Abstract
Purpose
We previously showed that E2F1 overexpression radiosensitizes prostate cancer cells in-vitro. Here, we demonstrate the radiosensitization efficacy of Ad-E2F1 in growing LNCaP (orthotopically) and PC3 (subcutaneously) nude mice xenograft tumors.
Methods and Materials
Adenoviral E2F1 was injected intra-tumorally in LNCaP (3×108 PFU) and PC3 (5×108 PFU) tumors treated with or without radiation. Tumor volumes (TV) were measured by MRI in LNCaP tumors, calipers in PC3 tumors and serum PSA levels by ELISA in LNCaP tumors. Apoptosis was measured by TUNEL staining and key proteins involved in cell death signaling were analyzed by Western blot.
Results
Intracellular overexpression of Ad-E2F1 had significant effect in the regression of TV and reducing the PSA relative to adenoviral luciferase (Ad-Luc) control. The in-vivo regressing effect of Ad-E2F1 on LNCaP tumor growth was significant (PSA-34 ng/ml/TV-142 mm3) compared to Ad-Luc control (PSA-59 ng/ml/TV-218 mm3; p<0.05). This effect was significantly enhanced by radiation therapy (PSA-16 ng/ml/TV-55 mm3 compared to Ad-Luc/PSA-42 ng/ml/TV-174 mm3; p<0.05). For PC3 tumors, the greatest effect was observed with Ad-E2F1 alone, there was little or no effect when RT was combined. However, addition of RT enhanced the level of in-situ apoptosis in PC3 tumors. Molecularly, Ad-E2F1 in a combination setting abrogated radiation induced BCL-2 protein and was associated with an increase in activated BAX, together caused a potent radiosensitizing effect irrespective of p53 and AR functional status.
Conclusions
We show here for the first time that ectopic overexpression of E2F1 in-vivo using an adenoviral vector significantly inhibits orthotopic p53wild-type LNCaP and subcutaneous p53null PC3 tumors in nude mice. Furthermore, we demonstrate that E2F1 strongly sensitizes LNCaP tumors to RT. These findings suggest that E2F1 overexpression can sensitize prostate tumor cells in-vivo independent of p53 or androgen receptor status.
Keywords: Ad-E2F1, Prostate cancer, Radiation, LNCaP, PC3
INTRODUCTION
Radiation therapy (RT) is a common treatment for prostate cancer; yet, for men with intermediate to high-risk disease, failure rates remain 25-40% over 5 years. We have found that overexpression of E2F1 significantly increases cell death in prostate cancer cells exposed in-vitro to radiation and this effect is independent of p53 and androgen receptor (AR) functional status (1, 2).
The E2F transcription factor family, a key target of retinoblastoma protein, has seven members E2F1–E2F7 (3). These transcription factors, with the exception of E2F7, regulate gene transcription by forming heterodimers with DP1 and DP2 (3). When E2F1 dissociates from hyper-phosphorylated retinoblastoma (RB) during the G0/early G1 phase, it activates the transcription of target genes, committing cells in late G1 phase to initiate cell cycle progression (3). Many human cancers that harbor pRb mutations have increased E2F1 expression (4). Since activated E2F1 transcription factor can promote both proliferation or apoptosis (5), depending on the cellular milieu and predominant signal, E2F1 can act as either an oncogene or tumor suppressor (6).
A delicate balance between cell proliferation and apoptosis determines development of cancer. The p53 and E2F1 signaling pathways are disrupted in prostate tumors (6, 7). Presence of endogenous mutant p53 in prostate cancer cells can impair the function of ectopic wt p53 introduced into the cells in a dominant negative manner. Genes such as proapoptotic E2F1 targets are particularly useful to bypass cell death blocked by loss or dominant gain of p53 function. Our earlier findings that E2F1 overexpression can induce apoptosis in p53wild-type LNCaP and p53null PC3 cells support this concept (1, 2). The therapeutic applications are, therefore, broad, since many advanced prostate cancers harbor p53 mutations or lack p53 expression (7, 8).
In the present study, we report the effect of overexpressing E2F1 combined with RT in LNCaP tumors (orthotopic) and PC3 tumors (subcutaneous). We show here for the first time that targeting E2F1 in-vivo using an adenoviral vector significantly inhibits orthotopic p53wild-type LNCaP and subcutaneous p53null PC3 tumors in nude mice. Furthermore, we demonstrate that E2F1 strongly sensitizes LNCaP tumors to RT. These findings suggest that E2F1 overexpression can sensitize prostate tumor cells in-vivo independent of p53 or androgen receptor status.
METHODS AND MATERIALS
Tumor Implantation
Male athymic nude mice (4–8 weeks old) were purchased (Harlan, Indianapolis, IN). All injections and surgeries were performed according to methods approved by the institutional animal care and use committee. Detailed surgical procedures and conditions were reported by us previously (9). We used two prostate xenograft tumor models in the present study 1) orthotopic LNCaP tumor model (5×105 cells in 24μL were injected into the dorsal prostate lobes) and 2) subcutaneous PC3 tumor model (3×106 PC3 cells inoculated in the flank).
Treatments
For the LNCaP xenograft, treatments started when the serum PSA levels reached 3-8 ng/mL. Adenoviral vector constructs incorporating the E2F1 full length were generated as described previously (10). Ad-E2F1 was given intra-tumorally at a dose of 3×108 PFU (single treatment) for LNCaP tumors. For PC3 tumors Ad-E2F1 was injected 5×108 PFU intra-tumorally when the tumor size reached 50mm3. Radiation therapy to the mouse prostate tumors were delivered after 24h and on the 5th day in two independent studies using methods described previously (9).
Treatment Groups and Response Assessment
The treatments groups were as follows: Ad-Luc (adenoviral-luciferase) control, Ad-Luc+RT (5Gy single fraction) and Ad-E2F1, Ad-E2F1+RT. After Ad-Luc and/or Ad-E2F1 treatment(s), animals were assigned to receive a single fraction dose of 5Gy. Tumor volumes (TV) by MRI and serum PSA were obtained weekly after treatment. The efficacy of the treatment was assessed by weekly MRI and PSA levels for 10 weeks. PSA≤25ng/ml was defined as freedom from biochemical failure (FFBF) and TV≤100mm3 was defined as freedom from tumor volume failure (FFTVF). For the PC3 tumors, TV was assessed weekly by performing caliper measurements in three orthogonal dimensions.
MRI protocols to image the mouse prostate tumors were explained in detail in our previous studies (9), to assess the TV weekly after the beginning of treatment. Briefly, TVs were measured by manually outlining the tumor margins using Bruker Paravision software, summing the number of voxels enclosed, and multiplying by the single voxel volume. Mice were euthanized by CO2 inhalation if the MRI TV reached 300 mm3 and/or serum PSA exceeded 80ng/mL. Tumors were immediately excised and prepared by fixation in 10% formalin, and TV (measured via calipers) and the weight of the excised tumor was recorded.
Beginning at two weeks, blood samples from all the treatment and the control groups were collected by weekly retro-orbital bleeding for serum PSA measurements using IMx automated PSA immunoassay kit as per manufacturers recommendations (Abbott Laboratories, Abbott Park, IL) and described by us previously (9).
Western Analyses
Equal amounts of protein from tumor tissue homogenates were resolved by SDS– PAGE electrophoresis and probed with anti-E2F1, PUMA (Calbiochem, San Diego, CA), anti-phosphor-p53 (ser15) (Cell Signaling Technologies), anti-p53, p21, p73, BAX and BCL-2 (DAKO A/S, Carpinteria, CA). For activated BAX analyses, procedure was described earlier (11). β-Actin (Santa Cruz, Biotechnology, Santa Cruz, CA) was used as an internal control.
Luciferase Assay
Luciferase expression was measured in tumors injected with Ad-Luc or Ad-E2F1 (3×108 PFUs) as described by us previously (2).
Immunohistochemical Analysis
Orthotopically induced LNCaP tumors were injected with different doses of Ad-E2F1 (1, 2, 3, 4 and 5×108 PFUs). The tumors were excised after 24h post injection, fixed in formalin, embedded in paraffin and processed for immunohistochemistry. For infectivity assessment, the entire paraffin-embedded treated-tumor tissue were serially sectioned, with each section was 50 micron apart and a total of 50 sections were obtained. Twenty sections were selected to represent the whole TV for immunohistochemical analyses of E2F1 expression, as described by us previously (9).
Analyses of In Situ Tumor Apoptosis by TUNEL
To quantify the relative numbers of cells with DNA fragmentation, TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay was performed for all the treatment groups using ApopTag® (S7100; Chemicon International, USA) according to manufacturer’s protocol. Results were expressed as number of TUNEL-positive cells/total number of cells counted.
Data Analysis and Statistics
The MRI measurements of tumor growth and PSA levels were reasonably described by first order rate process. The time series for each animal were fitted with a single exponential as described by us previously (9). Student’s t test was applied to the estimates of TV and PSA at 6 weeks and their doubling times. Further, the FFBF and FFTVF from the experimental pairs Ad-Luc and Ad-E2F1 for each of the treatments (alone, RT) were placed in 2×2 contingency tables and tested for significance using the chi-square test. For all statistical tests p<0.05 was considered significant.
RESULTS
Intratumoral Delivery of Ad-E2F1 and Stability of E2F1 Expression in Prostate Tumor Xenografts
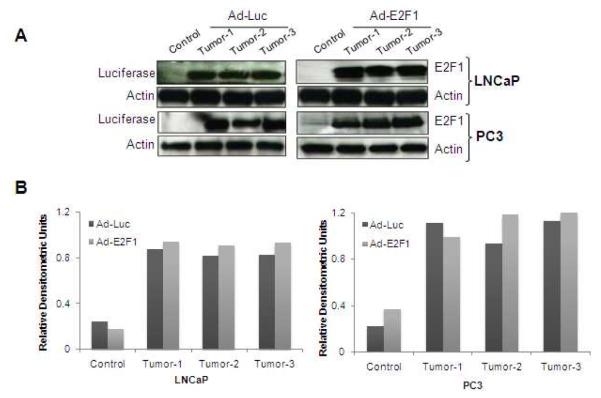
Infection of tumors with Ad-Luc or Ad-E2F1 showed that these tumors express luciferase and E2F1 proteins respectively suggesting efficient expression of these genes (Fig. 1A, B). We also confirmed that tumors injected with Ad-Luc had higher levels of luciferase activity by measuring the relative light units (data not shown). Of note, the basal levels of E2F1 in control LNCaP tumors were absent because of the short exposure, however, upon longer exposure weak basal levels of E2F1 was observed (data not shown).
Fig. 1.
(A) LNCaP and PC3 tumors injected either with Ad-E2F1 (3×108 PFUs) or Ad-Luc or no treatment were analyzed for the luciferase and E2F1 proteins by Western blot analysis and probed with antibodies against luciferase and E2F1. β-actin was used as a loading control. (B) Densitometric analysis of Figure A is represented as histograms.
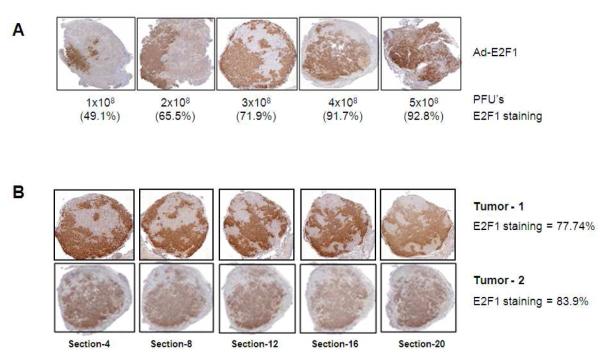
Different concentrations of Ad-E2F1 were administered intratumorally in multiple sites to orthotopic LNCaP tumors during a single surgical procedure and tumor expression evaluated. The proportion of E2F1 expressing cells in the serially sectioned tumors after the administration of 1, 2, 3, 4, and 5×108 PFU’s was determined (Fig. 2A). In tumors injected with 1×108 PFU, 49.1% of tumor cells in the entire tumor overexpressed E2F1. In comparison, in tumors injected with 5×108 PFU, nearly 93% of tumor cells overexpressed E2F1. Illustration of the transduction efficiency of Ad-E2F1 in the serial sections of tumors injected with Ad-E2F1 at 3×108 PFU is shown in Figure 2B. E2F1 expression was consistent throughout these representative tumors at 77-84% (Fig. 2B).
Fig. 2.
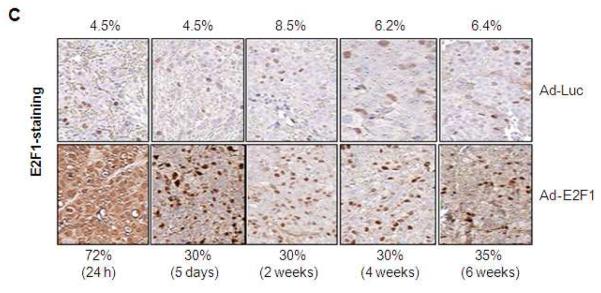
(A) Orthotopically grown LNCaP tumors injected with different doses of Ad-E2F1 (1, 2, 3, 4 and 5×108 PFUs) and stained for E2F1 by immunohistochemistry. The average percentages of cells in the tumor overexpressing E2F1 are shown under each representative slice. E2F1 expression ranges from 49 to 93% for the doses studied. (B) Spatial expression of E2F1 in Ad-E2F1 transduced LNCaP tumor. Two LNCaP tumors injected with Ad-E2F1 at 3×108 PFUs and stained for E2F1 using immunohistochemistry are shown. (C) Temporal expression of E2F1 in Ad-Luc/Ad-E2F1 transduced orthotopic LNCaP tumors. After 24h of infection, 5 days, 2, 4 and 6 weeks the tumors were excised from the mice and stained for E2F1 by immunohistochemistry.
We then tested the proportion of tumor cells expressing E2F1 over a period of time and found that 30-35% of the tumor cells overexpress E2F1 for up to 6 weeks after intra-tumoral injection, compared to 4.5-8.5% in the Ad-Luc controls (Fig. 2C).
Correlation between Tumor Volume by MRI, Caliper measurements and Serum PSA levels after Ad-E2F1 and RT
The therapeutic efficacy of adenoviral treatment of LNCaP tumors was assessed by MRI-based TV and serum PSA levels. In Figure 3A, representative coronal views of MRI images taken at 6 weeks after Ad-Luc or Ad-E2F1±RT treatment are shown. These representative tumors had volumes of 371mm3 for Ad-Luc (Fig. 3A-1) and 231mm3 for Ad-Luc+RT (Fig. 3A-2) 125mm3 for Ad-E2F1 (Fig. 3A-3) and 25mm3 for Ad-E2F1+RT (Fig. 3A-4). MRI volume was confirmed using physical measurements of excised LNCaP tumors by calipers immediately after MRI imaging. The relationship between MRI-based TV to caliper-based TV are shown in Figure 3B (R2 = 0.73, p<0.001). The R2 value for this correlation was significant and supports the use of this non-invasive imaging approach. We also compared MRI based TV with the PSA measurements (Fig. 3C), which showed more variability as anticipated (R2=0.67, p<0.05).
Fig. 3.
(A) MRI images of orthotropic prostate tumors. Representative coronal MRI views of (clockwise) Ad-Luc, Ad-E2F1, Ad-E2F1+RT and Ad-Luc+RT taken 6 weeks after treatment are displayed. (B) Correlation between the MRI and caliper TV measurements. (C) Correlation between MRI and serum PSA levels.
Ad-E2F1+RT Induced Tumor Regression in LNCaP Tumors
Eighty-seven mice with LNCaP tumors were divided into 6 treatment groups: Ad-Luc, Ad-E2F1, Ad-Luc+RT (24h), Ad-E2F1+RT (24h), Ad-Luc+RT (5d), Ad-E2F1+RT (5d). The time-related data collection resulted in a total of 842 MRI scans and 927 PSA measurements with averages of 9.7 (range 3 to 25) MRIs and 10.6 PSAs (range 4 to 26) per mouse. The TV and PSA measurements were obtained for 3–26 weeks after the initial Ad-Luc and Ad-E2F1 treatments and the average baseline TV and PSA values were 26.4mm3 and 6.6ng/ml, respectively.
LNCaP tumors were irradiated at 24h or on day 5 after Ad-E2F1 injection to examine whether there is a difference in the tumor response. This was explored to determine the effect of radiation delivery at time-points with different levels of E2F1. The expression of E2F1 on day 5 was approximately half of the one at 24h (Fig. 2C). Statistical analysis of TV and PSA for the two time-points with RT (24h and 5d) revealed no difference between the groups and for simplicity; the groups were pooled for further comparisons.
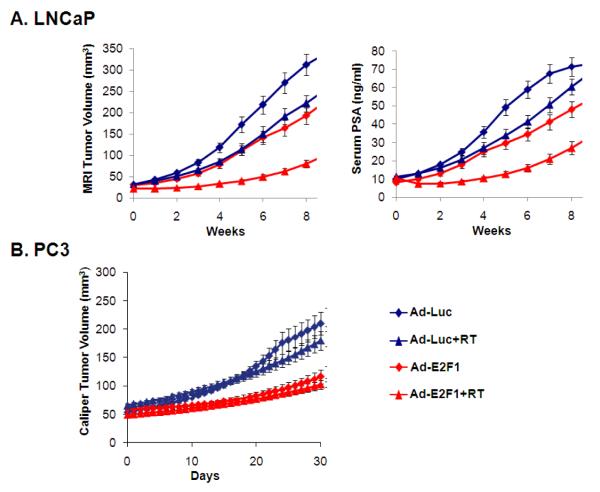
In vivo administration of Ad-E2F1 resulted in significant radiosensitization of LNCaP cells (Fig. 4A). The graphs represent the average TV and PSA (±SEM) obtained from the fitted data. It is clear that the combination treatment with Ad-E2F1+RT resulted in marked inhibition of tumor growth.
Fig. 4.
(A) Growth curves showing Ad-E2F1+RT treatments in orthotopically grown LNCaP tumors. The curves represent the average of numerical fits to the individual growth curves (n=16-17). Values for fast growing tumors are truncated at 100ng/ml for PSA and 400 mm3 for TV. (B) Growth curves showing Ad-E2F1+RT treatments in PC3 tumors. The curves represent the average of numerical fits to the individual growth curves (n=10).
The effect of Ad-Luc±RT and Ad-E2F1±RT on absolute PSA levels and TVs are shown in Table 1. The first two columns in Table 1 represent the fraction of mice with LNCaP tumors with PSA<25ng/ml and TV<100mm3 at 6 weeks; in the third column a combination endpoint, defined as the percentage of mice meeting both requirements for PSA and TV thresholds is presented. Adding RT to Ad-E2F1 significantly impedes tumor growth, with 85-93% of the mice in these groups having a PSA<25ng/ml and TV<100mm3. These results were significantly different from all three other groups in the experiment. The last two rows in Table 1 are the pooled measurements for Ad-Luc and Ad-E2F1. Again, all endpoints were more than 2 times higher for Ad-E2F1 than the Ad-Luc controls.
Table 1.
Percentage of mice with PSA < 25 ng/ml and tumor volume < 100 mm3.
| Group | LNCaP (6 weeks) | PC3 (4 weeks) | ||
|---|---|---|---|---|
| PSA<25 ng/ml | TV <100 mm3 | Combined | TV <100mm3 | |
| Ad-Luc | 31% (5/16) | 25% (4/16) | 25% (4/16) | 30% (3/10) |
| Ad-E2F1 | 53% (9/17) | 53% (9/17)‡ | 53% (9/17)‡ | 70% (7/10)‡ |
| Ad-Luc+RT | 22% (6/27) | 33% (9/27) | 18% (5/27) | 24% (6/25) |
| Ad-E2F1+RT | 85% (23/27)* | 93% (25/27)* | 85% (23/27)* | 68% (17/25)† |
| Ad-Luc (pooled)1 | 26% (11/43) | 30% (13/33) | 21% (9/43) | 26% (9/35) |
| Ad-E2F1 (pooled)1 | 73% (32/44)† | 77% (34/44)† | 73% (32/44)† | 69% (24/35)† |
Pooled data for the corresponding control (Ad-Luc) and test vector (Ad-E2F1)
p<0.05 compared to all groups above
p<0.05 compared to the group above
p<0.1 compared to the group above
The fitted curves were used to calculate the average estimated PSA and TV at 6 weeks, as well as doubling times (Table 2). The combination therapy resulted in significantly lower PSA levels and TV size relative to all groups in the experiment. The doubling times for Ad-E2F1+RT were significantly increased relative to the Ad-Luc+RT control group.
Table 2.
Tumor parameters as calculated by kinetic modeling of longitudinal data from PSA and tumor volume.
| Group | LNCaP | PC3 | ||||
|---|---|---|---|---|---|---|
| PSA at 6 wks (ng/ml) |
TV at 6 wks (mm3) |
PSA Doubling Time (wks) |
TV Doubling Time (wks) |
TV at 4 wks (mm3) |
TV Doubling Time (days) |
|
| Ad-Luc | 59±10 | 218±42 | 3.74±0.8 | 3.6±0.7 | 198±40 | 23±5 |
| Ad-E2F1 | 34±7† | 142±36 | 4.68±0.8 | 4.35±0.7 | 108±21† | 29±6 |
| Ad-Luc+RT | 42±6* | 174±25 | 2.88±0.4 | 3.36±0.5 | 230±28 | 18±2 |
| Ad-E2F1+RT | 16±3‡ | 55±9‡ | 5.12±0.7† | 5.57±0.7† | 97±13† | 34±4† |
PSA and TV values for fast growing tumors are truncated at 100 ng/ml and/or 500 mm3, respectively.
p<0.05 compared to the group above
p<0.05 compared to Ad-Luc group
p<0.05, compared to all groups above.
Ad-E2F1 Induced Tumor Regression in PC3 Prostate Tumor Xenografts
Seventy mice were divided into 4 treatment groups: Ad-Luc, Ad-E2F1, Ad-Luc+RT and Ad-E2F1+RT. The data collection resulted in a total of 1365 TV caliper measurements (average 19.5 measurements per mouse; range 6 to 29). These measurements were made for 19 - 88 days. The average baseline TV was 46.5mm3.
The effect of Ad-E2F1 in combination with RT is shown in Figure 4B. There was a much greater growth inhibitory effect from Ad-E2F1 alone, as compared to Ad-Luc; however, there was little additional tumor regression when radiation was added that was not significant. Administering Ad-E2F1 alone had marked effect in the freedom of failure measurements relative to Ad-Luc that was more pronounced in PC3 tumors compared to LNCaP tumors (Table 1). Yet for PC3, RT did not impact tumor growth when added to Ad-E2F1. The estimated TVs at 4 weeks were lower in the Ad-E2F1±RT groups and doubling times longer (Table 2, last two columns).
Ad-E2F1±RT Results in In Situ Tumor Apoptosis
After ascertaining the tumor regressing effect of Ad-E2F1, we evaluated the extent of in-situ apoptosis present in these prostate xenograft tumor cells from Ad-E2F1±RT treatment. Results are expressed as number of TUNEL-positive cells divided by the total number of cells analyzed in each tumor section. Ad-E2F1 infected tumors cells demonstrated significant TUNEL staining compared to the Ad-Luc control in both LNCaP and PC3 tumors (Fig. 5A). Further significant enhancement (p<0.001) was observed with radiation (5Gy, single fraction) administered 24h after Ad-E2F1 transduction, compared to Ad-E2F1 alone, Ad-Luc and Ad-Luc+RT groups. Similar results were observed when RT was delivered 5 days after Ad-E2F1/Ad-Luc injection (data not shown).
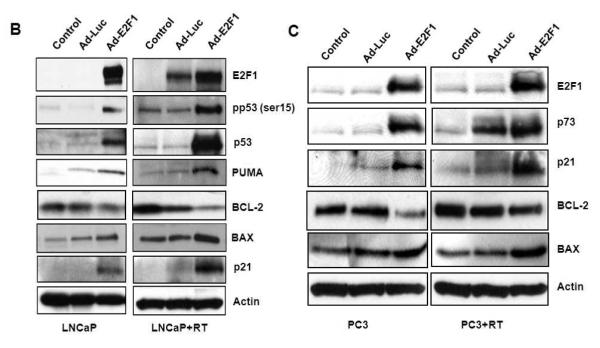
Fig. 5.

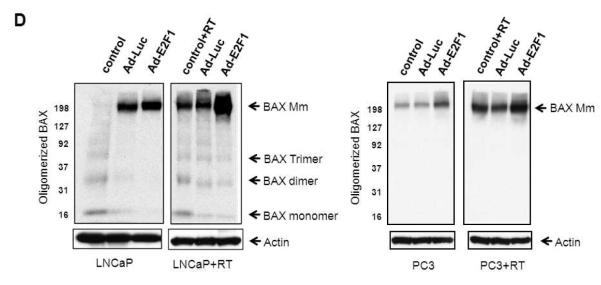
(A) Adenoviral E2F1 and RT treatments increased TUNEL staining in LNCaP and PC3 tumors. Results are expressed as number of TUNEL-positive cells divided by the total number of cells analyzed in each tumor section. (B, C) Ad-E2F1+RT induced apoptotic protein profile in LNCaP and PC3 tumors. Key apoptotic proteins from LNCaP and PC3 tumors were probed with specific antibodies. (D) Extracts from LNCaP and PC3 tumors treated with Ad-Luc or Ad-E2F1 and no treatment controls with or without radiation (5Gy) were subjected to Western blot using anti-BAX antibody for BAX oligomerization.
Ad-E2F1±RT Modifies the Expression of Key Apoptotic Proteins
The effect of Ad-E2F1±RT on key apoptotic genes was investigated by Western blot analyses (Fig. 5B&C). E2F1 expression was increased after Ad-E2F1 treatment in both LNCaP and PC3 tumors. Ad-E2F1 overexpression increased p53 protein levels in unirradiated LNCaP tumors; the highest relative increase in p53 was observed with Ad-E2F1+RT (Fig. 5B). Ad-E2F1 also caused an increase in activated, serine phosphorylated, p53 protein in unirradiated LNCaP tumors that was higher when RT was added.
Since PC3 tumors lack the expression of p53, we assessed the level of the p73 homologue. Ad-E2F1 significantly induced p73 and these levels were further increased with addition of RT (Fig. 5C). The expression of p53/p73 downstream targets: PUMA, p21 and BAX were also increased by Ad-E2F1 and enhanced further when RT was added. The activated state of BAX from Ad-E2F1 and RT treatment was also examined by assessing the presence of oligomerization. Ad-E2F1 overexpression and/or irradiation led to increased BAX oligomerization in both LNCaP and PC3 tumors, and more significant increase was observed when E2F1 was combined with RT (Fig. 5D). Concordantly, BCL-2 was lowered by Ad-E2F1 and remained lower than the Ad-Luc controls when RT was added.
DISCUSSION
Tumor regression effects by Ad-E2F1 expression in different solid tumors have been demonstrated by others (10, 12); however, the effects of in-vivo ectopic overexpression of E2F1 on prostate cancer growth or its application as a radiosensitizer has not been studied. In concordance with previous reports from non-prostate tumor models (13, 14), Ad-E2F1 injection alone reduced TV in orthotopic LNCaP tumors (Tables 1 and 2).
Ectopic overexpression of E2F1 renders apoptosis in several tumor types (15, 16). Agah and his colleagues reported that adenoviral delivery of E2F1 into myocardium overrides the G1/S checkpoint and induces apoptosis (17). In an another report, adenovirus-mediated E2F1 gene transfer has been shown to sensitize melanoma cells to chemotherapeutic agents, particularly topoisomerase II inhibitors, and enhance antitumor activity in a nude mouse model (18). E2F1 mediated tumor growth inhibition was observed in non-small cell lung tumors (19) and in leiomyosarcoma in-vitro and in-vivo (14). In our study increased apoptotic tumor cell death was observed in response to Ad-E2F1 alone in both LNCaP and PC3 tumor models. Taken together, these data suggest that E2F1 overexpression might render tumor regression, at least partly mediated through apoptosis.
There are no in-vivo studies that document radiosensitization from the ectopic expression of E2F1 in-vivo for prostate cancer. Our results show that adding radiotherapy to Ad-E2F1 treatment significantly inhibited LNCaP tumor growth synergistically, as compared to radiation or Ad-E2F1 alone (Table 2). We did not see radiosensitization of p53null PC3 cells; although Ad-E2F1 alone induced pronounced tumor growth inhibition. Although it was originally believed that p53 was essential for E2F1-mediated apoptosis, it is now clear that p53 function is not always required (10, 20). Studies on breast and ovarian carcinoma cells suggest that E2F1 mediated apoptosis does not require wild-type p53 (10). Our results using the PC3 model demonstrate that treatment with Ad-E2F1 is effective at inducing apoptosis and decreasing the TV, through p53-independent mechanisms (Figures 3b & 4a). Moreover, we observed a further increase in apoptosis when RT was added to Ad-E2F1; yet, no enhancement in tumor growth inhibition was seen over Ad-E2F1 alone. Apoptosis was measured at a single time point and may not be representative of much of a change in overall cell death. Nonetheless, there is evidence that ectopic E2F1 expression plus RT may be beneficial in some p53null tumor types, such as fibrosarcoma (21), and under the right conditions could contribute to increased tumor growth inhibition of prostate cancers that harbor p53 mutations or loss. At least for the many prostate tumors that do have p53 mutations or loss (7, 8), our evidence indicates that ectopic E2F1 overexpression may be quite effective.
In the present study, we consistently observed an increase in E2F1 in LNCaP tumors treated with Ad-Luc+RT in-vivo (Figure 5B); however, we did not observe this in-vitro (2). Adenovirus E4-6/7 protein has been shown to induce E2F1 protein expression (22), which could be stabilized by ATM, induced by radiation (23). It has been shown by studies that E2F1 can induce apoptosis at least by six different mechanisms, viz., (i) Direct interaction of E2F1 with p53 leading to enhanced p53 activity and this was demonstrated in this study (Figure 4B) (2, 24). (ii) Transcriptional activation of p53 homologue p73 (25). Although p73 transcriptional activity was not analyzed in this study, we observed increased protein levels of p73 and its target p21 in PC3 cells (Figure 4C). (iii) Transcriptional activation of caspases 3, 7, 8 and 9 (26). In our earlier studies, we showed that the activity of caspase 3 and 7 are induced with overexpression of E2F1 (2). (iv) Transcriptional activation of p14/ARF and stabilization of p53 (27). We observed increased protein stabilization of p53 in cells overexpressing E2F1 both in untreated and RT treated prostate cells (Figure 4B). (v) Transcriptional activation of Apaf-1, a protein that directly interacts with cytochrome C to initiate apoptotic proteolytic cascade is induced by E2F1 (28). (vi) E2F1 represses the transcription of Mcl-1, an anti-apoptotic protein (29).
BCL-2 is an upstream effector molecule that inhibits the apoptotic pathway and most cancers including prostate cancer cells overexpress BCL-2 (30, 31). BCL-2 forms a heterodimer with the apoptotic protein BAX and the ratio of BAX/BCL-2 is a decisive factor that plays an important role in determining whether cells will undergo apoptosis. We observed that Ad-E2F1 down-regulated BCL-2 protein and up-regulated levels of BAX protein in both LNCaP and PC3 xenografts, with and without radiation, suggesting the involvement of an intrinsic apoptotic pathway by which E2F1 overexpression and RT induces apoptosis and inhibits tumor growth in nude mice. BAX oligomerization and cytochrome C release is critical in the regulation of apoptosis by BCL-2 family of proteins (32). In our study, we demonstrate the presence of oligomerization of BAX in Ad-E2F1 and Ad-E2F1+RT treated LNCaP and PC3 tumor cells suggesting that BAX might mediate Ad-E2F1 and RT induced tumor inhibition and apoptosis.
The present findings provide evidence for presence of four different mechanisms mediating the E2F1-induced pro-apoptotic response. Our analysis of proteins involved in apoptosis and cell survival suggests that E2F1 overexpression might render tumor regression that is mediated partly from apoptotic mode of cell death. Mechanistically, these treatments abrogate the RT-induced BCL-2 expression and activate BAX to render apoptotic and tumor regressing effects. Despite, mounting evidence that manipulating E2F1 results in tumor inhibition, there is evidence that abnormalities in the regulation of the E2F1 pathway are a determinant of prostate cancer aggressiveness and patient outcome, and there is concern that the overexpression of E2F1 could promote cell proliferation and transformation (4). We and others have been investigating the potential of using truncated variants of E2F1 which lack the domains required for E2F1’s proliferative activity (Rb, DP binding domain and transactivation domain) for cancer therapy (24). Thus, E2F1 and its variants hold an immense promise to be developed into potential radioadjuvant therapies for treating different cancers including prostate cancer.
Acknowledgements
This publication was supported in part by Grants from the National Cancer Institute (CA 101984-01) and (CA-006927), Department of Defense (US Army Medical Research Grant (PC020427) and Varian Medical Systems (Palo Alto, Ca).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST NOTIFICATION No conflict of interest.
REFERENCES
- 1.Nguyen KH, Hachem P, Khor LY, et al. Adenoviral-E2F-1 radiosensitizes p53wild-type and p53null human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2005;63:238–246. doi: 10.1016/j.ijrobp.2005.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udayakumar TS, Hachem P, Ahmed MM, et al. Antisense MDM2 enhances E2F1-induced apoptosis and the combination sensitizes androgen-sensitive [corrected] and androgen-insensitive [corrected] prostate cancer cells to radiation. Mol Cancer Res. 2008;6:1742–1754. doi: 10.1158/1541-7786.MCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–142. doi: 10.1038/sj.cdd.4401324. [DOI] [PubMed] [Google Scholar]
- 4.Maddison LA, Sutherland BW, Barrios RJ, et al. Conditional deletion of Rb causes early stage prostate cancer. Cancer Res. 2004;64:6018–6025. doi: 10.1158/0008-5472.CAN-03-2509. [DOI] [PubMed] [Google Scholar]
- 5.Morris EJ, Ji JY, Yang F, et al. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature. 2008;455:552–556. doi: 10.1038/nature07310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libertini SJ, Tepper CG, Guadalupe M, et al. E2F1 expression in LNCaP prostate cancer cells deregulates androgen dependent growth, suppresses differentiation, and enhances apoptosis. Prostate. 2006;66:70–81. doi: 10.1002/pros.20314. [DOI] [PubMed] [Google Scholar]
- 7.Osman I, Drobnjak M, Fazzari M, et al. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin Cancer Res. 1999;5:2082–2088. [PubMed] [Google Scholar]
- 8.Ittmann M, Wieczorek R, Heller P, et al. Alterations in the p53 and MDM-2 genes are infrequent in clinically localized, stage B prostate adenocarcinomas. Am J Pathol. 1994;145:287–293. [PMC free article] [PubMed] [Google Scholar]
- 9.Stoyanova R, Hachem P, Hensley H, et al. Antisense-MDM2 sensitizes LNCaP prostate cancer cells to androgen deprivation, radiation, and the combination in vivo. Int J Radiat Oncol Biol Phys. 2007;68:1151–1160. doi: 10.1016/j.ijrobp.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt KK, Deng J, Liu TJ, et al. Adenovirus-mediated overexpression of the transcription factor E2F-1 induces apoptosis in human breast and ovarian carcinoma cell lines and does not require p53. Cancer Res. 1997;57:4722–4726. [PubMed] [Google Scholar]
- 11.Zagurovskaya M, Shareef MM, Das A, et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28:1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 12.Fueyo J, Gomez-Manzano C, Yung WK, et al. Overexpression of E2F-1 in glioma triggers apoptosis and suppresses tumor growth in vitro and in vivo. Nat Med. 1998;4:685–690. doi: 10.1038/nm0698-685. [DOI] [PubMed] [Google Scholar]
- 13.Dong YB, Yang HL, Elliott MJ, et al. Adenovirus-mediated E2F-1 gene transfer efficiently induces apoptosis in melanoma cells. Cancer. 1999;86:2021–2033. doi: 10.1002/(sici)1097-0142(19991115)86:10<2021::aid-cncr20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Vorburger SA, Hetrakul N, Xia W, et al. Gene therapy with E2F-1 up-regulates the protein kinase PKR and inhibits growth of leiomyosarcoma in vivo. Mol Cancer Ther. 2005;4:1710–1716. doi: 10.1158/1535-7163.MCT-05-0036. [DOI] [PubMed] [Google Scholar]
- 15.Denchi EL, Helin K. E2F1 is crucial for E2F-dependent apoptosis. EMBO Rep. 2005;6:661–668. doi: 10.1038/sj.embor.7400452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott MJ, Farmer MR, Atienza C, Jr., et al. E2F-1 gene therapy induces apoptosis and increases chemosensitivity in human pancreatic carcinoma cells. Tumour Biol. 2002;23:76–86. doi: 10.1159/000059708. [DOI] [PubMed] [Google Scholar]
- 17.Agah R, Kirshenbaum LA, Abdellatif M, et al. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100:2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong YB, Yang HL, Elliott MJ, et al. Adenovirus-mediated E2F-1 gene transfer sensitizes melanoma cells to apoptosis induced by topoisomerase II inhibitors. Cancer Res. 2002;62:1776–1783. [PubMed] [Google Scholar]
- 19.Kuhn H, Liebers U, Gessner C, et al. Adenovirus-mediated E2F-1 gene transfer in nonsmall-cell lung cancer induces cell growth arrest and apoptosis. Eur Respir J. 2002;20:703–709. doi: 10.1183/09031936.02.00294502. [DOI] [PubMed] [Google Scholar]
- 20.Nip J, Strom DK, Fee BE, et al. E2F-1 cooperates with topoisomerase II inhibition and DNA damage to selectively augment p53-independent apoptosis. Mol Cell Biol. 1997;17:1049–1056. doi: 10.1128/mcb.17.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruschy M, Wirbelauer C, Glanzmann C, et al. E2F-1 has properties of a radiosensitizer and its regulation by cyclin A kinase is required for cell survival of fibrosarcoma cells lacking p53. Cell Growth Differ. 1999;10:141–146. [PubMed] [Google Scholar]
- 22.Schaley J, O’Connor RJ, Taylor LJ, et al. Induction of the cellular E2F-1 promoter by the adenovirus E4-6/7 protein. J Virol. 2000;74:2084–2093. doi: 10.1128/jvi.74.5.2084-2093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin WC, Lin FT, Nevins JR. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001;15:1833–1844. [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh JK, Fredersdorf S, Kouzarides T, et al. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 25.Irwin M, Marin MC, Phillips AC, et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 26.Nahle Z, Polakoff J, Davuluri RV, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 27.Parisi T, Pollice A, Di Cristofano A, et al. Transcriptional regulation of the human tumor suppressor p14(ARF) by E2F1, E2F2, E2F3, and Sp1-like factors. Biochem Biophys Res Commun. 2002;291:1138–1145. doi: 10.1006/bbrc.2002.6591. [DOI] [PubMed] [Google Scholar]
- 28.Moroni MC, Hickman ES, Lazzerini Denchi E, et al. Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 29.Croxton R, Ma Y, Song L, et al. Direct repression of the Mcl-1 promoter by E2F1. Oncogene. 2002;21:1359–1369. doi: 10.1038/sj.onc.1205157. [DOI] [PubMed] [Google Scholar]
- 30.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Revelos K, Petraki C, Gregorakis A, et al. Immunohistochemical expression of Bcl2 is an independent predictor of time-to-biochemical failure in patients with clinically localized prostate cancer following radical prostatectomy. Anticancer Res. 2005;25:3123–3133. [PubMed] [Google Scholar]
- 32.Mikhailov V, Mikhailova M, Pulkrabek DJ, et al. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]