Abstract
Background
The aim of this study was to compare the socioeconomic profile of patients undergoing liver resection for colorectal liver metastasis (CLM) in a regional hepatopancreatobiliary unit with that of the local population. A further aim was to determine if degree of deprivation is associated with tumour recurrence after resection.
Methods
A retrospective analysis of patients undergoing liver resection for CLM was performed. Geodemographic segmentation was used to divide the population into five categories of socioeconomic status (SES).
Results
During a 7-year period, 303 patients underwent resection for CLM. The proportion of these patients in the two least deprived categories of SES was greater than that of the local population (50.2% versus 40.2%) and the proportion in the two most deprived categories was lower (18.3% versus 30.1%) (P < 0.001). There was no difference in recurrence rate (P = 0.867) or disease-free survival among categories of SES (P = 0.913). Multivariate analysis demonstrated no association between SES and tumour recurrence (P = 0.700).
Conclusions
Liver resection for CLM is performed more commonly among the least socioeconomically deprived population than among the most deprived. However, degree of deprivation was not associated with tumour recurrence after resection.
Introduction
The incidence of primary colorectal cancer is associated with low socioeconomic status (SES) in the UK, where the age-standardized incidence is 11% higher in men living in the most deprived areas of England compared with those living in the least deprived,1 although no difference has been demonstrated in women. Similar associations have been found in the USA, where individuals with higher levels of deprivation have been found to have a greater risk for the development of colorectal cancer even when other risk factors are controlled for.2 Population studies have also shown that low SES is associated with a worse outcome amongst patients with colorectal cancer.3–5 Approximately a quarter of patients with colorectal cancer will develop colorectal liver metastases (CLM) at the time of presentation6 and a further 25–30% will develop CLM within 2–3 years of diagnosis.7 Little is known of the impact of SES on the risk for CLM and on outcomes of liver resection: a single UK study demonstrated no association between social class and longterm outcome following resection.8 However, this study did not account for potential bias caused by patient selection for liver surgery. Patients with primary colorectal cancer often present symptomatically and are at risk of colonic obstruction, and population studies have shown that 60–80% of patients with primary colorectal cancer will be offered surgery.9 However, the proportion of patients with CLM who are offered surgery is far lower, at 10–20%.10,11 Patients who develop CLM must overcome a number of potential obstacles before undergoing liver surgery. They must survive surgery for primary colorectal cancer; they require longterm surveillance imaging to detect metachronous lesions; they must be referred to a hepatobiliary unit; they must be medically fit for surgery, and their metastases must be technically resectable. Socioeconomic factors may influence a patient's ability to overcome these obstacles following surgery for primary colorectal cancer, which may potentially skew the population of patients submitted to surgery for CLM in comparison with that of the population suffering primary colorectal cancer. A crude comparison of outcomes according to SES may therefore be less valid for CLM as patients may be more stringently selected than those undergoing surgery for primary colorectal cancer.
The primary aim of this study was to compare levels of socioeconomic deprivation in patients undergoing liver resection for CLM in a regional hepatopancreatobiliary (HPB) unit with those of the local population. A secondary aim was to determine if SES is associated with disease-free and overall survival.
Materials and methods
A retrospective analysis of a prospectively maintained database of all patients submitted to liver resection for CLM between July 2005 and March 2012 was undertaken. Patient details, laboratory data and operative details were retrieved. Synchronous metastases were defined as those diagnosed prior to or within 2 months of primary surgery. All patients underwent tumour staging with computed tomography (CT) prior to liver surgery. Preoperative magnetic resonance imaging (MRI) and positron emission tomography (PET) scans were performed at the discretion of the referring clinician. The physiological score was calculated using the POSSUM (physiological and operative severity score for the enumeration of mortality and morbidity) scoring system.12 Postoperative surveillance CT scans were performed at 6-monthly intervals for 3 years after liver resection and annually for another 2 years. All patients included in disease-free survival analyses underwent a minimum of one surveillance CT scan and the date of tumour recurrence was recorded.
Socioeconomic status was calculated using ACORN®,13 a commercially available geodemographic segmentation tool. This tool divides UK households into five categories in order of increasing deprivation, characterized as representing: wealthy achievers; the urban prosperous; the comfortably off; those of moderate means, and the hard-pressed. The smallest unit of population for which information is available is based on postcode. Full postcodes allow an accurate geographical breakdown because the median size of a residential postcode in the UK is 13 households or 31 residents.13 The deprivation category is based on data collected from multiple sources including property value, type, occupancy and usage. Further information relating to residents is obtained and includes data on date of birth, ethnicity and receipt of social benefits, along with data on spending habits and lifestyle. Population density data are obtained from the National Census.
Patient survival curves were constructed using the Kaplan–Meier method and differences in survival were assessed using the log-rank method. Patients were excluded from survival analyses if they underwent planned non-curative resections or did not receive surveillance imaging. Comparisons between groups according to SES were performed using the chi-squared test or Mann–Whitney U-test, as appropriate. Potential associations between pre- and intraoperative factors, as well as histological outcome and tumour recurrence, were tested using univariate logistic regression or the chi-squared test, as appropriate. Variables in the univariate analysis for which differences achieved a P-value of <0.25 were included in the multivariate regression model.14 Differences were considered to be significant at P < 0.05. Univariate and multivariate analyses were carried out using R Version 2.1.14.15
Patient consent was not required for this study following confirmation from the South West Health Research Authority that under the harmonized Guidance Approval for Research Ethics Committees (RECs), REC review is not required because patient data were collected in the course of normal hospital care and were anonymized for research purposes.
Results
Data relating to 303 liver resections performed for CLM over a period of 7 years were analysed. Clinicopathological characteristics and operative details of the group are displayed in Table 1. The proportions of residents of Devon and Cornwall in the first and second (least deprived) (40.2%) and fourth and fifth (most deprived) (30.1%) SES categories differed from those of the UK (37.4% and 35.1%, respectively) (P < 0.001) (Table 2). Socioeconomic data were unavailable for eight patients undergoing liver resection, leaving 295 for analysis. Of these 295 patients submitted to liver resection for CLM, the proportions of patients from the first and second (least deprived) categories (50.2%) and fourth and fifth (most deprived) categories (18.3%) of SES differed from the proportions in the local population (40.2% and 30.1%, respectively) (P < 0.001).
Table 1.
Preoperative and operative characteristics of 303 patients undergoing liver resection for colorectal liver metastases
| Value | ||
|---|---|---|
| Age, years, median (range) | 67 (33–90) | |
| Gender, n (%) | Female | 113 (37.3%) |
| Male | 190 (62.7%) | |
| T stage of primary, n (%) | 0 | 3 (< 1%) |
| 1 | 7 (2.3%) | |
| 2 | 19 (6.3%) | |
| 3 | 174 (57.4%) | |
| 4 | 91 (30.0%) | |
| Unavailable | 9 (3.0%) | |
| N stage of primary, n (%) | 0 | 133 (43.9%) |
| 1 | 101 (33.3%) | |
| 2 | 64 (21.1%) | |
| Unavailable | 5 (1.7%) | |
| Site of primary, n (%) | Colonic | 152 (50.2%) |
| Rectal | 151 (49.8%) | |
| Timing, n (%) | Synchronous | 144 (47.5%) |
| Metachronous | 159 (52.5%) | |
| Preoperative MRI, n (%) | Yes | 166 (54.8%) |
| No | 137 (45.2%) | |
| Preoperative PET, n (%) | Yes | 208 (68.6%) |
| No | 95 (31.4%) | |
| Preoperative liver-directed chemotherapy, n (%) | Yes | 151 (49.8%) |
| No | 152 (50.2%) | |
| Preoperative diabetes, n (%) | Yes | 28 (9.2%) |
| No | 275 (90.8%) | |
| BMI, kg/m2, median (range) | 27 (16–54) | |
| ASA class, n (%) | 1 | 24 (7.9%) |
| 2 | 211 (69.6%) | |
| 3 | 68 (22.4%) | |
| Neutrophil : lymphocyte ratio, median (range) | 2.58 (0.50–17.25) | |
| Preoperative albumin, g/dl, median (range) | 44 (26–52) | |
| POSSUM physiological score, median (range) | 16 (12–32) | |
| Operation, n (%) | Right hemihepatectomy | 129 (42.6%) |
| Extended right | 13 (4.3%) | |
| Left hemihepatectomy | 35 (11.6%) | |
| Extended left | 3 (1.0%) | |
| Left lateral sectorectomy | 31 (10.2%) | |
| Wedge resection | 79 (26.1%) | |
| Other | 13 (4.3%) | |
| RFA included, n (%) | Yes | 19 (6.3%) |
| No | 284 (93.7%) | |
| Wedge resection included, n (%) | Yes | 122 (40.3%) |
| No | 181 (59.7%) | |
| Number of segments resected, median (range) | 4 (1–6) | |
| Repeat operation, n (%) | Yes | 33 (10.9%) |
| No | 270 (89.1%) | |
| Curative resection, n (%) | Yes | 284 (93.7%) |
| No | 19 (6.3%) | |
| Number of tumours, median (range) | 1 (1–10) | |
| Maximum tumour diameter, mm, median (range) | 35 (3–155) | |
| Resection margin, n (%) | R0 | 232 (76.6%) |
| R1 | 71 (23.4%) | |
MRI, magnetic resonance imaging;
PET, positron emission tomography;
BMI, body mass index;
ASA, American Society of Anesthesiologists;
RFA, radiofrequency ablation.
Table 2.
Distribution of population categorized by socioeconomic status in the UK, and in Devon and Cornwall, and in those undergoing liver resection for colorectal liver metastases. Socioeconomic status was unclassified for eight patients. (Comparison between the proportion of residents of Devon and Cornwall and those undergoing liver resection: P < 0.001)
| Deprivation category | UK residents, n (%) | Residents of Devon and Cornwall, n (%) | Patients undergoing liver resection, n (%) |
|---|---|---|---|
| 1 Wealthy achievers (least deprived) | 14 967 871 (24.8%) | 580 065 (34.6%) | 137 (46.4%) |
| 2 Urban prosperous | 7 594 891 (12.6%) | 93 708 (5.6%) | 11 (3.7%) |
| 3 Comfortably off | 16 656 466 (27.6%) | 497 182 (29.7%) | 93 (31.5%) |
| 4 Moderate means | 8 449 324 (14.0%) | 271 357 (16.2%) | 31 (10.5%) |
| 5 Hard-pressed (most deprived) | 12 715 861 (21.1%) | 232 757 (13.9%) | 23 (7.8%) |
| Total | 60 384 413 | 1 676 069 | 295 |
The clinicopathological and operative characteristics of the 148 least deprived (categories 1 and 2) and 54 most deprived (categories 4 and 5) patients are displayed in Table 3. The use of PET scans was greater in the least deprived than in the most deprived group (75.0% versus 46.3%) and the proportion of patients with American Society of Anesthesiologists (ASA) class 1 status was higher in the least deprived (10.8%) compared with the most deprived (1.9%) group.
Table 3.
Preoperative and operative characteristics of the 148 least (categories 1 and 2) and 54 most (categories 4 and 5) socioeconomically deprived patients undergoing liver resection for colorectal liver metastases
| Least deprived patients |
Most deprived patients |
|||
|---|---|---|---|---|
| Categories 1 + 2 (n = 148) | Categories 4 + 5 (n = 54) | P-value | ||
| Age, years, median (range) | 67 (34–88) | 67 (33–90) | 0.562 | |
| Gender, n (%) | Female | 56 (37.8%) | 23 (42.6%) | 0.625 |
| Male | 92 (62.2%) | 31 (57.4%) | ||
| T stage of primary, n (%) | 0 | 2 (1.4%) | 0 | 0.060 |
| 1 | 4 (2.7%) | 1 (1.9%) | ||
| 2 | 12 (8.1%) | 2 (3.7%) | ||
| 3 | 71 (48.0%) | 39 (72.2%) | ||
| 4 | 52 (35.1%) | 11 (20.4%) | ||
| Unavailable | 7 (4.7%) | 1 (1.9%) | ||
| N stage of primary, n (%) | 0 | 64 (43.2%) | 24 (44.4%) | 0.781 |
| 1 | 50 (33.7%) | 16 (29.6%) | ||
| 2 | 30 (20.3%) | 13 (24.1%) | ||
| Unavailable | 4 (2.7%) | 1 (1.9%) | ||
| Site of primary, n (%) | Colonic | 77 (52.0%) | 29 (53.7%) | 0.874 |
| Rectal | 71 (48.0%) | 25 (46.3%) | ||
| Timing | Synchronous | 71 (48.0%) | 21 (38.9%) | 0.268 |
| Metachronous | 77 (52.0%) | 33 (61.1%) | ||
| Preoperative MRI, n (%) | Yes | 83 (56.1%) | 29 (53.7%) | 0.873 |
| No | 65 (43.9%) | 25 (46.3%) | ||
| Preoperative PET, n (%) | Yes | 111 (75.0%) | 25 (46.3%) | <0.001 |
| No | 37 (25.0%) | 29 (53.7%) | ||
| Preoperative liver-directed chemotherapy, n (%) | Yes | 77 (52.0%) | 25 (46.3%) | 0.526 |
| No | 71 (48.0%) | 29 (53.7%) | ||
| Preoperative diabetes, n (%) | Yes | 16 (10.8%) | 3 (5.6%) | 0.413 |
| No | 132 (89.2%) | 51 (94.4%) | ||
| BMI, kg/m2, median (range) | 27 (17–39) | 27 (19–54) | 0.859 | |
| ASA class, n (%) | 1 | 16 (10.8%) | 1 (1.9%) | 0.030 |
| 2 | 104 (70.3%) | 36 (66.7%) | ||
| 3 | 28 (18.9%) | 17 (31.5%) | ||
| Neutrophil : lymphocyte ratio, median (range) | 2.38 (0.50–10.10) | 2.85 (0.94–17.25) | 0.161 | |
| Preoperative albumin, g/dl, median (range) | 44 (29–51) | 43 (34–51) | 0.102 | |
| POSSUM physiological score, median (range) | 16 (12–32) | 17 (13–30) | 0.720 | |
| RFA included, n (%) | Yes | 11 (7.4%) | 4 (7.4%) | 1.000 |
| No | 137 (92.6%) | 50 (92.6%) | ||
| Wedge resection included, n (%) | Yes | 68 (45.9%) | 21 (38.9%) | 0.425 |
| No | 80 (54.1%) | 33 (61.1%) | ||
| Number of segments resected, median (range) | 4 (1–6) | 3 (1–6) | 0.617 | |
| Repeat operation, n (%) | Yes | 15 (10.1%) | 4 (7.4%) | 0.786 |
| No | 133 (89.9%) | 50 (92.6%) | ||
| Curative resection, n (%) | Yes | 136 (91.9%) | 53 (98.1%) | 0.191 |
| No | 12 (8.1%) | 1 (1.9%) | ||
| Number of liver metastases, median (range) | 2 (1–10) | 1 (1–8) | 0.317 | |
| Maximum diameter of metastases, mm, median (range) | 30 (3–120) | 35 (5–120) | 0.063 | |
| Resection margin, n (%) | R0 | 115 (77.7%) | 44 (81.5%) | 0.698 |
| R1 | 33 (22.3%) | 10 (18.5%) | ||
MRI, magnetic resonance imaging;
PET, positron emission tomography;
BMI, body mass index;
ASA, American Society of Anesthesiologists;
RFA, radiofrequency ablation.
Data for 18 patients were excluded from the disease-free survival analysis because their resections were non-curative or they did not complete a staged resection. Data for a further 11 patients were excluded because these patients died without undergoing surveillance imaging. This left a total of 266 patients for analysis. The median length of follow-up was 1.07 years (range: 0.14–6.59 years) in the least deprived categories and 1.14 years (range: 0.21–7.36 years) in the most deprived categories (P = 0.511). The median number of surveillance scans performed was three (range: one to nine) in both the least and most deprived groups (P = 0.938). Tumour recurrence occurred in 163 patients; there was no difference in recurrence rate [77/133 (57.9%) versus 30/50 (60.0%); P = 0.867] or median time to recurrence between patients in the two least deprived (0.56 years; range: 0.14–2.74 years) and two most deprived (0.61 years; range: 0.21–3.91 years) categories (P = 0.305). There was no difference among the disease-free survival curves for each category of SES (P = 0.913) (Fig. 1).
Figure 1.
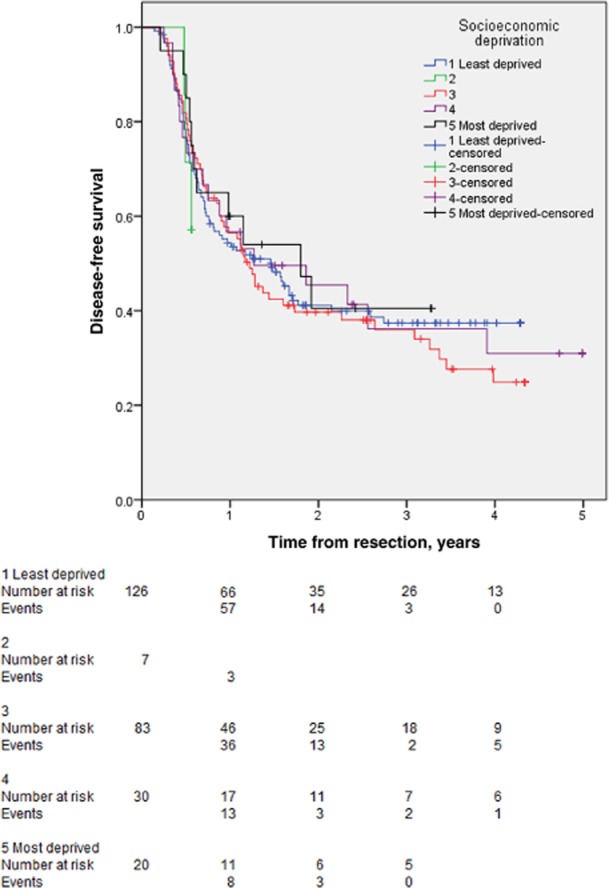
Kaplan–Meier curves for disease-free survival in 303 patients with colorectal liver metastases submitted to liver resection stratified according to socioeconomic status. Categories 1–5 represent, respectively: wealthy achievers; the urban prosperous; the comfortably off; those of moderate means, and the hard-pressed
Among those patients who underwent planned curative resections and for whom socioeconomic data were available, including those in whom no surveillance imaging was performed (n = 277), there were a total of 96 deaths during the study period (34.7%). There was no significant difference in mortality rate between patients in the two least deprived categories (42/136, 30.9%) and those in the two most deprived categories (18/53, 34.0%) (P = 0.729). Twelve patients died within 90 days of surgery (4.3%), but there was no significant difference in 90-day mortality between patients in the two least deprived (4/136, 2.9%) and those in the two most deprived (3/53, 5.7%) categories (P = 0.403). There was no difference in overall survival curves across categories of SES (P = 0.190) (Fig. 2).
Figure 2.
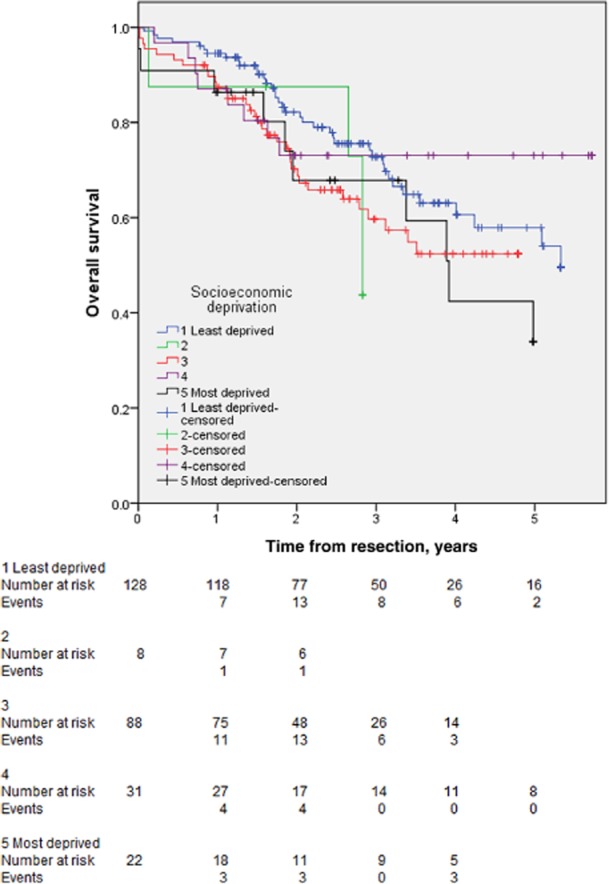
Kaplan–Meier curves for overall survival in 303 patients with colorectal liver metastases submitted to liver resection stratified according to socioeconomic status. Categories 1–5 represent, respectively: wealthy achievers; the urban prosperous; the comfortably off; those of moderate means, and the hard-pressed
Multivariate analysis of factors potentially associated with tumour recurrence (Table 4) demonstrated no association between SES and tumour recurrence (P = 0.700). Only the number of liver metastases (P = 0.014) and maximum tumour diameter (P = 0.001) were associated with tumour recurrence. Each additional liver metastasis increased the risk for recurrence by a factor of 1.28, and each additional millimetre in tumour diameter had a small effect, increasing the risk for recurrence by a factor of 1.02.
Table 4.
Univariate and multivariate analyses of factors associated with tumour recurrence following liver resection for colorectal liver metastases in 266 patients
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Not recurred (n = 103) | Recurred (n = 163) | P-value | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | |||
| Agea, years, median (range) | 65 (36–88) | 67 (33–87) | 0.872 | |||||
| Gender, n (%) | Male | 67 (65.0%) | 105 (64.4%) | 0.863 | ||||
| Female | 36 (35.0%) | 58 (35.6%) | ||||||
| SES category, n (%) | 1 (least deprived) | 52 (50.5%) | 74 (45.4%) | 0.700 | ||||
| 2 | 4 (3.9%) | 3 (1.8%) | ||||||
| 3 | 27 (26.2%) | 56 (34.4%) | ||||||
| 4 | 11 (10.7%) | 19 (11.7%) | ||||||
| 5 (most deprived) | 9 (8.7%) | 11 (6.7%) | ||||||
| T stage of primary, n (%) | 0, 1 or 2 | 10 (9.7%) | 18 (11.0%) | 0.147a | 0, 1, 2 versus 3 | 0.504 | 0.79 (0.40–1.56) | |
| 3 | 61 (59.2%) | 93 (57.1%) | 3 versus 4 | 0.706 | 1.11 (0.68–1.80) | |||
| 4 | 29 (28.2%) | 47 (28.8%) | ||||||
| N stage of primary, n (%) | 0 | 50 (48.5%) | 67 (41.1%) | 0.828 | ||||
| 1 | 34 (33.0%) | 53 (32.5%) | ||||||
| 2 | 18 (17.5%) | 40 (24.5%) | ||||||
| Site of primary colorectal tumour, n (%) | Colon | 55 (53.4%) | 75 (46.0%) | 0.389 | ||||
| Rectum | 48 (46.6%) | 88 (54.0%) | ||||||
| Timing, n (%) | Synchronous | 46 (44.7%) | 78 (47.9%) | 0.584 | ||||
| Metachronous | 57 (55.3%) | 85 (52.1%) | ||||||
| Preoperative chemotherapy, n (%) | 47 (45.6%) | 85 (52.1%) | 0.412 | |||||
| Preoperative diabetes, n (%) | 7 (6.8%) | 15 (9.2%) | 0.523 | |||||
| BMI, kg/m2a, median (range) | 27 (19–54) | 27 (16–50) | 0.518 | |||||
| ASA class, median (range) | 2 (1–3) | 2 (1–3) | 0.566 | |||||
| Preoperative albumin, g/dla, median (range) | 44 (34–50) | 44 (26–52) | 0.949 | |||||
| POSSUM physiological scorea, median (range) | 17 (12–32) | 16 (12–32) | 0.378 | |||||
| Neutrophil : lymphocyte ratio (preop)a, median (range) | 2.4 (0.5–17.3) | 2.6 (0.7–9.1) | 0.211b | 1.09 (0.95–1.26) | 0.079 | 1.09 (0.94–1.28) | ||
| Preoperative MRI, n (%) | 59 (57.3%) | 87 (53.4%) | 0.640 | |||||
| Preoperative PET, n (%) | 68 (66.0%) | 110 (67.5%) | 0.930 | |||||
| Wedge resection included, n (%) | 31 (30.1%) | 67 (41.1%) | 0.117b | 0.062 | 1.70 (0.98–2.94) | |||
| RFA included, n (%) | 6 (5.8%) | 10 (6.1%) | 0.959 | |||||
| Number of segments resecteda, median (range) | 4 (1–6) | 4 (1–6) | 0.222b | 1.10 (0.94–1.28) | 0.120 | 1.00 (0.94–1.07) | ||
| Repeat operation, n (%) | 11 (10.7%) | 18 (11.0%) | 0.105b | 0.824 | 1.04 (0.39–2.77) | |||
| Number of tumoursa, median (range) | 1 (1–7) | 2 (1–10) | 0.007b | 1.29 (1.07–1.55) | 0.014 | 1.28 (1.06–1.56) | ||
| Largest tumour diameter, mma, median (range) | 28 (3–155) | 35 (6–150) | 0.002b | 1.02 (1.01–1.03) | 0.001 | 1.02 (1.00–1.04) | ||
| Resection margin <1 mm (R1), n (%) | 18 (17.5%) | 45 (21.2%) | 0.212b | 0.372 | 1.03 (0.94–1.07) | |||
In univariate analysis continuous variables were tested with logistic regression. Categorical variables were tested with the chi-squared test.
Significant at the level of <0.25 for univariate analysis and tested in multivariate analysis.
95% CI, 95% confidence interval; SES, socioeconomic status; BMI, body mass index; ASA, American Society of Anesthesiologists; MRI, magnetic resonance imaging; PET, positron emission tomography; RFA, radiofrequency ablation.
Discussion
The principal finding of this study is that the SES of patients undergoing liver resection for CLM is not representative of that of the local population because the proportion of patients from the least deprived categories is higher than expected and that of patients from the most deprived categories is lower than expected. Amongst patients undergoing liver resection for CLM, the degree of socioeconomic deprivation had no effect on tumour recurrence after resection.
The finding that people from the least deprived categories of SES account for a higher proportion of patients undergoing liver surgery for CLM than they do in the local population is significant and in keeping with the present authors' clinical observations. The comparison is subject to bias as the incidence of colorectal cancer is influenced by SES and the disease is more common in populations with greater levels of deprivation.1 This would tend therefore to increase the differences observed in the proportions of the different population categories submitted to liver surgery in comparison with those within the local population because CLM would be expected to occur more commonly amongst patients of lower SES. There are many potential reasons why patients with the least deprivation are more likely to undergo surgery for CLM, despite being at lower risk for the development of colorectal cancer. Patients with higher levels of deprivation are more likely to suffer postoperative complications and death following primary colorectal cancer surgery16 and are likely to have more or more severe comorbidities that render them unfit for further surgery. Socioeconomic status is associated with educational attainment,17 and patients with greater deprivation may be less aware of the potential benefits of treatment for metastatic disease. This may affect patients' willingness to engage with longterm surveillance to detect metachronous disease and to seek referral to an HPB unit. There is also an element of discretion by clinical practitioners in many stages of the patient pathway prior to surgery for CLM, which may be influenced by perceptions of degree of socioeconomic deprivation.
Interestingly, there was a large disparity in the use of staging PET scans, which were performed in 74.5% of patients from the least deprived groups compared with only 30.4% of patients from the most deprived. This may be partly explained by the higher incidence of T4 primary tumours amongst the least deprived patients, which is one of the indications for PET scans in national guidelines,6 but is not otherwise explicable by the other measures of disease burden used in this study.
There was no difference in objective measures of health between patients in the highest and lowest categories of SES, as shown by the presence of preoperative diabetes, physiological score or body mass index. This may reflect the greater selection of patients from more deprived groups, in whom the rate of these markers of poor health might be expected to be higher. There was, however, a small difference in subjective measures of health as determined by ASA grade.
To categorize SES, this study used the ACORN® system, which has been used in a number of epidemiological studies.18–21 This system has advantages in that economic data are drawn from a wide range of sources in addition to property values. Other studies addressing the influence of SES on health care outcomes have used the income domain of the Index of Multiple Deprivation (IMD) score22 and the Townsend index.23 These systems have been used simultaneously in previous studies24,25 and neither method has been shown to be superior. Moreover, the difficulties of analysing and interpreting socioeconomic data have been described.26 However, the systems allow for the valid and simultaneous comparison of different populations in contexts in which potential bias and inaccuracy will affect the populations under study equally.
In a manner reflecting the findings of previous work,8 degree of socioeconomic deprivation was not shown to be associated with either 90-day mortality or disease recurrence. The most likely explanation for this to be derived from the present data is not that SES does not affect these outcomes, but that greater selection occurs amongst patients of lower SES to favour patients who are likely to have better outcomes.
The difference in the rates of liver resection for CLM according to SES may reflect selection on the basis of objective health measures. However, further study is required to confirm this and to ensure equity of access to specialized hepatobiliary services within a publicly funded health care system. Similar differences may be found in other countries, especially those with systems of predominantly private health insurance, and the selection of patients for surgery on the basis of SES may influence the comparison of outcomes between countries.
Acknowledgments
The authors are grateful to Bowel Cancer West (registered charity number 1140271) for its charitable grant to obtain the ACORN® socioeconomic deprivation data. Bowel Cancer West had no other involvement in the collection, analysis and interpretation of data, the writing of the manuscript or the decision to submit for publication.
Conflicts of interest
None declared.
References
- National Cancer Intelligence Network. 2008. pp. 1–34. Cancer Incidence by Deprivation, England, 1995–2004.
- Doubeni C, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y. Socioeconomic status and the risk of colorectal cancer. Cancer. 2012;118:3636–3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller H, Sandin F, Robinson D, Bray F, Klint S, Linklater KM. Colorectal cancer survival in socioeconomic groups in England: variation is mainly in the short term after diagnosis. Eur J Cancer. 2012;48:46–53. doi: 10.1016/j.ejca.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Aarts MJ, Lemmens VEPP, Louwman MWJ, Kunst AE, Coebergh JWW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46:2681–2695. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Eloranta S, Lambert PC, Cavalli-Bjorkman N, Andersson TM-L, Glimelius B, Dickman PW. Does socioeconomic status influence the prospect of cure from colon cancer – a population-based study in Sweden 1965–2000. Eur J Cancer. 2010;46:2965–2972. doi: 10.1016/j.ejca.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl. 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos KA, Bird N. Current diagnostic and therapeutic approaches for colorectal cancer liver metastasis. Hippokratia. 2008;12:132–138. [PMC free article] [PubMed] [Google Scholar]
- Neal CP, Mann CD, Sutton CD, Garcea G, Ong SL, Steward WP. Evaluation of the prognostic value of systemic inflammation and socioeconomic deprivation in patients with resectable colorectal liver metastases. Eur J Cancer. 2009;45:56–64. doi: 10.1016/j.ejca.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Whitehouse LE, Farrell T, Nickerson C, Thomas JD, Quirke P. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without the English NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107:757–764. doi: 10.1038/bjc.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasco G, Gallerani E. Treatment of liver metastases from colorectal cancer: what is the best approach today? Dig Liver Dis. 2001;33:438–444. doi: 10.1016/s1590-8658(01)80018-9. [DOI] [PubMed] [Google Scholar]
- Andreou A, Aloia T, Brouquet A, Vauthey J-N. Recent advances in the curative treatment of colorectal liver metastases. Gastrointest Cancer Res. 2011;4(Suppl):2–8. [PMC free article] [PubMed] [Google Scholar]
- Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- CACI Ltd. 2013. Acorn Technical Document. Available at http://acorn.caci.co.uk/downloads/Acorn-Technical-document.pdf (last accessed 11 June 2014)
- Agresti A. An Introduction to Categorical Data Analysis, 2nd edn. Hoboken, NJ: John Wiley; 2002. [Google Scholar]
- R Foundation for Statistical Computing. 2011. R Version 170. Available at http://www.r-project.org/ (last accessed 6 June 2014)
- Smith JJ, Tilney HS, Heriot AG, Darzi AW, Forbes H, Thompson MR. Social deprivation and outcomes in colorectal cancer. Br J Surg. 2006;93:1123–1131. doi: 10.1002/bjs.5357. [DOI] [PubMed] [Google Scholar]
- Department for Children, Schools and Families. 2009. Deprivation and Education – The evidence on pupils in England, foundation stage to key stage 4. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/222172/DCSF-RTP-09-01.pdf (last accessed 30 September 2013)
- Beswick S, Affleck P, Elliott F, Gerry E, Boon A, Bale L. Environmental risk factors for relapse of melanoma. Eur J Cancer. 2008;44:1717–1725. doi: 10.1016/j.ejca.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SM, Carey IM, DeWilde S, Richards N, Cook DG. Trends and inequities in beta-blocker prescribing for heart failure. Br J Gen Pract. 2008;58:862–869. doi: 10.3399/bjgp08X376195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, Jones ME, Schoemaker MJ, Hemming J, Thomas D, Williamson J. The Breakthrough Generations Study: design of a longterm UK cohort study to investigate breast cancer aetiology. Br J Cancer. 2011;105:911–917. doi: 10.1038/bjc.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWilde S, Carey IM, Emmas C, Richards N, Cook DG. Trends in the prevalence of diagnosed atrial fibrillation, its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart. 2006;92:1064–1070. doi: 10.1136/hrt.2005.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department for Communities and Local Government London DCLG. 2010. pp. 1–4. English Indices of Deprivation 2010, pp. .
- Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality in the North. London: Routledge; 1988. [Google Scholar]
- Cheyne L, Taylor A, Milton R, Fear J, Callister MEJ. Social deprivation does not affect lung cancer stage at presentation or disease outcome. Lung Cancer. 2013;81:247–251. doi: 10.1016/j.lungcan.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Frater A, Roderick P, Moon G, Halloran S. Do geodemographic typologies explain variations in uptake in colorectal cancer screening? An assessment using routine screening data in the south of England. J Public Health (Oxf) 2010;32:572–581. doi: 10.1093/pubmed/fdq025. [DOI] [PubMed] [Google Scholar]
- Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–1023. [PMC free article] [PubMed] [Google Scholar]


