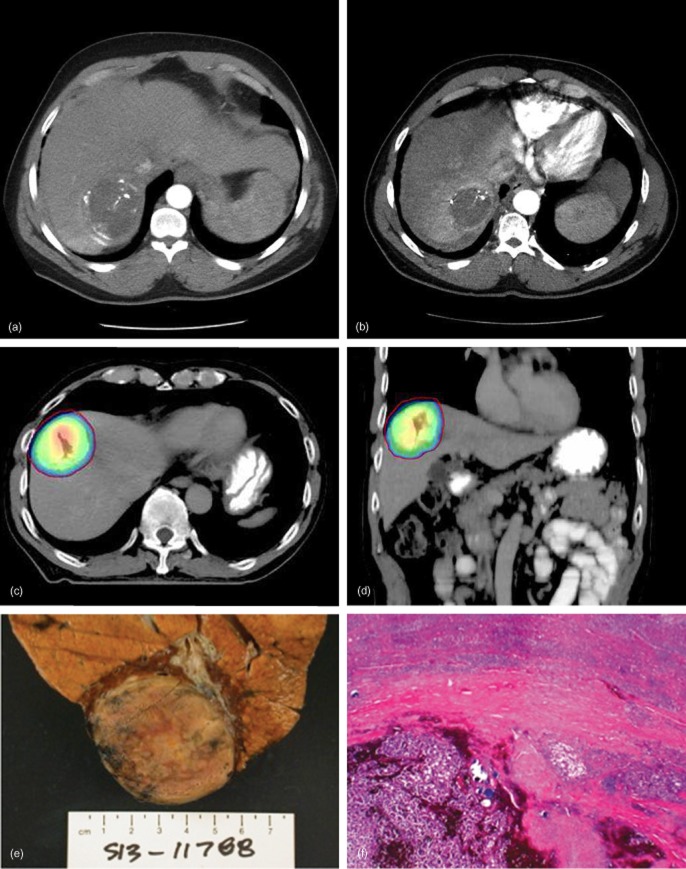
(a) Axial computed tomography (CT) scan demonstrating a hepatocellular carcinoma (HCC) lesion treated with lipiodol-based transarterial chemoembolization (TACE). (b) Axial CT scan obtained 4 months after TACE plus stereotactic body radiotherapy (SBRT) illustrating significant HCC tumour (and surrounding tissue) involution. (c) Axial CT SBRT plan showing highly conformal dose distribution around the target. (d) Coronal CT SBRT plan showing highly conformal dose distribution around the target. (e) Gross pathologic photograph of an HCC specimen obtained at the time of liver transplantation from a patient treated with TACE + SBRT. (f) Histopathology of the HCC specimen in (e) demonstrates complete tumour necrosis and no viable HCC. (Haematoxylin and eosin stain; original magnification ×4)
Radiographic measurements
Diagnosis of HCC
Hepatocellular carcinoma is diagnosed according to the visualization of an arterially enhancing lesion of 1–2 cm in size with portal venous washout and a pseudocapsule on delayed-phase CT, or the visualization of an arterially enhancing lesion of >2 cm in size with portal venous washout or a pseudocapsule on delayed-phase CT.19,20
Necrosis of the HCC tumour
Tumour response was assessed via the modified response evaluation criteria in solid tumours (mRECIST) as previously described.17 There are four categories of tumour response according to mRECIST, which indicate: complete response; partial response; stable disease, and progressive disease.21
Sarcopoenia
Psoas cross-sectional area was used as a surrogate marker of fragility.22 The area of the right and left psoas muscle was measured at the level of the fourth lumbar vertebral body on cross-sectional imaging. The enclosed region was then used to calculate the cross-sectional area of the psoas muscle.
Cirrhosis
A modified caudate to right lobe ratio of >0.9,23,24 nodular transformation of the liver and sequela of portal venous hypertension25 were used as radiographic indicators of cirrhosis.
Data analysis
Data on patient demographics, clinical history, laboratory findings and HCC tumour characteristics were collected. Liver disease was quantified according to the Model for End-stage Liver Disease (MELD) score and Child–Pugh score. Pre-TACE variables collected by CT and magnetic resonance imaging (MRI) included number of lesions, size of tumours and the total diameter of the three largest tumours in patients with multifocal HCC. Tumour stage was measured according to BCLC staging, including BCLC-B subtypes.26 Data collected from post-TACE CT and MRI included HCC tumour necrosis as measured according to mRECIST criteria.21 To allow the use of common statistical procedures, the analysis was restricted to examination of the index HCC tumour, which was defined as the largest tumour. (The use of more than one tumour per patient in the analysis would have violated the common assumption of independent data observations.) The hepatoma–arterial embolization (HAP) score27 was used as a prognostic tool to compare outcomes following initial TACE interventions between groups. The Assessment for Retreatment with TACE (ART) score28 was used as a prognostic tool to enable the comparison of outcomes following repeat TACE interventions between groups.
A two-sample t-test was used to compare means between groups. The primary analytic approach for dichotomous variables utilized chi-squared analyses. Kaplan–Meier curves were constructed to evaluate patient survival. Survival probabilities were analysed using the Wilcoxon test because it is more sensitive in detecting differences at shorter survival times. Observations were censored at the time of surgical resection or transplantation. For all inferences, the probability of a type I error (α) was set at 0.05. All analyses were conducted using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient demographics
A total of 161 patients with HCC tumours of ≥3 cm treated with TACE as an initial locoregional oncologic therapy were identified. Only TACE was used in 124 HCC patients, and TACE followed by SBRT was used in 37 HCC patients. There were no significant differences in age, gender, race or the aetiology of liver disease between the TACE-alone and TACE + SBRT groups (Table 1). Although all patients had underlying liver disease, 12.9% of the TACE-alone group and 16.2% of the TACE + SBRT group did not have obvious cirrhosis as evidenced by platelet counts of >100 and failure to meet cirrhosis cross-sectional imaging characteristics. There were no significant differences in underlying liver function as measured by the MELD score, Child–Pugh score, BCLC-B subtype classification, or in muscle wasting as quantified by sarcopoenia measurements. Furthermore, there were no differences between the groups in the predictors of TACE outcomes as quantified by the HAP and ART scores (Table 1).
Table 1.
Baseline demographics of patients with hepatocellular carcinoma of ≥3 cm treated with transarterial chemoembolization (TACE) alone or TACE plus stereotactic body radiotherapy (SBRT)
| Variable | TACE-only group (n = 124) | TACE + SBRT group (n = 37) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 62.2 ± 9.0 | 64.4 ± 12.7 | 0.405 |
| Male, % | 75.8% | 72.8% | 0.829 |
| Race, % | 0.494 | ||
| Black | 16.7% | 21.6% | |
| White | 75.8% | 75.7% | |
| Other | 7.5% | 2.7% | |
| Aetiology of liver diseasea, % | |||
| Alcohol | 24.4% | 18.9% | 0.657 |
| HBV | 7.3% | 8.1% | 1.000 |
| HCV | 44.7% | 51.4% | 0.5733 |
| NASH | 20.2% | 18.9% | 1.000 |
| Haemochromatosis | 2.4% | 5.4% | 0.324 |
| Portal hypertensionb, % | 0.862 | ||
| Platelet count ≤ 100, cirrhosis | 46.0% | 46.0% | |
| Platelet count > 100, cirrhosis | 41.1% | 37.8% | |
| Platelet count > 100, no cirrhosis | 12.9% | 16.2% | |
| Sarcopoenia measurement, cm2, mean ± SD | 10.6 ± 3.4 | 11.2 ± 3.8 | 0.247 |
| MELD score, mean ± SD | 11.2 ± 4.2 | 10.2 ± 3.6 | 0.211 |
| Child–Pugh score, mean ± SD | 6.7 ± 1.5 | 6.3 ± 1.2 | 0.291 |
| HAP score, mean ± SD | 1.2 ± 0.8 | 1.3 ± 0.9 | 0.525 |
| BCLC-B subtypes, % | 0.287 | ||
| B1 | 22.0% | 16.7% | |
| B2 | 36.4% | 55.6% | |
| B3 | 13.6% | 8.3% | |
| B4 | 28.0% | 19.4% | |
| ART score, mean ± SD | 1.3 ± 1.7 | 1.6 ± 2.1 | 0.923 |
Some patients had liver disease of more than one aetiology.
Cirrhosis diagnosis is based upon cross-sectional imaging characteristics.
ART, Assessment for Retreatment with TACE; BCLC, Barcelona Clinic Liver Cancer; HAP, hepatoma–arterial embolization; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-stage Liver Disease; NASH, non-alcoholic steatohepatitis; SD, standard deviation.
Tumour characteristics
There were no significant differences in α-fetoprotein measurements between the TACE-alone and TACE + SBRT groups. Tumour characteristics, as quantified by pretreatment cross-sectional imaging, were also comparable between the groups (Table 2). The mean ± standard deviation (SD) number of tumours (2.1 ± 1.6 in the TACE-alone group and 1.8 ± 1.1 in the TACE + SBRT group; P = 0.6), and distribution of unifocal and multifocal HCC (52.9% and 47.1%, respectively, in the TACE-alone group, and 54.1% and 45.9%, respectively, in the TACE + SBRT group; P = 1.0) were nearly identical between groups. There was a trend towards an increased size of the largest HCC tumour in the TACE + SBRT group (5.8 ± 3.0 cm in the TACE-alone group and 6.1 ± 2.4 cm in the TACE + SBRT group; P = 0.09), although the total tumour diameter was nearly identical in both groups (7.7 ± 4.9 cm in the TACE-alone group and 7.8 ± 3.3 cm in the TACE + SBRT group; P = 0.2). There were no significant differences in the mRECIST measures of HCC tumour necrosis following initial TACE (P = 0.7) (Table 2).
Table 2.
Tumour and treatment characteristics in patients with hepatocellular carcinoma (HCC) of ≥3 cm treated with transarterial chemoembolization (TACE) alone or TACE plus stereotactic body radiotherapy (SBRT)
| Variable | TACE-only group (n = 124) | TACE + SBRT group (n = 37) | P-value |
|---|---|---|---|
| α-fetoprotein, ng/ml, mean ± SD | 19.8 ± 192.1 | 32.7 ± 456.0 | 0.534 |
| Tumour characteristics | |||
| Number of tumours, mean ± SD | 2.1 ± 1.6 | 1.8 ± 1.1 | 0.571 |
| HCC type, % | 1.000 | ||
| Unifocal disease | 52.9% | 54.1% | |
| Multifocal disease | 47.1% | 45.9% | |
| Size of largest tumoura, cm, mean ± SD | 5.8 ± 3.0 | 6.1 ± 2.4 | 0.094 |
| Total tumour diameterb, cm, mean ± SD | 7.7 ± 4.9 | 7.8 ± 3.3 | 0.206 |
| Response according to mRECISTc, % | 0.688 | ||
| Complete | 27.2% | 30.3% | |
| Partial | 52.2% | 57.6% | |
| Stable | 13.0% | 9.1% | |
| Progressive | 7.6% | 3.0% | |
| Sorafenib pre- or post-d, % | 36.1% | 41.9% | 0.677 |
| Type of TACE, % | 0.707 | ||
| Lipiodol | 54.8% | 59.5% | |
| Drug-eluting beads | 42.7% | 40.5% | |
| TACE treatments, n, mean ± SD | 1.3 ± 0.6 | 1.3 ± 0.7 | 0.906 |
| Curative interventions, % | |||
| Liver resection | 6.7% | 0% | 0.348 |
| Liver transplantation | 15.5% | 12.1% | 0.784 |
| Local recurrence,% | 25.8% | 10.8% | 0.042 |
Axial diameter of largest tumour.
Sum of axial diameters of three largest lesions.
Tumour necrosis measurements are from 30 days post-TACE.
Sorafenib use defined as within 90 days pre- or post-TACE.
SD, standard deviation.
Sorafenib was used in 36.1% of TACE-alone patients and 41.9% of TACE + SBRT patients (P = 0.7). Liver resection was performed infrequently in the TACE-alone group and not at all in the TACE + SBRT group. Liver transplantation was performed in 15.5% of the TACE-alone group and 12.1% of the TACE + SBRT group (P = 0.8) (Table 2).
Transarterial chemoembolization procedures
The usage of lipiodol and drug-eluting beads (DEBs)-based TACE was similar between groups (P = 0.7). Further, the total number of TACE procedures administered was similar between groups (P = 0.9) (Table 2).
Stereotactic body radiotherapy procedures
The characteristics of SBRT treatment and associated toxicities are detailed in Table 3. Most patients were treated with one (n = 18) or two (n = 15) TACE procedures prior to SBRT. Generally, SBRT was started within 2 weeks of TACE, although in three patients the interval between TACE and SBRT was >3 weeks. Respiratory gating was employed during SBRT in all but three patients, in whom the PTV excursion was not large enough to warrant its use. Sorafenib was not administered concurrently in any patient. Rates of SBRT-related toxicities were low; only one instance each of Grade 2 and Grade 3 gastrointestinal toxicity occurred. No patient developed Grade 2 or higher haematologic toxicities, and one patient developed significant rib pain following treatment. One patient died within 4 weeks following SBRT; the cause of death was presumed to reflect pulmonary sepsis rather than radiation-induced liver disease (RILD).
Table 3.
Characteristics and toxicities of stereotactic body radiotherapy (SBRT) in patients with hepatocellular carcinoma also treated with transarterial chemoembolization (TACE)
| Patients, n | |
|---|---|
| SBRT patients | 37 |
| TACE procedures | |
| 1 | 18 |
| 2 | 15 |
| 3 or more | 4 |
| Time from TACE to SBRT | |
| ≤2 weeks | 28 |
| 2–3 weeks | 6 |
| >3 weeks | 3 |
| Respiratory gating | |
| Yes | 34 |
| No | 3 |
| Use of fiducials | |
| Yes | 9 |
| No | 28 |
| Duration of SBRT | |
| ≤7 days | 21 |
| 8–9 days | 9 |
| ≥10 days | 7 |
| SBRT dose | |
| 36 Gy in three fractions | 3 |
| 45 Gy in three fractions | 26 |
| 60 Gy in three fractions | 3 |
| Others | 5 |
| Toxicities | |
| Gastrointestinal | |
| Grades 0 and 1 | 35 |
| Grade 2 | 1 |
| Grade 3 | 1 |
| Haematologic | |
| Grades 0 and 1 | 37 |
| Bone and soft tissue | |
| Grades 0 and 1 | 36 |
| Grade 2 | 1 |
| Hepatic | |
| Grades 0 and 1 | 37a |
One death occurred 4 weeks following SBRT as a result of pulmonary sepsis.
Local recurrence and patient survival
Local recurrences were observed in 32 of 124 (25.8%) patients in the TACE-alone group and in four of 37 (10.8%) patients in the TACE + SBRT group (P = 0.042) (Table 2). There was no 30-day mortality in either the TACE-alone or the TACE + SBRT group. Data for 90-day mortality reflected the deaths of seven patients in the TACE-alone group and none in the TACE + SBRT group (P = 0.35). After censoring for liver transplantation, Kaplan–Meier curves were constructed for each group (Fig. 3). Superior survival was observed in the TACE + SBRT group compared with the TACE-alone group (Wilcoxon test, P = 0.02). Median survival estimated from the Kaplan–Meier curves was 20 months in the TACE-alone group and 33 months in the TACE + SBRT group.
Figure 3.
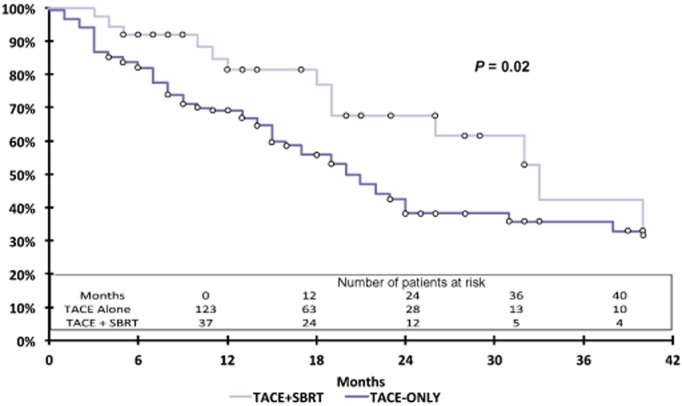
Kaplan–Meier curves for overall survival in patients with hepatocellular carcinoma tumours of ≥3 cm treated with transarterial chemoembolization (TACE) alone or TACE plus stereotactic body radiotherapy (SBRT) (Wilcoxon test, P = 0.02)
Discussion
This retrospective study suggests a survival advantage for patients with HCC tumours of ≥3 cm treated with TACE + SBRT over TACE alone. Patients treated with SBRT following TACE experienced a median survival 13 months longer than that of patients treated with TACE alone. The SBRT was well tolerated; no deaths as a result of RILD and no instances of significant morbidity were observed (toxicities: Grade 4, n = 0; Grade 3, n = 1; Grade 2, n = 2; Grade 1, n = 145). These favourable survival outcomes observed with TACE + SBRT support the supposition that the respective strengths and weaknesses of TACE and SBRT are complementary: TACE preferentially induces central HCC tumour necrosis and SBRT preferentially treats the periphery of the HCC tumour. Interestingly, although the initial analysis included HCC tumours of all sizes treated with TACE and SBRT, smaller HCC tumours did not appear to derive a survival advantage. Exploratory correlation analysis suggested a survival advantage in tumours of ≥3 cm. Clinical practice at the study centre favours the ablation of HCC lesions of <3 cm unless these HCC tumours are in an unfavourable position (adjacent to major biliary structures) or in patients with significant comorbidities. Thus the lack of a survival advantage in HCC lesions of <3 cm may reflect a negative selection bias, or it may be that the additive effects of SBRT are only observed when HCC tumours have achieved a certain size. The mechanism of improved survival may simply derive from improved local control as evidenced by the significantly lower rate of local recurrence observed in the TACE + SBRT group.
Although more technically difficult to administer than other forms of radiotherapy, SBRT is more simple and expeditious for the patient and is predicted to be more clinically efficacious than conventional radiotherapy approaches.29 Unlike conventional radiotherapy schedules in which treatment is administered on 5 days per week for 5–6 weeks, SBRT in this study was predominately delivered in three fractions of 15 Gy over a 7-day period. Such a regimen is attractive to patients who must travel long distances and increases the likelihood that patients will receive radiotherapy at a centre with hepatobiliary expertise. Treatment with SBRT also spares more uninvolved liver parenchyma from high-dose radiation compared with conventional radiotherapy, which is very important given that 85–95% of cases of HCC in Western centres arise in the setting of cirrhosis.1,2 Two recent meta-analyses (consisting almost exclusively of studies from Eastern centres) have looked at the outcomes of combining TACE with different forms of radiotherapy (but not SBRT).30,31 The meta-analyses concluded that there was a significant improvement in overall survival with the use of TACE combined with radiation therapy.30,31 This improvement in survival was achieved without an incremental increase in the rate of serious adverse effects.30,31
An alternative interpretation of the present data is that SBRT alone provided the survival advantage, not the TACE + SBRT. Case series without concurrent comparison control groups have reported in-field tumour control rates of up to 90% in select HCC patients using SBRT alone.11,13,32 Consistent with BCLC recommendations, the protocol at the present centre typically starts with the administration of TACE in non-resectable HCC lesions of ≥3 cm.3,4 Furthermore, for destination therapy, it is the current institution's practice to combine different locoregional modalities when it is feasible to do so to improve outcomes, given the frequent local failure rates of TACE alone and radiotherapy alone.
Another aspect of HCC treatment that is unresolved by these data concerns the size limits and characteristics of the HCC tumour that can be effectively treated by TACE in combination with SBRT. Is there a cut-off size after which TACE + SBRT is no longer effective? What impact does segmental or lobar venous vascular invasion have? Kang et al. reported a study conducted in Seoul, South Korea, in which 47 patients with inoperable HCC achieved a 76.6% rate of complete or partial response to salvage SBRT following one to five TACE procedures.12 In this case series, the HCC lesions measured up to 10 cm in greatest axial diameter and 25% of tumours demonstrated portal venous invasion.12 This study highlights the promising results of combining TACE and SBRT, even in large tumours with unfavourable characteristics. The present authors also have anecdotal experience of a handful of HCC patients with major vascular invasion or tumour thrombus who have exceeded their expected survival after treatment with TACE + SBRT.
The accurate localization of and delivery of radiation to the HCC tumour in SBRT is technologically demanding. One of the biggest technical challenges refers to localizing the tumour using orthogonal kV (static plain film) imaging or cone-beam CT (unenhanced) prior to treatment delivery. The practice of the present authors is to use either lipiodol-based TACE or radio-opaque fiducials in patients in whom adjuvant SBRT is planned. The lipiodol is radio-opaque and can be identified to some extent on plain films, but especially well on unenhanced CT. In TACE patients treated with DEBs, this group has employed percutaneous placement of radio-opaque fiducials (Polymark 1 × 3 mm; Cortex Manufacturing, Inc., Portland, OR, USA). Typically, three fiducials are placed near the tumour prior to CT simulation. Simulation scans are used to generate a digitally constructed radiograph, which also depicts the lipiodol contrast or fiducials in relationship to the HCC tumour. Lipiodol or fiducials are identified by imaging at the time of SBRT. By overlaying the current image on the digitally constructed ‘planning’ radiograph by applying shifts along the x-, y- and z-axes, the targets on the two images (the digitally reconstructed ‘planning’ image and the real-time image) are made to overlap precisely, thereby allowing the very accurate delivery of radiotherapy to planned fields. Modern radiotherapy treatment machines are capable of synchronizing radiation delivery with preselected phases of the patient's respiratory cycle (‘respiratory gating’), thereby further improving accuracy. Various studies to evaluate newer methods of target delineation, such as real-time fiducial tracking, as well as refinements in radiation techniques, such as ‘radiation dose-painting’, in an effort to improve treatment delivery and limit toxicities are currently ongoing.33,34
As with all retrospective studies, this report has limitations. Of greatest concern is the potential patient selection bias. This refers to the question of whether the patients selected to receive SBRT simply represented a group of patients who were expected to do better, which might explain the apparent survival advantage of TACE + SBRT. To address this concern, the present authors planned to use propensity scores to match covariates in the TACE-alone and TACE + SBRT groups. However, the covariates did not differ significantly between the groups at baseline (Tables 1 and 2), thus eliminating the need for propensity scores.35 From a clinical perspective, some faculty members in the UAB tumour clinic were supportive of adjuvant SBRT, whereas others had concerns regarding the efficacy of this therapy. Referrals for adjuvant SBRT ebbed and flowed according to the successes and failures of this treatment modality. These practice patterns account for much of the dispersion of patients between the TACE-alone and TACE + SBRT groups. However, it is likely that patient selection bias can only be conclusively addressed in a randomized clinical trial. Other limitations of this report include its retrospective design, the fact that it represents a single-centre experience, and the relatively small number of patients treated with TACE + SBRT. Despite these limitations, the present data represent one of the largest experiences (and one of the very few controlled series) of HCC patients treated with TACE + SBRT at a Western centre.
In conclusion, this retrospective study suggests a survival advantage for patients with HCC tumours of ≥3 cm treated with TACE + SBRT over TACE alone. The confirmation of this observation will require this concept to be tested in a prospective, randomized clinical trial.
Conflicts of interest
None declared.
References
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruix J, Sherman M Practice Guidelines Committee American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. 2013. National Comprehensive cancer Network guidelines for hepatobiliary cancers. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (last accessed 4 January 2014)
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev. 2006;32:594–606. doi: 10.1016/j.ctrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- Choi BO, Jang HS, Kang KM, Lee SW, Kang YN, Chai GY. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154–158. doi: 10.1093/jjco/hyi236. [DOI] [PubMed] [Google Scholar]
- Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Longterm outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transplant. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- Piro AJ, Taylor CC, Belli JA. Interaction between radiation and drug damage in mammalian cells. I. Delayed expression of actinomycin D/x-ray effects in exponential and plateau phase cells. Radiat Res. 1975;63:346–362. [PubMed] [Google Scholar]
- Dorn DP, Bryant MK, Zarzour J, Smith JK, Redden DT, Saddekni S. Chemoembolization outcomes for hepatocellular carcinoma in cirrhotic patients with compromised liver function. HPB. 2013;16:648–655. doi: 10.1111/hpb.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant MK, Dorn DP, Zarzour J, Smith JK, Redden DT, Saddekni S. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB. 2013;16:327–335. doi: 10.1111/hpb.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh BD, Schefter TE, Cardenes HR, Stieber VW, Raben D, Timmerman RD. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- Englesbe MJ, Terjimanian MN, Lee JS, Sheetz KH, Harbaugh CM, Hussain A. Morphometric age and surgical risk. J Am Coll Surg. 2013;216:976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology. 224:769–774. doi: 10.1148/radiol.2243011495. [DOI] [PubMed] [Google Scholar]
- Lee JK, Sagel SR, Stanley RJ. Computed Body Tomography with MRI Correlation. 4th edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 2003. pp. 894–897. [Google Scholar]
- Ito K, Mitchell DG, Siegelman ES. Cirrhosis: MR imaging features. Magn Reson Imaging Clin N Am. 2002;10:75–92. doi: 10.1016/s1064-9689(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D. A simple prognostic scoring system for patients receiving transarterial embolization for hepatocellular cancer. Ann Oncol. 2013;24:2565–2570. doi: 10.1093/annonc/mdt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Muller C. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- Solberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: executive summary. Pract Radiat Oncol. 2012;2:2–9. doi: 10.1016/j.prro.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Huang J, Zhang T, Wu H. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS ONE. 2013;8:e68453. doi: 10.1371/journal.pone.0068453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184–194. doi: 10.1016/j.radonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Yang JC, Wexler LH, Meyers PA, Happersett L, La Quaglia MP, Wolden SL. Intensity-modulated radiation therapy with dose-painting for paediatric sarcomas with pulmonary metastases. Pediatr Blood Cancer. 2013;60:1616–1620. doi: 10.1002/pbc.24502. [DOI] [PubMed] [Google Scholar]
- Azcona JD, Li R, Mok E, Hancock S, Xing L. Development and clinical evaluation of automatic fiducial detection for tumour tracking in cine megavoltage images during volumetric modulated arc therapy. Med Phys. 2013;40:031708. doi: 10.1118/1.4791646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]