Abstract
Background
The aim of the authors was to reassess the impact of a positive surgical margin (R1) after a liver resection for colorectal liver metastases (CLMs) on survival in the era of modern chemotherapy, through their own experience and a literature review.
Methods
Inclusion criteria were: R1 or R0 resection with no local treatment modalities, extra-hepatic metastases or other cancer.
Results
Among 337 patients operated between 2000 and 2010, 273 patients were eligible (214 R0/59 R1). The mean follow-up was 43 ± 29 months. Compared with a R0 resection, a R1 resection offered a lower 5-year overall (39.1% versus 54.2%, P = 0.010), disease-free (15.2% versus 31.1%, P = 0.021) and progression-free (i.e. time to the first non-curable recurrence; 33.1% versus 47.3%, P = 0.033) survival rates. Metastases in the R1 group were more numerous, larger and more frequently synchronous. Independent factors of poor survival were: number, size and short-time interval of CLM occurrence, N status, rectal primary, absence of adjuvant chemotherapy, but not a R1 resection. With the more-systematic administration of chemotherapy since 2005, the intergroup difference in progression-free survival disappeared (P = 0.264).
Conclusion
A R1 resection had no prognostic value per se but reflected a more severe disease. The recent change in the prognostic value of a R1 resection may be linked to the beneficial effect of chemotherapy.
Introduction
Recent advances in chemotherapy and surgical techniques have enabled surgeons to extend the indication for resection of colorectal liver metastases (CLM). Multiple, massive and unfavourably located CLMs can now be resected, leading to complex hepatic resections that are more likely to incur a R1 resection. Historically, microscopically incomplete R1 resections have been associated with an elevated risk of recurrence at the surgical margin1,2 and significantly lower survival rates.3,4 Until the 1990s, a 10-mm margin was advocated because of the existence of micrometastases up to 10 mm around the tumour.5 Nevertheless, at that time, patients did not receive peri-operative chemotherapy. In the early 2000s, Elias et al.6 showed that the prognosis did not depend on the margin width (as long as the margin was negative). In 2003, the French guidelines for clinical practice recommended a 5-mm margin instead of a 10-mm margin.7 In 2008, de Haas et al. showed no difference in overall survival (OS) between R0 and R1 patients and defined the R1 ‘by necessity’ conditioning the resectability.8 In contrast, Dhir et al.'s9 2011 meta-analysis showed that a 10-mm negative margin was associated with better survival than a 1-to 10-mm negative margin was. Another recent study showed that patients with positive resection margins did not survive for more than 10 years.10 There are several possible explanations for these discrepancies: (i) distant recurrences as well as margin recurrences both impact survival, (ii) a repeat hepatectomy or local treatment of a margin recurrence counterbalances the poor prognosis of a R1 resection and (iii) variations in the inclusion period and inconsistent consideration of whether peri-operative chemotherapy had been administered precluded an analysis of the impact of continuous improvements in chemotherapy over time.
The aim of the present study was to analyse a population of patients operated on in the authors' university medical centre in the 2000s, focusing on a period of more systematic administration of chemotherapy (2005–2010), and to determine the influence of margin status on survival in the era of modern liver surgery and chemotherapy regimens.
Patients and method
Study population
An analysis of the authors' prospectively-completed computerized database established that 337 patients had been operated on for CLMs in the authors' institution between 2000 and 2010. Patients were eligible for the study if they met the following criteria: a complete macroscopic resection with clearly described surgical margins in a histopathological examination, no evidence of concomitant extrahepatic disease, no simultaneous use of local treatments (e.g. radiofrequency ablation) and no history of other types of cancer. In patients who received peri-operative chemotherapy, 5-fluorouracil was administered with leucovorin, oxaliplatin or irinotecan. Pre-operative chemotherapy was administered when liver metastases were initially unresectable (i.e. inability to leave an adequate remnant liver volume after complete removal of all CLMs) or in a neoadjuvant setting in patients with synchronous CLMs (CLMs having appeared within the previous 6 months) or marginally resectable CLMs (≥5 bilateral nodules).8 As a rule, most hepatic resections were performed 1 month after the end of chemotherapy.11 Only chemotherapy administered within the 3 months preceding surgery was considered as neoadjuvant chemotherapy. With a few exceptions, only patients with down-sized or stable disease after chemotherapy were candidates for a liver resection. Given that the number of patients having undergone chemotherapy was significantly greater in recent years, the authors also compared two time periods, before and after 2005, also the year in which targeted therapies such as bevacizumab and cetuximab were introduced.
Pre-operative evaluation
Pre-operative staging included a physical examination, routine blood tests and serum tumour marker assays [carcinoembryonic antigen (CEA) and/or carbohydrate antigen (CA) 19-9], colonoscopy, abdominal imaging by multislice computed tomography (CT) and/or magnetic resonance imaging (MRI) of the liver in the month before a laparotomy, and chest imaging by routine radiography or CT. An 18F-FDG-PET scan was performed in patients with more severe disease (e.g. multiple, synchronous, bilobar CLMs).12 Disease resectability was determined in a multidisciplinary assessment by a team of surgeons and medical oncologists. Unresectability was usually based on an insufficient remnant liver volume.13 The type of hepatic resection was planned pre-operatively on the basis of the CLMs' characteristics in pre-chemotherapy and pre-operative CT scans.
Liver resection
The surgical techniques and the various vascular control methods used to reduce the intra-operative bleeding have been described elsewhere.13,14 A hepatic parenchymal transection was performed mostly with a compact ultrasonic surgical aspirator (CUSA; Dissectron®; Integra LifeSciences, Plainsboro, NJ, USA) or, if not, with a Kelly clamp crushing technique. The surgical goal was to achieve complete resections with a tumour-free margin for all the initially-identified tumour deposits (including missing metastases). If a tumour-free margin could not be obtained, the resection was still performed when macroscopically-complete resection of all metastases could be achieved. In accordance with the International Union Against Cancer guidelines,15 a R0 resection was defined as any microscopically-complete resection with a margin ≥1 mm, and a R1 resection was defined as a complete macroscopic resection with microscopic tumour invasion of the resection margin (tumor free margin <1 mm). Three-month post-operative morbidity and mortality were rated according to the Clavien–Dindo classification.16 Complications were defined as severe when they required repeat surgery or resulted in organ failure or patient death (grades III to V16).
Follow-up
Administration of adjuvant chemotherapy was discussed in a multidisciplinary staff meeting and by taking account of the patient's history and histopathological data. Patients were monitored by the referring surgeon and/or oncologist, with a physical examination, liver biochemistry assays, CEA/CA19-9 assays and imaging of the abdomen every 3–6 months and a chest CT every year. The goal of this regular follow-up was to offer curative treatment in the event of recurrence where possible. A diagnosis of recurrence was based on elevated CEA/CA19-9 levels and characteristic imaging findings. The authors also analysed patterns of recurrence and the corresponding treatments.
Statistical analysis
Continuous data were expressed as the mean ± standard deviation and compared using the independent-sample t-test or Mann–Whitney U-test, as appropriate. Categorical data were compared using a chi-squared test or Fisher exact test, as appropriate. The study's primary endpoint was the survival time after a hepatectomy. OS was defined as the time interval between the hepatectomy and death or last follow-up. Disease-free survival (DFS) was defined as the time interval between the hepatectomy and first post-operative recurrence or death. In order to consider the pattern of recurrence and curability, the authors analysed the progression-free survival (PFS) defined in that purpose as the time interval between the hepatectomy and either the first recurrence that could not be treated curatively or death.8 The latter parameter was investigated in order to assess putative relationships between the pattern of recurrence, previous chemotherapy and the likelihood of curative treatment. Survival rates were calculated according to the Kaplan–Meier method and compared in a log-rank test. To identify independent survival factors, factors with P < 0.2 and their first interactions were included in a backward step-by-step Cox proportional hazards model. Predictive independent factors of a R1 resection were identified using a logistic regression method. For assessing the effect of group (R0, R1) on each outcome, we used a propensity score method17 to adjust the analysis for observable differences between R1 and R0 patients. To compute the propensity scores, we used multivariate logistic regression with group (R0 and R1) as the dependent variable and independent variables selected from the multivariate analysis, as significantly associated with one of the three outcomes.18 Lastly, the effect of group on each outcome (OS, DFS and PFS) was assessed using a Cox multivariate regression model with group and propensity score as dependent variables. All analyses were performed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
Patients
Out of a total of 337 patients, 64 (18.9%) were excluded because of a macroscopically incomplete (R2) resection (n = 6), concurrent extrahepatic disease (n = 28), a history of other cancers (n = 14), concomitant local treatment (n = 10) or because the final histological diagnosis did not correspond to CLMs (n = 6). Of the 273 eligible patients (81.1%), 59 patients (21.6%) had an R1 resection. Comparison of demographics, treatment and potential predictive variables of an R1 resection are shown in Table 1. Overall, patients in the R1 group displayed a higher number of metastases (which were often synchronous and bilaterally distributed) and/or a larger metastasis size, reflecting worse tumour biology.
Table 1.
Clinicopathological features, operative procedures and post-operative outcome
| R0 (n = 214) | R1 (n = 59) | P | |
|---|---|---|---|
| Age | 62.7 ± 10.2 | 62.7 ± 10.4 | 0.976 |
| Primary tumour | |||
| Colonic origin | 158 (73.8%) | 46 (78%) | 0.518 |
| Rectal origin | 56 (26.2%) | 13 (22%) | |
| CLM | |||
| Synchronous | 115 (53.8%) | 40 (67.8%) | 0.054 |
| Bilobar | 36 (16.8%) | 17 (28.8%) | 0.039 |
| CEA ≥10 UI/l | 98 (45.8%) | 37 (62.7%) | 0.021 |
| Peri-operative chemotherapy | 181 (84.6%) | 52 (88.1%) | 0.494 |
| Neoadjuvant chemotherapy | 133 (62.1%) | 43 (72.9%) | 0.127 |
| Number of cycles | 8.5 ± 5.2 (2–32) | 10.8 ± 6.2 (2–28) | 0.010 |
| LV5-FU2 | 5 (3.75%) | 0 (0%) | |
| FOLFOX or FOLFIRI | 87 (65.4%) | 28 (65.1%) | |
| FOLFOX + FOLFIRI | 16 (12%) | 5 (11.6%) | |
| FOLFOX or FOLFIRI + Biotherapy | 21 (15.8%) | 9 (20.9%) | |
| FOLFOX + FOLFIRI + Biotherapy | 4 (3%) | 1 (2.3%) | |
| Adjuvant chemotherapy | 127 (59.3%) | 31 (52.5%) | 0.349 |
| Number of cycles | 7.4 ± 3.3 | 6.7 ± 2.8 | 0.192 |
| LV5-FU2 | 12 (9.4%) | 4 (12.9%) | |
| FOLFOX or FOLFIRI | 80 (63%) | 17 (54.8%) | |
| FOLFOX + FOLFIRI | 10 (7.9%) | 3 (0.95%) | |
| FOLFOX or FOLFIRI + Biotherapy | 24 (18.9%) | 7 (22.6%) | |
| FOLFOX + FOLFIRI + Biotherapy | 1 (0.8%) | 0 (0%) | |
| Radiological response to chemotherapy | 0.840 | ||
| Response (%) | 79 (59.4%) | 25 (58.1%) | |
| Stabilization (%) | 45 (33.8%) | 16 (37.2%) | |
| Progression (%) | 9 (6.8%) | 2 (4.7%) | |
| Type of hepatic resection | |||
| Anatomical or Wedge | 168 (78.5%) | 37 (62.7%) | 0.013 |
| Anatomical + Wedge | 46 (21.5%) | 22 (37.3%) | |
| Major (≥3 segments) | 131 (61.2%) | 39 (66.1%) | 0.493 |
| Extended (≥5 segments) | 24 (11.2%) | 14 (23.7%) | 0.014 |
| Operative time (min) | 283 (80–645) | 323 (120–780) | 0.030 |
| Portal triad clamping | 79 (36.9%) | 26 (44.1%) | 0.317 |
| Total ischaemia (min) | 20.1 ± 44.5 (0–110) | 20.5 ± 23.7 (0–93) | 0.962 |
| Blood loss (ml) | 470 ± 360 (10–2400) | 597 ± 549 (10–3500) | 0.110 |
| Intra-operative transfusion | 6 (2.8%) | 5 (8.5%) | 0.064 |
| Transection CUSA | 168 (78.5%) | 44 (74.6%) | 0.521 |
| Number of CLM(s) | 2.6 ± 2.5 | 3.3 ± 2.6 | 0.048 |
| Cumulated diameter (cm) | 4.7 ± 3.4 (1.3–20) | 6.1 ± 3.3 (1.8–13.2) | 0.016 |
| Largest CLM size (cm) | 3.5 ± 2.3 | 4.7 ± 3 | 0.070 |
| At least one CLM ≥30 mm | 117 (54.7%) | 39 (66.1%) | 0.116 |
| Overall morbidity rate | 80 (37.4%) | 26 (44.1%) | 0.351 |
| Severe morbidity rate (grade III–V) | 25 (11.7%) | 11 (18.6%) | 0.162 |
| Mortality rate (Clavien V) | 4 (1.9%) | 2 (3.4%) | 0.613 |
Data were expressed as the mean ± standard deviation; Results with borderline or statistical significance were indicated in bold; CLM, colorectal liver metastasis; CEA, carcinoembryonic antigen.
Recurrence patterns and treatments
After a mean follow-up period of 43 ± 29 months, recurrence had occurred in 184 (67.4%) of the 273 patients (Table 2). Recurrence at the resection margin was associated with another type of intrahepatic recurrence in 4 of the 10 patients in the R1 group and 6 of the 12 patients in the R0 group (P = 0.485). When considering only patients who received neoadjuvant chemotherapy, the difference in recurrence rates at the resection margin was no longer significant between R0 (N = 10, 14.9%) and R1 groups (N = 5; 19.2%; P = 0.754).
Table 2.
Pattern and treatment of recurrences
| R0 (n = 214) | R1 (n = 59) | P | |
|---|---|---|---|
| No recurrence | 75 (35%) | 14 (23.7%) | 0.101 |
| Recurrence | 139 (65%) | 45 (76.3%) | |
| Site of recurrence(s) | |||
| Hepatic | 45 (32.4%) | 19 (42.2%) | |
| Extra hepatic | 50 (36.0%) | 13 (28.9%) | 0.465 |
| Both | 44 (31.6%) | 13 (28.9%) | |
| Time to recurrence (months) | 27.8 ± 28.4 | 20.8 ± 27.5 | 0.029 |
| Hepatic recurrence | 89 (41.6%) | 32 (54.2%) | 0.083 |
| Surgical margin | 12 (13.4%) | 10 (31.3%) | 0.025 |
| First recurrence curative treatment (analysis by patient) | 63 (45.3%) | 16 (35.6%) | 0.250 |
| First hepatic recurrence curative treatment (analysis by site) | |||
| Iterative hepatectomy | 33 (37.1%) | 8 (25%) | 0.216 |
| Including surgical margin recurrence | 6 (6.7%) | 2 (6.2%) | 0.156 |
| Radiofrequency | 2 (2.2%) | 4 (12.5%) | 0.042 |
| Including surgical margin recurrence | 1 (8.3%) | 2 (20%) | 0.429 |
| First extra-hepatic recurrence curative treatment (analysis by site) | |||
| Surgery | 30 (31.9%) | 4 (15.4%) | 0.098 |
| Radiofrequency | 1 (1.1%) | 4 (15.4%) | 0.008 |
| Status at last follow up | |||
| Dead | 82 (38.3%) | 32 (54.2%) | 0.028 |
| Alive without recurrence | 67 (31.3%) | 11 (18.6%) | 0.057 |
| Alive without disease (including iterative curative treatment of recurrence) | 101 (47.2%) | 21 (35.6%) | 0.112 |
| Overall survival (months) | 44.6 ± 28.5 (1–133) | 38.8 ± 30.3 (1–122) | 0.050 |
Data were expressed as the mean ± standard deviation; Results with borderline or statistical significance were indicated in bold.
The five-year OS, PFS and DFS rates for the population as a whole were 51.3%, 46.1% and 28.4%, respectively. The 5-year survival rates for the R1 group were markedly lower than those for the R0 group (OS: 39.1% versus 54.2%, respectively, P = 0.010; DFS: 15.2% versus 31.1%, P = 0.021; PFS: 33.1% versus 47.3%, P = 0.033). With the more systematic use of chemotherapy since 2005 (chemotherapy rates of 88.6%, in association with targeted therapies in 12.8% of patients, versus 79.6% before 2005; P = 0.044), a comparison of 141 R0 patients and 34 R1 patients revealed a significant difference for OS (P = 0.024) and a trend towards a significant difference for DFS (P = 0.081), whereas the difference disappeared for PFS (i.e. time to the first non-curable recurrence; P = 0.264). Univariate analysis and multivariate analysis of factors associated with survival are shown in Table 3. To counter bias as a result of differences in covariate distribution between patients with R1 and R0 resections, a propensity score analysis was performed using multivariable logistic regressions with the group (R0/R1) considered as a dependent variable and 13 independent variables selected from the multivariate analysis (Table 3). There were no statistically significant intergroup differences in terms of the OS rate [hazard ratio (HR): 1.3; 95% confidence interval (CI): 0.79–2.1; P = 0.312], the DFS rate (HR: 1.3; 95% CI: 0.89–1.9; P = 0.171) or the PFS rate (HR: 1.3; 95% CI: 0.83–2; P = 0.240). Hence, in a multivariate analysis, margin status was not a significant predictor of survival.
Table 3.
Univariate (UV) and multivariate (MV) analysis of 5-year overall (OS), disease-free (DFS) and progression-free (PFS) survivals
| n | OS (%) | UV (P-value) | MV (P-value) HR [95%CI] | DFS (%) | UV (P-value) | MV (P-value) HR [95%CI] | PFS (%) | UV (P-value) | MV (P-value) HR [95%CI] | |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | 273 | 51 | 28 | 46 | ||||||
| ≥70 years | 203 | 47 | 0.199 | 29 | 0.569 | 45 | 0.670 | |||
| <70 years | 70 | 52 | 27 | 45 | ||||||
| ASA ≤2 | 234 | 52 | 0.424 | 26 | 0.085 | 45 | 0.828 | |||
| ASA >2 | 39 | 42 | 42 | 41 | ||||||
| Colon | 204 | 55 | 0.118 | 0.054 | 32 | 0.008 | NS | 50 | 0.015 | 0.013 |
| Rectum | 69 | 40 | 1.5 [0.99–2.2] | 18 | 33 | 1.6 [1.1–2.4] | ||||
| CEA | ||||||||||
| <10 | 138 | 57 | 0.140 | 30 | 0.649 | 54 | 0.168 | |||
| ≥10 | 135 | 44 | 27 | 41 | ||||||
| Chemo | ||||||||||
| Neoadjuvant | 176 | 47 | 0.042 | NS | 39 | 0.001 | NS | 52 | 0.003 | NS |
| No | 97 | 57 | 22 | 40 | ||||||
| Adjuvant | 158 | 58 | 0.006 | 0.048 | 25 | 0.889 | 49 | 0.219 | ||
| No | 115 | 41 | 0.6 [0.4–0.9] | 31 | 41 | |||||
| Peri-operative | 233 | 51 | 0.662 | 25 | 0.026 | NS | 45 | 0.376 | ||
| No | 40 | 46 | 30 | 50 | ||||||
| Hepatectomy | ||||||||||
| Major | 170 | 55 | 0.209 | 26 | 0.183 | 42 | 0.246 | |||
| Minor | 103 | 48 | 30 | 50 | ||||||
| ≥5 seg | 38 | 53 | 0.003 | NS | 15 | 0.003 | NS | 28 | 0.001 | NS |
| <5 seg | 235 | 35 | 30 | 47 | ||||||
| Operative time | 0.037 | NS | 0.160 | 0.064 | ||||||
| Blood loss | 0.004 | NS | 0.019 | NS | 0.002 | NS | ||||
| Transfusion | 11 | 40 | 0.087 | 0 | 0.002 | NS | 15 | 0.001 | NS | |
| No | 262 | 50 | 29 | 44 | ||||||
| Complication | 106 | 43 | 0.007 | NS | 24 | 0.442 | 35 | 0.082 | NS | |
| No | 167 | 56 | 29 | 50 | ||||||
| Unilobar | 220 | 50 | 0.990 | 30 | 0.135 | 44 | 0.212 | |||
| Bilobar | 53 | 50 | 18 | 55 | ||||||
| Stage T1 or T2 | 30 | 62 | 0.160 | 33 | 0.276 | 55 | 0.210 | |||
| Stage T3 or T4 | 233 | 49 | 27 | 43 | ||||||
| Stage | ||||||||||
| N0 | 128 | 63 | 0.008 | 0.008 | 39 | 0.005 | NS | 56 | 0.005 | 0.046 |
| N+ | 145 | 44 | 1.7 [1.1–2.7] | 20 | 38 | 1.4 [1.1–2.1] | ||||
| Stage | ||||||||||
| M0 | 118 | 49 | 0.160 | 35 | 0.002 | NS | 52 | 0.012 | NS | |
| M1 | 155 | 54 | 22 | 40 | ||||||
| Time interval of CLM occurrence | 0.075 | NS | 0.001 | 0.005 | 0.004 | 0.038 | ||||
| 0.6 [0.3–0.8] | 0.6[0.6–0.9] | |||||||||
| Number of CLM | 0.001 | 0.003 | 0.001 | 0.006 | 0.001 | 0.018 | ||||
| 1.1 [1.1–1.2] | 1.1 [1.1–1.1] | 1.1 [1.1–1.1] | ||||||||
| Size of larger metastasis | 0.001 | 0.001 | 0.001 | 0.006 | 0.001 | 0.003 | ||||
| 1.1 [1.1–1.2] | 1.1 [1.1–1.2] | 1.1 [1.1–1.2] | ||||||||
| Margin | ||||||||||
| R1 | 59 | 39 | 0.010 | NS | 15 | 0.025 | NS | 33 | 0.037 | NS |
| R0 | 214 | 54 | 31 | 47 | ||||||
| <10 mm | 142 | 58 | 0.940 | 27 | 0.475 | 46 | 0.871 | |||
| ≥10 mm | 72 | 49 | 35 | 48 | ||||||
| <4 mm | 94 | 46 | 0.715 | 24 | 0.460 | 47 | 0.813 | |||
| ≥4 mm | 120 | 57 | 34 | 46 |
Differences in survival between the R0 and R1 groups were estimated using Cox's proportional hazards model and expressed as hazard ratios (HR) with 95% confidence (CI); Results with borderline or statistical significance were indicated in bold. NS, non significant (by multivariate analysis).
Discussion
In the current study population, a positive R1 surgical margin was associated with a poor prognosis by univariate analysis. However, a R1 resection was also associated with more advanced metastatic disease and more complex resections. In a multivariate analysis, the independent predictors of poor survival were related to aggressive tumour biology such as severe metastatic burden (CLM size, number and synchronicity), the N status of the colorectal tumour and the presence of a rectal primary tumour, but not a R1 resection. Moreover, while the survival time to the first non-curable recurrence was worse in the R1 group when considering the overall population, this difference disappeared after 2005 with the more systematic use of chemotherapy and the introduction of targeted therapies, suggesting a chemotherapy-induced change in pattern and curability of recurrences.
For several reasons the prognostic impact of margin status seems questionable. First, in the current population, the outcome in the R0 group did not appear to depend on the margin width, in agreement with previous studies.2,19 Second, it can be assumed that the parenchymal transection technique, i.e. ultrasonic dissector used in more than 75% of patients, has attenuated the impact of a R1 resection. Indeed, the CUSA may crush and suck an additional 2–4 mm of margin, transforming a R0 intra-operative resection into a R1 pathological resection,2 resulting in an overestimation of the proportion of R1 resections.2 In a recent study, the presence of a positive surgical resection margin did not influence local and distant recurrence rates as long as a liver resection was performed with a CUSA ® by an experienced hepatobiliary surgeon.20 Third, in the R1 group, a R1 resection was not a prognostic factor after adjustment for metastatic severity, whereas recurrence at the resection margin in the R1 group was associated with another type of intra-hepatic recurrence in 40% of the patients. In the current series, unlike the margin status, the parameter of tumour biology, such as CLM size, number and synchronicity were independent predictors of poor survival, whether or not patients had received neoadjuvant chemotherapy. Hence, the negative impact of R1 status on overall survival may be related to the more aggressive tumour biology (making resection of the tumour with negative surgical margins more difficult) rather than the residual presence of microscopic tumour cells at the surgical margin.21
The authors furthermore reviewed the largest series having reported on survival rates after a CLM resection.1,2,5,6,8,22–34 Their assessment first showed that the impact of a R1 resection on survival has become progressively questionable over the last 10 years (Fig. 1). This contrasts with the more severe clinical and histopathological characteristics35 and the increasing complexity of a hepatic resection for CLMs.36,37 Increasingly efficient chemotherapy may have changed the long-term outcome after a R1 resection, especially in patients with advanced metastatic disease. In three recent studies on neoadjuvant chemotherapy,24,33,34 there were no differences in survival rates between R0 and R1 resection groups, in particular in patients with optimal morphological or histopathological responses.24 In contrast, the OS with R1 resection was still worse than with R0 for patients with suboptimal responses to chemotherapy.24,33 This beneficial effect of neoadjuvant chemotherapy could be related to the chemotherapy-induced concentric shrinkage of the tumour;38 with chemotherapy, no micrometastases were found more than 4 mm beyond the periphery of the tumour compared with 10 mm without chemotherapy.27 Accordingly, the frequency of microscopic invasion in patients having received pre-hepatectomy chemotherapy was lower than in patients not having received chemotherapy,33,39 thus altering the prognostic impact of margin status. In the current study, the recurrence rate at the surgical margin decreased from 31.3% to 19.2% in the group R1 with the use of neoadjuvant chemotherapy, and subsequently became comparable to that of the R0 group.
Figure 1.
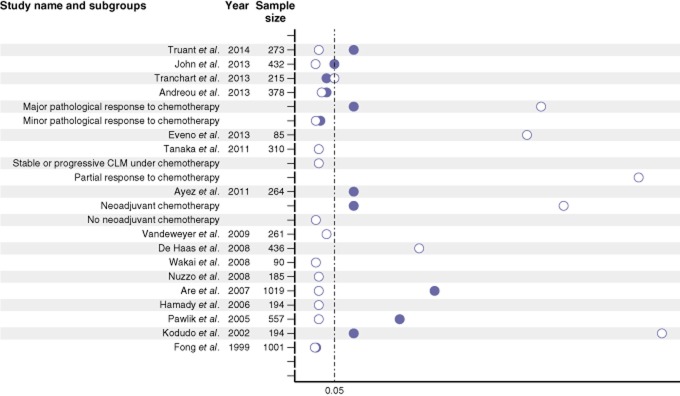
Literature review on the main studies specifically dedicated to margin status for colorectal liver metastase (CLM) for the past 10 years and comparing R0 to R1 resections. Circles represent the P-values of the univariate analysis (white circle) and/or multivariate analysis (black circle). The dotted line represents a significance threshold 0.05
Above all, two recent reports have suggested that peri-operative chemotherapy is related to the pattern of recurrence, which in turn may be related to long-term survival.40,41 Thus, Vigano et al.37 reported that the long-term outcome of a liver resection for CLMs had improved over a 20-year period (even in patients with negative prognostic factors) and suggested that this improvement was related to a reduction in recurrence, better chemotherapy of recurrence and a higher resection rate. De Jong et al. also reported that between 1982 and 2008, adjuvant chemotherapy favourably influenced recurrence rates and patterns after a curative-intent resection of CLMs.42 In contrast, in two recent studies that still found a R1 resection to be independently associated with a poorer survival, the authors did not comment on whether peri-operative chemotherapy could have changed the pattern of recurrence and thus the feasibility of curative treatment.23,24 In our population, the use of adjuvant chemotherapy was independently associated with an improved outcome. Moreover, in spite of the greater disease severity in the R1 group, the difference in PFS (i.e. the survival time to the first non-curable recurrence) between R0 and R1 groups disappeared in patients operated on after 2005 (i.e. once peri-operative chemotherapy and, in some cases, targeted therapies had been administered more systematically). This time period effect on survival suggests that more effective chemotherapy may be associated with a less ominous pattern of recurrence – suggesting the killing of a microscopic residual tumour left behind at the time of surgery, on the resection margin or elsewhere – offering a greater chance of curative treatment.
In conclusion, R1 margin status may be a surrogate indicator of advanced and/or more extensive disease rather than an independent predictor of survival. In today's patients with severe metastatic disease, R1 status' lack of prognostic impact reflects improvements in chemotherapy. Neoadjuvant chemotherapy may help to narrow surgical margins, whereas adjuvant chemotherapy may cure residual micrometastatic disease; both of these approaches should increase the likelihood of curative repeat resection in the event of recurrence. Taken as a whole, these data suggest that peri-operative chemotherapy should not be questioned in patients with severe metastatic disease.
Conflicts of interest
None declared.
References
- Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86:332–337. doi: 10.1046/j.1365-2168.1999.01030.x. [DOI] [PubMed] [Google Scholar]
- Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- Elias D, Cavalcanti A, Sabourin JC, Lassau N, Pignon JP, Ducreux M. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998;24:174–179. doi: 10.1016/s0748-7983(98)92878-5. [DOI] [PubMed] [Google Scholar]
- Adam R, Auclerc G, Bruel J-M, Cherqui D, Domergue J, Dorval E. Quelles métastases hépatiques sont résécables d'emblée? Gastroenterol Clin Biol. 2003;27:11–13. [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234–242. doi: 10.1097/SLA.0b013e318223c609. [DOI] [PubMed] [Google Scholar]
- Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol. 2011;2011:763245. doi: 10.5402/2011/763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh FK, Tilney HS, Tekkis PP, John TG, Rees M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037–1042. doi: 10.1038/sj.bjc.6603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truant S, Huglo D, Hebbar M, Ernst O, Steinling M, Pruvot FR. Prospective evaluation of the impact of [18F]fluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg. 2005;92:362–369. doi: 10.1002/bjs.4843. [DOI] [PubMed] [Google Scholar]
- Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O. Remnant liver volume to body weight ratio > or =0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Boleslawski E, Decanter G, Truant S, Bouras AF, Sulaberidze L, Oberlin O. Right hepatectomy with extra-hepatic vascular division prior to transection: intention-to-treat analysis of a standardized policy. HPB (Oxford) 2012;14:688–699. doi: 10.1111/j.1477-2574.2012.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Wittekind CH. TNM Classification of Malignant Tumours. Hoboken: John Wiley & Sons; 2002. [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB (Oxford) 2010;12:43–49. doi: 10.1111/j.1477-2574.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeweyer D, Neo EL, Chen JW, Maddern GJ, Wilson TG, Padbury RT. Influence of resection margin on survival in hepatic resections for colorectal liver metastases. HPB (Oxford) 2009;11:499–504. doi: 10.1111/j.1477-2574.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchart H, Chirica M, Faron M, Balladur P, Lefevre LB, Svrcek M. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg. 2013;37:2647–2654. doi: 10.1007/s00268-013-2186-3. [DOI] [PubMed] [Google Scholar]
- Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveno C, Karoui M, Gayat E, Luciani A, Auriault ML, Kluger MD. Liver resection for colorectal liver metastases with peri-operative chemotherapy: oncological results of R1 resections. HPB (Oxford) 2013;15:359–364. doi: 10.1111/j.1477-2574.2012.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17:1324–1329. doi: 10.1245/s10434-009-0770-4. [DOI] [PubMed] [Google Scholar]
- Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008;15:2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- Kokudo N, Miki Y, Sugai S, Yanagisawa A, Kato Y, Sakamoto Y. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I. R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy. Eur J Surg Oncol. 2011;37:336–343. doi: 10.1016/j.ejso.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2011;19:1618–1627. doi: 10.1245/s10434-011-2114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. 752–755. [DOI] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P. Impact of expanding criteria for resectability of colorectal metastases on short-and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- Vigano L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol. 2012;19:2035–2044. doi: 10.1245/s10434-011-2186-1. [DOI] [PubMed] [Google Scholar]
- Ng JK, Urbanski SJ, Mangat N, McKay A, Sutherland FR, Dixon E. Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer. 2008;112:362–371. doi: 10.1002/cncr.23184. [DOI] [PubMed] [Google Scholar]
- Parikh AA, Gentner B, Wu TT, Curley SA, Ellis LM, Vauthey JN. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Hill CR, Chagpar RB, Callender GG, Brown RE, Gilbert JE, Martin RC., 2nd Recurrence following hepatectomy for metastatic colorectal cancer: development of a model that predicts patterns of recurrence and survival. Ann Surg Oncol. 2011;19:139–144. doi: 10.1245/s10434-011-1921-y. [DOI] [PubMed] [Google Scholar]
- D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]


