Abstract
We conducted a phase I study to determine (a) the maximum tolerated dose of peri-radiation therapy temozolomide (TMZ) and (b) the safety of a selected hypofractionated intensity modulated radiation therapy (HIMRT) regimen in glioblastoma multiforme (GBM) patients. Patients with histological diagnosis of GBM, Karnofsky performance status (KPS)≥60 and adequate bone marrow function were eligible for the study. All patients received peri-radiation TMZ; 1 week before the beginning of radiation therapy (RT), 1 week after RT and for 3 weeks during RT. Standard 75 mg/m2/day dose was administered to all patients 1 week post-RT. Dose escalation was commenced at level I: 50 mg/m2/day, level II: 65 mg/m2/day and level III: 75 mg/m2/day for 4 weeks. HIMRT was delivered at 52.5 Gy in 15 fractions to the contrast enhancing lesion (or surgical cavity) plus the surrounding edema plus a 2 cm margin. Six men and three women with a median age of 67 years (range, 44–81) and a median KPS of 80 (range, 80–90) were enrolled. Three patients were accrued at each TMZ dose level. Median follow-up was 10 months (range, 1–15). Median progression free survival was 3.9 months (95% confidence interval [CI]: 0.9–7.4; range, 0.9–9.9 months) and the overall survival 12.7 months (95% CI: 2.5–17.6; range, 2.5–20.7 months). Time spent in a KPS ≥70 was 8.1 months (95% CI: 2.4–15.6; range, 2.4–16 months). No instance of irreversible grade 3 or higher acute toxicity was noted. HIMRT at 52.5 Gy in 15 fractions with peri-RT TMZ at a maximum tolerated dose of 75 mg/m2/day for 5 weeks is well tolerated and is able to abate treatment time for these patients.
Keywords: Concurrent, Glioblastoma multiforme, Hypofractionated, Temozolomide
1. Introduction
Glioblastoma multiforme (GBM) is one of the most aggressive and most common glial tumors with an incidence of 5/100,000/ year [1]. The median survival is 12.1 months with surgery and radiation therapy (RT) alone [2]. The addition of temozolomide (TMZ) chemotherapy has resulted in a median survival of 14.6 months [2]. Standard RT for GBM was arrived at in the 1970s and consists of 60 Gy delivered in 30 fractions of 2 Gy each for a total of 6 weeks [3–5]. Clearly in the last 10–15 years there have been great advances in imaging of the area to be irradiated as well as advances in the more precise delivery of radiation using three dimensional (3D) conformal and intensity modulated radiation therapy (IMRT) techniques [6]. IMRT is widely regarded as a technique that enables delivery of very conformal radiation to a target while limiting radiation deposition on surrounding structures at risk; IMRT has been considered and used for GBM.
Considering the limited life expectancy of this group of patients and that conventional treatment may occupy a significant amount of their survival time it would be beneficial to evaluate the hypofractionation schedules delivered with the newest RT techniques to try to shorten the time that patients spend receiving treatment, thereby decreasing patients’ inconvenience and potentially improving the quality of life of their limited survival time. It is not surprising then that there has been a rekindled interest in exploring hypofractionation regimens that, even if equivalent in effectiveness, would be preferable to conventional ones because of their shorter duration in patients with a terminal disease [7–18]. Moreover hypofractionation is associated with reduced costs compared to standard fractionation delivered with the same algorithm. Hypofractionation has been accepted for elderly or poor performance patients [16, 19]. Recently several unconventional fractionation schedules consisting of four to 20 fractions have been proposed for GBM [7–18]. As TMZ has become the standard chemotherapy to be given concomitantly to RT and as TMZ may potentiate the effects of RT, including the undesirable ones, it is imperative to assess the safety of TMZ concomitant with the higher dose of radiation per fraction in hypofractionation regimens [2, 18, 20, 21]. Several treatment regimens of concurrent and post-RT TMZ in combination of various RT fractionation schedules have been proposed [2, 9, 12, 14–16, 18]. However, the data on safety and efficacy of hypofractionated RT with pre-RT, concurrent and post-RT TMZ is lacking. This paper aimed to evaluate in a phase I study the safety of a chemoradiation treatment using hypofractionation intensity modulated radiotherapy (HIMRT) and an escalating peri-RT TMZ dose.
2. Materials and methods
After approval by the Institutional Review Board, a traditional 3 + 3 phase I study [22] was conducted to assess the scope and tolerability of HIMRT with concurrent and adjuvant TMZ. The study was designed to enroll a minimum of three and maximum of 18 patients. All patients who met the inclusion criteria for study and also consented to participate in the trial were required to sign a written informed consent form. The inclusion criteria for the study were de novo GBM and anaplastic astrocytoma, tumors must not involve brain stem or optic chiasm, tumor was diagnosed following biopsy or surgery, age >18 years, Karnofsky performance status (KPS)≥60, adequate bone marrow reserve, normal renal function, and normal liver function. Patients with prior treatment of their brain tumor were excluded. All patients underwent comprehensive standard pre-treatment evaluation.
2.1. RT
RT was started within 4–6 weeks after surgery or biopsy. IMRT was delivered using a linear accelerator with 6 MV photons. Volumetric CT scans fused to volumetric contrast MRI to delineate the target were used for treatment planning. Gross target volume (GTV) was defined as the contrast enhancing area and/or the surgical cavity. Clinical target volume (CTV) was defined as GTV plus surrounding edema (defined by T2-weighted image). A 2 cm margin was added to define the planned target volume (PTV). Our proposed hypofractionation scheme was designed by calculating a 3 week regimen that would have acute (tumor) effects equivalent to 5906 cGy of conventional (2 Gy) fractionation, assuming alpha: beta ratio of 10. Late effects, assuming alpha:beta ratio of 2, were calculated to be equivalent to 7219 cGy at conventional 2 Gy fractions. A total dose of 52.5 Gy over 15 fractions (3.5 Gy per fraction) over 3 consecutive weeks (5 fractions per week) was delivered to the PTV.
2.2. TMZ
A standard phase I 3 + 3 design was followed for dose escalation. TMZ was administered for 5 weeks: 1 week before beginning RT, for 3 weeks during RT, and for 1 week after completion of RT. The dose escalation study was designed to enroll three patients per cohort in successive dose levels. Three escalating dose levels of TMZ were planned; dose level I was 50 mg/m2/day for the first 4 weeks and 75 mg/m2/day for the last 1 week of treatment; dose level II was 65 mg/m2/day for the first 4 weeks and 75 mg/m2/ day for the last 1 week of treatment; and dose level III was 75 mg/m2/day over the entire 5 weeks of treatment. Dose limiting toxicity (DLT) was defined as any adverse event qualifying as irreversible grade 3 and any grade 4–5 toxicity as per the revised USA National Cancer Institute Common Toxicity Criteria (version 3.0) [23]. Dose escalation was to be halted when the maximum tolerated dose (MTD) was reached; MTD was defined as one dose level below the dose at which DLT was observed in one-third or more patients. If one of the three patients in a dose cohort experienced DLT, three more patients were added to the cohort. If no DLT was observed in the group after 5 weeks of treatment then an additional three patients were accrued at the next higher dose level. If two of the three patients at any dose level exhibited DLT then the study was to terminate.
Adjuvant TMZ was commenced 4 weeks after completion of RT. The initial dose of 150 mg/m2/day was used for the first cycle and then increased to 200 mg/m2/day with the second cycle, provided that toxicity was acceptable. Adjuvant TMZ was continued for 5 consecutive days every 28 days for at least six cycles or until the disease progression or DLT was reached. Avastin (Genentech, San Francisco, CA, USA) was started when there was radiological progression of disease after the completion of HIMRT and concurrent TMZ. Oral trimethoprim–sulfamethoxazole was prescribed during concurrent chemoradiation to mitigate the risk of Pneumocystis carinii pneumonia due to TMZ-induced lymphocytopenia. Antiemetic prophylaxis with prochlorperazine and/or a 5 hydroxytryptamine-3 antagonist was typically prescribed prior to concurrent and adjuvant TMZ. All patients continued to receive appropriate treatment of other chronic diseases during and after the protocol therapy.
2.3. Follow-up
All patients were evaluated for any adverse events with laboratory evaluation including serum chemistries and hematologic profile weekly or earlier as needed during the 5 weeks of chemoradiotherapy (primary endpoint). After the initial 5 week period, follow-up visits were arranged monthly or earlier as needed. Neuroradiologic progression (contrast MRI), KPS, hematological analysis and other indicators were evaluated at each follow-up visit. The time to neuroradiological evidence of tumor recurrence or progression, survival time, and time spent in a KPS ≥70 were evaluated as the secondary endpoints.
2.4. Statistical analysis
Mean, standard deviation, median and range for continuous variables, and frequency for discrete data were calculated for patient demographics. The maximum grade for each type of toxicity was recorded for each patient, and frequency tables were provided. Progression free survival (PFS) and overall survival (OS) were used for survival data analysis. Clinical and/or radiographic PFS was defined as the interval from date of definitive (histological) diagnosis to date of clinical and/or radiographic progression, whichever was earlier. OS was determined from the date of diagnosis to death from any cause. Time spent in a KPS ≥70 was calculated from date of diagnosis to KPS decline (KPS <70) or censored at the last date the patient was known with KPS ≥70. Survival curves were estimated using the method of Kaplan–Meier and were displayed graphically.
3. Results
A total of nine patients were enrolled between 2009 and 2012. The median age of the three female and six male patients was 67 years (range, 44–81). Eight patients had a solitary lesion and one patient had multicentric GBM. Gross tumor resection was achieved in two and partial resection in six patients while one patient underwent biopsy. All the lesions were histologically diagnosed as GBM (World Health Organization grade IV). Mean CTV treated was 93.88 cm3 (range, 9.04–330.7 cm3) and mean PTV treated was 205.72 cm3 (range, 82.5–375.8 cm3) (Table 1).
Table 1.
Demographics and treatment details of patients with glioblastoma multiforme
| Total | 9 |
|---|---|
| Sex | |
| Female | 3 (33%) |
| Male | 6 (67%) |
| Age, years | |
| Median | 67 |
| Range | 44–81 |
| Karnofsky performance score at presentation | |
| 80 | 3 (33%) |
| 90 | 6 (67%) |
| Histology proven GBM | 9 (100%) |
| Surgical resection | |
| Gross total resection | 3 (33%) |
| Partial resection | 5 (56%) |
| Biopsy | 1 (11%) |
| Tumor volume treated | |
| − CTV | |
| Mean | 93.88 cm3 |
| Range | 9.04–330.7 cm3 |
| − PTV | |
| Mean | 205.72 cm3 |
| Range | 82.5–375.8 cm3 |
| Adjuvant chemotherapy | |
| TMZ | 9 (100%) |
| TMZ + Avastina | 4 (44%) |
| Thrombocytopenia with adjuvant TMZ | 2 (22%) |
| Tumor lateralization | |
| Left | 5 (56%) |
| Right | 3 (33%) |
| Multiple | 1 (11%) |
| Tumor location | |
| Frontal | 3 (33%) |
| Fronto-temporal | 1 (11%) |
| Temporal | 3 (33%) |
| Parietal | 1 (11%) |
| Multiple | 1 (11%) |
CTV = clinical target volume, GBM = glioblastoma multiforme, PTV = planned target volume, TMZ = temozolomide.
Genentech, San Francisco, CA, USA.
3.1. TMZ and RT response
All but one patient completed HIMRT and TMZ as per protocol. We were able to accomplish the dose escalation protocol without DLT. Following completion of protocol, all but one patient received adjuvant TMZ chemotherapy (one patient died of a non-neurological cause 1 month after the completion of RT). Median number of adjuvant TMZ cycles was five (range, zero–11). Avastin was administered in four patients and one patient received a combination of Avastin and irinotecan.
3.2. Toxicity
The regimen of 5 weeks of TMZ and 3 weeks of HIMRT delivered at the proposed dose was well tolerated. There were no instances of irreversible grade 3 or higher acute hematologic or non-hematologic toxicity. One patient had reversible grade 3 fatigue and somnolence. Acute grade 1 or 2 toxicities are listed in Table 2. One patient required interruption in the post-RT TMZ due to scalp infection; the adjuvant TMZ was then continued as per schedule. One patient developed grade 3 seizure and left motor weakness, which was thought to be secondary to disease progression. One patient developed grade 3 deep venous thrombosis, which was not thought to be related to the treatment. Another two patients experienced grade 4 persistent late thrombocytopenia after receiving at least nine cycles of adjuvant TMZ. Further adjuvant cycles were discontinued in these two patients.
Table 2.
Toxicity grading for adverse effects of cancer treatment seen in this cohort [23]
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Fatigue | 4 | 3 | 1 | 0 |
| Headache | 2 | 2 | 0 | 0 |
| Constipation | 3 | 2 | 0 | 0 |
| Nausea | 2 | 0 | 0 | 0 |
| Partial seizure | 0 | 1 | 0 | 0 |
| Motor deficits | 1 | 1 | 0 | 0 |
| Numbness | 1 | 0 | 0 | 0 |
| Insomnia | 0 | 1 | 0 | 0 |
| Somnolence | 0 | 0 | 1 | 0 |
| Vision changes | 3 | 0 | 0 | 0 |
| Dry skin | 3 | 0 | 0 | 0 |
| Hypertension | 0 | 1 | 0 | 0 |
| Memory deficits | 1 | 0 | 0 | 0 |
| Confusion | 1 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 |
| Oral candidiasis | 1 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 |
| Alopecia | 1 | 0 | 0 | 0 |
| Incontinence | 1 | 0 | 0 | 0 |
| Stomach cramps | 1 | 0 | 0 | 0 |
| High ALT | 4 | 0 | 0 | 0 |
| Low calcium | 1 | 0 | 0 | 0 |
| Imbalance | 1 | 0 | 0 | 0 |
| High glucose | 0 | 0 | 1 | 0 |
| Leucopenia | 1 | 0 | 0 | 0 |
| Anemia | 2 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 2 |
ALT = alanine aminotransferase.
3.3. Follow-up and survival
The median follow-up time was 10 months (range, 1–15 months). Median PFS was 3.9 months (95% confidence interval [CI]: 0.9–7.4; mean, 4.6 months; range, 0.9–9.9 months) (Fig. 1) and the median OS was 12.7 months (95% CI: 2.5–17.6; mean, 11.7 months; range, 2.5–20.7 months). The 1 year survival rate was 50% (Fig. 2). The median time spent in a KPS ≥70 was 8.1 months (95% CI: 2.4–15.6; mean, 8.4 months; range, 2.4–16 months) and the 1 year rate of time spent in KPS ≥70 was 25% (Fig. 3).
Fig. 1.
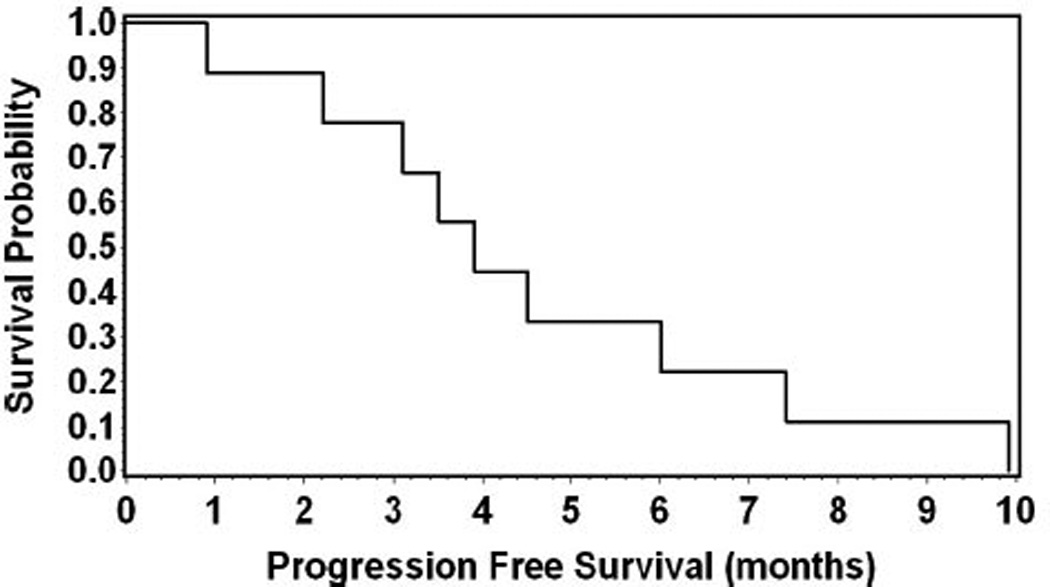
Kaplan–Meier analysis for progression free survival.
Fig. 2.
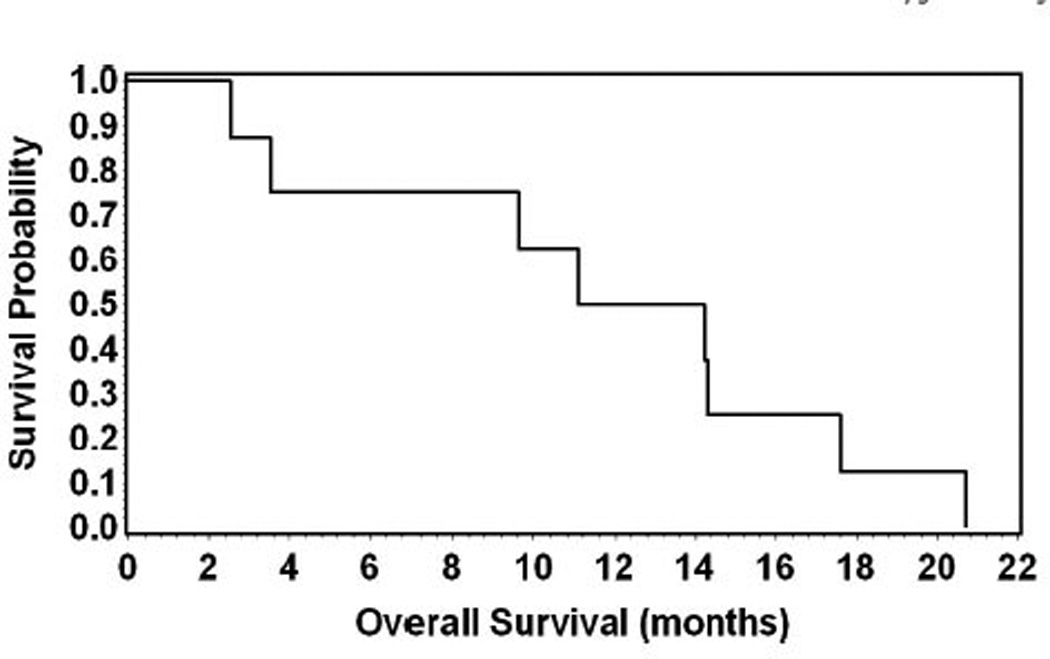
Kaplan–Meier analysis for overall survival.
Fig. 3.
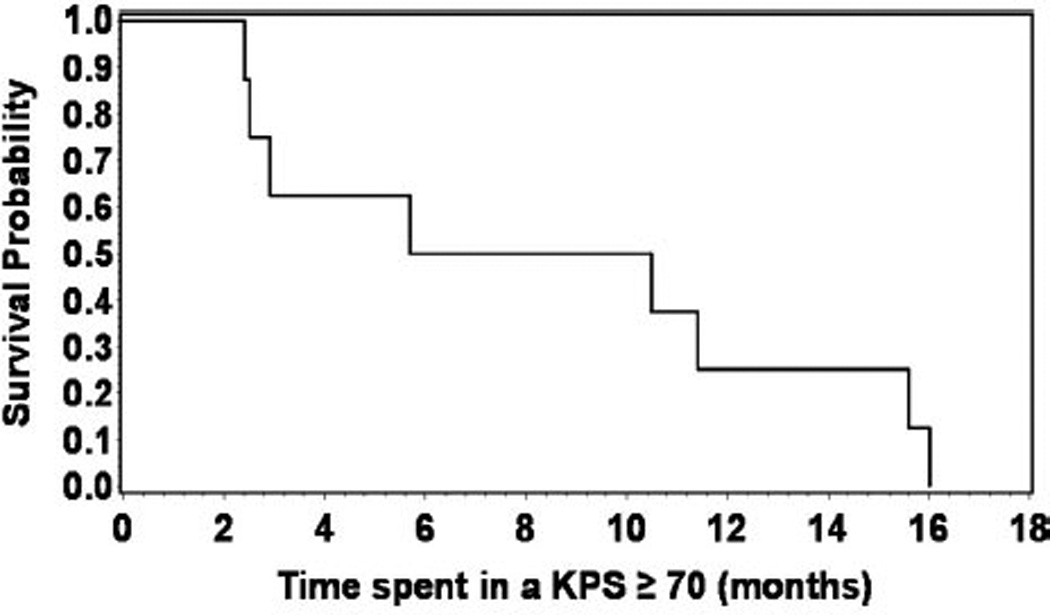
Kaplan–Meier analysis for time spent in a Karnofsky performance status (KPS)≥70.
4. Discussion
The present study was conducted with the goal of administrating a safe dose of chemoradiation that would shorten overall treatment time compared to conventional fractionation, reduce treatment cost and enable delivering increasing dose of radiation per fraction that might increase cell killing. To our knowledge this is the first prospective phase I standard 3 + 3 design study demonstrating the dose of peri-RT TMZ up to 75 mg/m2/day combined with HIMRT at 52.5 Gy in 15 fractions is safe and well tolerated in GBM patients. A few studies have investigated the role of combined hypofractionated radiotherapy with a concurrent TMZ chemotherapy regimen. In a retrospective study of 112 elderly (≥60 years) GBM patients receiving 3D conformal RT (40 Gy in 15 fractions) with concurrent and adjuvant TMZ (57 patients), Cao et al. suggested that the TMZ did not confer a survival benefit in elderly patients with GBM. In addition 9% of patients receiving concurrent TMZ developed grade 3–4 hematological toxicity [7]. Terasaki et al. conducted a prospective trial of 3D conformal RT delivering 45 Gy in 15 fractions with concomitant and adjuvant TMZ in 26 patients with GBM [18]. The 6 month PFS was 65% and median survival was 15.6 months. More importantly the chemoradiation treatment was well tolerated with no grade 3–4 toxicity. However, these authors must have been dealing with unusually small GBM considering that the median diameter of T1-weighted enhancing area was only 5.7 mm (range, 3.6–7.8 mm), despite 10 patients having had only biopsy and eight patients only undergoing partial resection. In a prospective phase I trial of 16 patients with GBM, Chen et al. used IMRT [8]. They employed a dose escalation pattern to a total dose of 60 Gy, 20 daily doses of 3 Gy (three patients), 15 daily doses of 4 Gy (three patients), 12 daily doses of 5 Gy (four patients) and 10 daily doses of 6 Gy (six patients). Concurrent TMZ was given for 28 continuous days starting on first day of RT to all patients. Radiation necrosis (RN) requiring reoperation was observed in 3/16 patients (19%) one each belonging to the 3, 4 and 6 Gy fractionation protocol; 3/16 (19%) and 10/15 (67%) patients developed acute and late grade 3–4 hematological toxicity (one patient developed acute gastrointestinal bleeding not related to treatment preventing adjuvant TMZ). One (7%) patient became blind 7 months after RT with 60 Gy in 15 fractions (grade 4 late non-hematological toxicity).
Another prospective phase II study reported 20 elderly (≥65 years) patients with GBM treated with 40 Gy in 15 fractions using 3D conformal RT and a stereotactic boost delivered in one to three fractions. Concomitant and adjuvant TMZ was used with no acute or late grade 3–4 toxicity [9]. Reddy et al. reported on a phase II prospective trial of 24 newly diagnosed patients with GBM treated with IMRT to 60 Gy in 10 daily fractions with concurrent and adjuvant TMZ [15]. The surgical cavity plus residual tumor shown on T1-weighted brain MRI, with largest diameter of ≤6 cm, was irradiated with 10 fractions of 6 Gy over 2 weeks and 30 Gy in 3 Gy fractions was delivered to the abnormality seen on the T2-weighted MRI. Median OS was 16.6 months; 3/24 (12.5%) patients experienced acute grade 3–4 toxicity (two hematological and one non-hematological: gastrointestinal bleeding preventing adjuvant TMZ) while 11/23 (47%) had grades 3–4 late hematological toxicity. Moreover, 6/24 patients (25%) underwent repeated surgery at a median of 10.3 months after HIMRT for suspected tumor recurrence; four patients had ≥80% necrosis (two had 100% necrosis) and two had between 40 and 70% necrosis [15]. Median GTV plus 5 mm margin and CTV plus 5 mm margin irradiated in the study by Reddy et al. was 97.9 and 258 cm3, respectively [15]. Another retrospective study involved 35 GBM patients treated with IMRT at 60 and 40 Gy to the GTV and PTV, respectively, in 20 fractions with concurrent and adjuvant TMZ. There were no acute grade 3–4 toxicities while one (3%) patient developed late grade 3–4 non-hematological toxicity [14]. In a prospective IMRT dose-escalation study 19 GBM patients were treated with 60–65 Gy to PTV1 (enhancing T1-weighted area and/or surgical cavity + 15 mm margin) and 45 Gy to PTV2 (T2-weighted abnormality + 25 mm margin) with concurrent and adjuvant TMZ. Three of the 19 patients (16%) developed acute hematological toxicity [13]. Minniti et al. published a prospective study involving 43 elderly GBM patients treated using 3D conformal RT with 30 Gy in 6 fractions over 2 weeks [11]. No concurrent TMZ was given; during adjuvant TMZ 28% and 18% of patients developed grade 3 and 4 toxicities, respectively. The present study demonstrated the safety of peri-RT TMZ with the proposed HIMRT regimen. We were able to safely escalate the TMZ dose to the standard 75 mg/m2/day. TMZ has demonstrated in vitro and in vivo increased tumor cell radiosensitivity by an inhibition of DNA repair leading to an increase in mitotic catastrophe [21]. To our knowledge, no conclusive evidence on the radiosensitizing effects of concurrent TMZ in GBM patients is available. We administered 1 week pre-RT TMZ in an attempt to enhance the possible TMZ radiosensitizing effects. The rationale of using pre-RT TMZ has been demonstrated in human GBM-derived cell lines that either possess or lack O6-methylguanine-DNA-methyltransferase (MGMT) activity [24]. These authors concluded that pre-RT TMZ sensitizes the cell line and leads to formation of double-strand breaks within 45 min of radiation exposure [24]. The 1 week pre-RT TMZ would then increase the radiosensitivity and enable increased cell killing with RT. Another study on the primary GBM xenograft demonstrated that 1 week pre-RT TMZ or concomitant RT/TMZ therapy was more effective than post-RT TMZ in prolonging survival in about half of mice with human GBM-xenografts with suppressed MGMT activity [25]. Continued TMZ 1 week after RT was designed to maximize tumor cell toxicity of RT, in addition to alkylating damage to tumor cells that the TMZ can induce on its own. We did not stratify the patients based on their MGMT status, as this was a phase I prospective study conducted with the goal of evaluating the MTD of peri-RT TMZ with proposed hypofractionated regimen.
Hypofractionation offers the advantages of (a) reduced treatment time that is clearly beneficial to patients with a terminal disease, (b) decreased cost, and (c) radiobiological advantages such as increased cell killing associated with increased dose per fraction and reduced accelerated repopulation [14, 18]. IMRT has dosimetric advantages making it possible to treat targets close to vital structures by its ability to be extremely conformal, thereby decreasing the amount of radiation deposited on structures at risk while maintaining coverage of the target; at the same time the increased dose heterogeneity and increased integral dose associated with IMRT may theoretically increase the radionecrosis rate [14, 18]. Designing an optimal hypofractionation regimen involves a balancing act between reducing the number of fractions and increasing the dose per fraction while at the same time being able to exploit the demonstrated benefits of concurrent TMZ. The risk of RN increases significantly with increasing total radiation dose, fraction size, irradiated tumor volume and with the addition of chemotherapy [26–28]. Our PTV was substantial (median, 204.72 cm3; range, 82.5–375.8 cm3) however we had no incidence of radionecrosis. This is in contrast to radionecrosis rates reported by other studies. Reddy et al. administered 60 Gy in a 10 fraction regimen and six (25%) patients with RN required reoperation [15]. Similarly, Chen et al. reported three (19%) patients with RN who required reoperation [8]. Fractionation dose was 60 Gy in 10 fractions in one patient, 15 fractions in another and 20 fractions in the third. Both series prescribed to a volume (median GTV + 5 mm margin was 97 cm3 [15] and 87 cm3 [8] and the median CTV + 5 mm margin was 258 cm3 [15] and 248 cm3 [8]) that was similar to ours. Both series included concurrent TMZ [8, 15]. In general, dose regimens delivering 10 fractions of 5–6 Gy per fraction (with and without TMZ) [8, 9, 15] have demonstrated increased risk of RN compared to the 15–20 fraction dose regimen with and without concurrent/adjuvant TMZ [14, 17, 18].
Our proposed chemoradiation regimen enables delivery of radiation to the sizeable target tumor volume without any instance of RN. Also, the incidence of grade 3–4 acute or late hematological and non-hematological toxicity compares well to other studies using hypofractionation and concurrent/adjuvant TMZ, with reported incidence rates of 8–20% acute and 3–73% late toxicity [7–9, 13–15, 18].
One of the limitations of this study is the small sample size. Further study with larger number of patients or perhaps a multicentric randomized prospective trial is needed. This preliminary study demonstrates that peri-RT TMZ up to the dose of 75 mg/m2/day combined with HIMRT at 52.5 Gy in 15 fractions is safe and well tolerated and is able to abate treatment time for GBM patients.
Footnotes
Conflicts of interest/disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol. 2002;64:259–273. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Bleehen NM, Stenning SP. A medical research council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The medical research council brain tumour working party. Br J Cancer. 1991;64:769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro WR, Young DF. Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol. 1976;33:494. doi: 10.1001/archneur.1976.00500070036007. [DOI] [PubMed] [Google Scholar]
- 5.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati M, Bernardo A, Ramsinghani N, et al. Stereotactic radiotherapy of central nervous system and head and neck lesions, using a conformal intensity-modulated radiotherapy system (Peacocktrade mark system) Skull Base. 2001;11:109–119. doi: 10.1055/s-2001-14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao JQ, Fisher BJ, Bauman GS, et al. Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience. J Neurooncol. 2012;107:395–405. doi: 10.1007/s11060-011-0766-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Damek D, Gaspar LE, et al. Phase I trial of hypofractionated intensitymodulated radiotherapy with temozolomide chemotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81:1066–1074. doi: 10.1016/j.ijrobp.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Floyd NS, Woo SY, Teh BS, et al. Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;58:721–726. doi: 10.1016/S0360-3016(03)01623-7. [DOI] [PubMed] [Google Scholar]
- 10.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6- week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 11.Minniti G, De Sanctis V, Muni R, et al. Hypofractionated radiotherapy followed by adjuvant chemotherapy with temozolomide in elderly patients with glioblastoma. J Neurooncol. 2009;91:95–100. doi: 10.1007/s11060-008-9689-z. [DOI] [PubMed] [Google Scholar]
- 12.Monjazeb AM, Ayala D, Jensen C, et al. A phase I dose escalation study of hypofractionated IMRT field-in-field boost for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82:743–748. doi: 10.1016/j.ijrobp.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morganti AG, Balducci M, Salvati M, et al. A phase I dose-escalation study (ISIDE-BT-1) of accelerated IMRT with temozolomide in patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2010;77:92–97. doi: 10.1016/j.ijrobp.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 14.Panet-Raymond V, Souhami L, Roberge D, et al. Accelerated hypofractionated intensity-modulated radiotherapy with concurrent and adjuvant temozolomide for patients with glioblastoma multiforme: a safety and efficacy analysis. Int J Radiat Oncol Biol Phys. 2009;73:473–478. doi: 10.1016/j.ijrobp.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Reddy K, Damek D, Gaspar LE, et al. Phase II trial of hypofractionated IMRT with temozolomide for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;84:655–660. doi: 10.1016/j.ijrobp.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Roa W, Xing JZ, Small C, et al. Current developments in the radiotherapy approach to elderly and frail patients with glioblastoma multiforme. Expert Rev Anticancer Ther. 2009;9:1643–1650. doi: 10.1586/era.09.128. [DOI] [PubMed] [Google Scholar]
- 17.Sultanem K, Patrocinio H, Lambert C, et al. The use of hypofractionated intensity-modulated irradiation in the treatment of glioblastoma multiforme: preliminary results of a prospective trial. Int J Radiat Oncol Biol Phys. 2004;58:247–252. doi: 10.1016/s0360-3016(03)00819-8. [DOI] [PubMed] [Google Scholar]
- 18.Terasaki M, Eto T, Nakashima S, et al. A pilot study of hypofractionated radiation therapy with temozolomide for adults with glioblastoma multiforme. J Neurooncol. 2011;102:247–253. doi: 10.1007/s11060-010-0306-6. [DOI] [PubMed] [Google Scholar]
- 19.Phillips C, Guiney M, Smith J, et al. A randomized trial comparing 35 Gy in ten fractions with 60 Gy in 30 fractions of cerebral irradiation for glioblastoma multiforme and older patients with anaplastic astrocytoma. Radiother Oncol. 2003;68:23–26. doi: 10.1016/s0167-8140(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 21.Kil WJ, Cerna D, Burgan WE, et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–938. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 22.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 24.Bobola MS, Kolstoe DD, Blank A, et al. Minimally cytotoxic doses of temozolomide produce radiosensitization in human glioblastoma cells regardless of MGMT expression. Mol Cancer Ther. 2010;9:1208–1218. doi: 10.1158/1535-7163.MCT-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson BL, Grogan PT, Mladek AC, et al. Radiosensitizing effects of temozolomide observed in vivo only in a subset of O6-methylguanine-DNA methyltransferase methylated glioblastoma multiforme xenografts. Int J Radiat Oncol Biol Phys. 2009;75:212–219. doi: 10.1016/j.ijrobp.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink J, Born D, Chamberlain MC. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12:276–285. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 27.Leibel SA, Sheline GE. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66:1–22. doi: 10.3171/jns.1987.66.1.0001. [DOI] [PubMed] [Google Scholar]
- 28.Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]


