Summary
Reliable clinical or molecular predictors of benefit from azacitidine therapy in patients with myelodysplastic syndromes (MDS) are not defined. Doubling of platelet count at start of second cycle of azacitidine therapy compared to baseline has been associated with achieving response and survival advantage in a Dutch cohort. To validate this observation, we analyzed a larger cohort of North American patients whose data was collected in a prospective clinical trial with a longer median follow-up. We found a significant association between platelet count doubling after first cycle of azacitidine therapy and probability of achieving objective response. Among patients with MDS or oligoblastic acute myeloid leukemia (<30% bone marrow blasts, n=102), there was a statistically significant reduction in risk of death for patients who achieved platelet count doubling (n=23, median OS, 21.0 months) compared to those who did not (n=79, median OS, 16.7 months, adjusted HR (no/yes)=1.88, 95% CI, 1.03-3.40, P=0.04). Nonetheless, the addition of this platelet count doubling variable did not improve the survival prediction provided by the revised International Prognostic Scoring System or the French Prognostic Scoring System. Identification of reliable and consistent predictors for clinical benefit for azacitidine therapy remains an unmet medical need and a top research priority.
Keywords: Myelodysplastic syndromes (MDS), azacitidine, Revised International Prognostic Scoring System (IPSS-R), French Prognostic Scoring System (FPSS), prognostic models
Background
Azacitidine has emerged as the only agent with a proven survival benefit in patients with high-risk (HR) myelodysplastic syndromes (MDS) with a median overall survival (OS) prolongation of 9.5 months over conventional care regimens (Fenaux et al, 2009). Although azacitidine has become the recommended first-line treatment for patients with HR-MDS, there are many limitations to this therapy (Zeidan et al, 2013a). Only 50-60% of patients achieve objective responses with azacitidine therapy, and prolonged treatment of 4 to 6 months is required before a failure to achieve a response can be declared (Fenaux et al, 2009, Silverman et al, 2002, Silverman et al, 2006). Additionally, almost all patients who respond to azacitidine will eventually progress, the majority within 2 years, and no cures are achievable with the drug (Zeidan et al, 2013b). Once primary or secondary resistance to azacitidine occurs, the survival is dismal with a median OS of less than 6 months (Prebet et al, 2011).
Therefore, selection of patients who are not likely to derive benefit from azacitidine at baseline or shortly after initiation of therapy has become a top clinical and research priority (Faltas et al, 2013, Zeidan et al, 2013c). Identification of such patients who are unlikely to benefit from azacitidine would potentially spare the 4 to 6 months of ineffective therapy, potentially significant side effects, unnecessary costs, and wasted precious time before considering clinical trials or other aggressive interventions (Zeidan & Komrokji, 2013). Despite several reports to establish biomarkers (e.g. TET2 mutations) or prediction models, no biomarkers or model have consistently selected patients at baseline who are likely or unlikely to obtain clinical benefit from azacitidine therapy (Itzykson et al, 2011a, Sekeres et al, 2012).
In a recent publication in the British Journal of Haematology, a group from the Netherlands studied a cohort of 90 azacitidine-treated patients with MDS, chronic myelomonocytic leukemia (CMML) and acute myeloid leukemia (AML) and identified platelet doubling time after the first cycle of azacitidine therapy as an independent positive predictor of overall survival (OS) (van der Helm et al, 2011). In that cohort, patients received a median of five cycles (range 1–19) of azacitidine (administered at the standard schedule of 75 mg/m2/d for 7 day every 28 days) and achieved a median OS of 13.0 months (range, 9.8-16.2). In univariate analysis, a two-fold increase or more in platelet count at the start of the second azacitidine cycle compared to the start of the first cycle was associated with superior survival (hazard ratio [HR], <2-fold increase vs. >2-fold increase)=7.8, 95% confidence interval [95% CI], 1.1-57.4, P=0.04). In multivariate analysis adjusting for the presence of peripheral blood [PB] blasts and International Prognostic Scoring System [IPSS] poor cytogenetics category, the HR with respect to platelet count doubling was 5.4 (95%CI, 0.73-39.9, P=0.10) (van der Helm et al, 2011). To our knowledge, this association has not been subsequently validated. In order to validate this observation, we used a cohort of patients who were treated with azacitidine in a large North American Leukemia Intergroup Trial.
Methods
Study cohort
In the E1905 trial, 150 patients with MDS (n=93), CMML (n=5), or AML with myelodysplasia-related changes (AML-MRC, n=52) were randomized to azacitidine monotherapy (50 mg/m2/day on days 1-10 of each 28-day cycle) or a combination regimen of azacitidine (same schedule) with the histone deacetylase inhibitor entinostat (4 mg/m2/day on days 3 and 10 of each cycle) (Prebet et al, 2014). OS was not significantly different between the 2 groups (median OS: 18 months for the azacitidine group vs. 13 months in the combination group, P=0.09). Additionally, there was no significant difference in response rates (Hematologic normalization [HN] defined as complete response [CR] + partial remission [PR] + trilineage Hematological Improvement [HI-T]) which occurred in 32% of patients in the azacitidine group compared to 27% in the azacitidine-entinostat group (P=0.80)].
In a nested retrospective analysis of this cohort, we analyzed OS by platelet doubling after the first cycle of azacitidine therapy. Similar to the index paper, we identified platelet doubling as two-fold or larger increase in the platelet count at the start of cycle 2 compared to the start of cycle 1 of azacitidine. Patients without complete information for the determination of platelet doubling were excluded from the analysis (n=24). Among all patients included in this analysis, we have defined an overall cohort (n=126) and a subcohort of patients with MDS or oligoblastic AML (defined as those with AML and bone marrow blasts up to 30%, n=102) who started protocol treatment.
Statistical analysis
Patient demographic and disease characteristics were compared using Wilcoxon rank-sum test and Fisher's exact test as appropriate. Overall response was dichotomized as either having a response (defined as achieving CR, PR, or any HI) or not having a response. OS was defined as time from registration to death from any causes with follow-up censored at the date of last contact. The variable platelet doubling was binary coded, with 1 indicating at least a two-fold increase in platelet count at the start of Cycle 2 treatment compared to the start of Cycle 1 treatment and 0 otherwise. The effect of this factor on response and OS was analyzed using the logistic regression model and a landmark analysis, respectively. The landmark survival analysis was performed with the landmark time-point chosen as the start of cycle 2 (i.e., 28 days after study entry, 1 cycle = 28 days). Hazard ratio (HR) for death was computed using Cox proportional hazards (PH) models with survival time measured from the start of cycle 2. Confounding factors (with p<0.10) were fitted into the multivariable Cox models to further evaluate the effect of platelet doubling. P values were all two-sided. A level of 5% was considered statistically significant. We also used Akaike's information criterion (AIC; a measure indicating the relative quality of a statistical model based on a given set of data) to assess for the incremental improvement in survival prediction by adding the platelet count doubling variable to two validated MDS prognostic scores.
Results
Study cohort
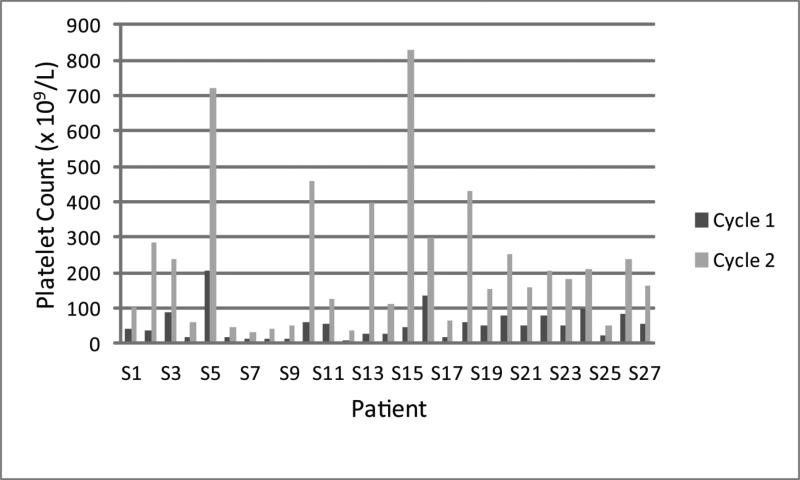
Table 1 shows the baseline characteristics and demographics of the entire study cohort (n=126) and as stratified by achieving platelet count doubling. There was no significant difference in age, gender, performance status, or the type of malignancy between those who achieved (n=27) and those who did not achieve (n=99) platelet count doubling. For those 27 patients who achieved platelet count doubling, the median baseline platelet count was 50 × 109/L (standard deviation [SD], 43.8 × 109/L, range, 7-207 × 109/L) and increased to a median of 165 × 109/L (SD, 201.3 × 109/L, range, 34-830 × 109/L) at start of cycle 2 of azacitidine [Figure 1]. The majority of patients who achieved platelet doubling had unfavorable IPSS cytogenetics compared to those who did not achieve platelet count doubling (65.4% vs. 35.7%, P=0.01). Patients who did not achieve platelet doubling had a higher prevalence of PB blasts at baseline compared to those patients who achieved platelet doubling, although the difference was not statistically significant (35.8% vs. 16%, P=0.09). There was no statistically significant difference in the proportion of patients who achieved platelet count doubling between those who received azacitidine therapy (17 out 62 [27.4%]) and those who received azacitidine+entinostat (10 out of 64 [15.6%], P=0.13).
Table 1.
Baseline patient demographics and disease characteristics by platelet doubling status in the overall cohort (n=126).
| Platelet Doubling (N=126) | P valuea | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| N (99) | % (78.6) | N (27) | % (21.4) | ||
| Age (median, range) | 72.0 (25-85) | 69.0 (48-87) | 0.69 | ||
| Gender | 0.81 | ||||
| Male | 70 | 70.7 | 18 | 66.7 | |
| Female | 29 | 29.3 | 9 | 33.3 | |
| ECOG performance status | 1.00 | ||||
| <2 | 87 | 87.9 | 24 | 88.9 | |
| ≥2 | 12 | 12.1 | 3 | 11.1 | |
| Disease | 0.98 | ||||
| MDS High/Intermediate-2 | 44 | 44.4 | 13 | 48.1 | |
| MDS Low/Intermediate-1 | 19 | 19.2 | 5 | 18.5 | |
| CMML | 4 | 4.0 | 1 | 3.7 | |
| Oligoblastic AML | 16 | 16.2 | 5 | 18.5 | |
| Non-oligoblastic AML | 16 | 16.2 | 3 | 11.1 | |
| Cytogenetics risk group by IPSS | 0.01 | ||||
| Favorable | 32 | 38.1 | 5 | 19.2 | |
| Intermediate | 17 | 20.2 | 0 | 0 | |
| Unfavorable | 30 | 35.7 | 17 | 65.4 | |
| Unacceptable for analysis | 5 | 6.0 | 4 | 15.4 | |
| Missing | 15 | - | 1 | - | |
| Presence of peripheral blood blasts | 0.09 | ||||
| No | 61 | 64.2 | 21 | 84.0 | |
| Yes | 34 | 35.8 | 4 | 16.0 | |
ECOG: Eastern Cooperative Oncology Group, IPSS: International Prognostic Scoring System, MDS: Myelodysplastic syndromes, CMML: Chronic myelomonocytic leukemia, AML-MRC: Acute myeloid leukemia with myelodysplasia-related changes.
Figure 1.
A histogram of the platelet count at baseline and at start of cycle 2 of therapy for the 27 patients who achieved platelet count doubling.
Platelet doubling association with response and survival
To account for the effect of cytogenetics and PB blast presence at baseline on the relationship between platelet doubling and response and OS, and similar to the index paper, we included those two variables together with treatment arm in the logistic regression and Cox regression models. Given the effects of platelet doubling on either response or OS were similar between the two treatment arms (i.e., no 2-way interaction effect), our analysis combined the two treatment arms. As can be seen in Table 2, the odds ratio (OR) of eventually achieving an objective response was significantly higher for those patients who had platelet count doubling by the start of cycle 2 in both univariate and multivariable logistic regression models for the overall cohort and the 2 subcohorts.
Table 2.
Odds ratio (OR) for overall response with respect to platelet doubling (no vs. yes) using logistic regression models.
| Cohort | Platelet Doubling | Overall Response | Univariable Model | Multivariable Modela | ||||
|---|---|---|---|---|---|---|---|---|
| Yes (N) | No (N) | OR (95% CI) | P value (Wald) | N | OR (95% CI) | P value (Wald) | ||
| Overall | No | 46 (46%) | 53 (54%) | 0.30 (0.12, 0.78) | 0.01 | 105 | 0.35 (0.11, 1.14) | 0.08 |
| Yes | 20 (74%) | 7 (26%) | ||||||
| MDS/oligoblastic AML | No | 38 (48%) | 41 (52%) | 0.26 (0.09, 0.77) | 0.02 | 87 | 0.29 (0.08, 1.03) | 0.056 |
| Yes | 18 (78%) | 5 (22%) | ||||||
MDS: Myelodysplastic syndromes, AML Acute myeloid leukemia.
Adjusted by cytogenetics risk group and presence of peripheral blood blasts.
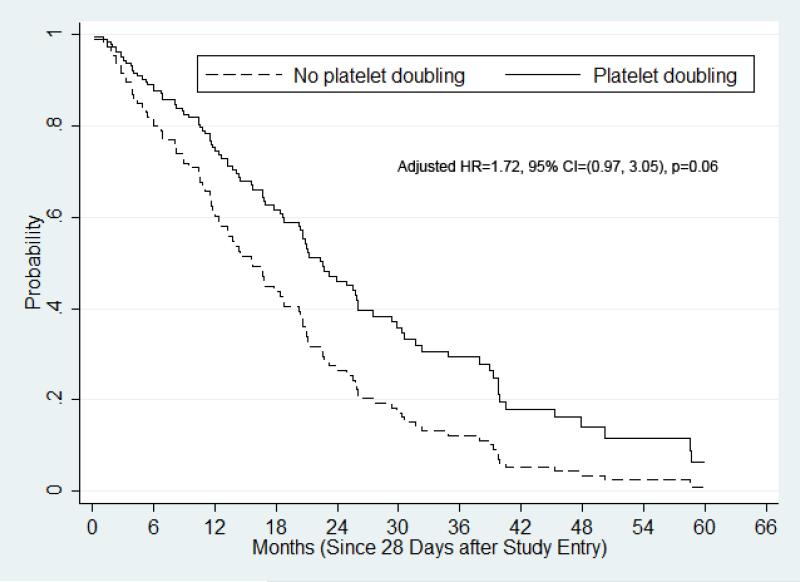
Figures 2 and 3 display the adjusted survival curves (measured beginning 28 days after study entry) using Cox PH regression with respect to platelet count doubling for various cohorts. In univariate analysis of the overall cohort (n=126), there was a statistically-insignificant trend for OS prolongation in patients who achieved platelet count doubling (n=27, median OS, 18.9 months) compared to their counterparts (n=99, median OS, 14.5 months, HR (no/yes)=1.34, 95% CI, 0.85-2.11, P=0.21). In multivariable analysis of the overall cohort that accounted for cytogenetic risk group and presence PB blasts, the trend for OS prolongation became stronger (adjusted HR [no/yes]=1.72, 95% CI, 0.97-3.05, P=0.06, Figure 2).
Figure 2.
Adjusted overall survival by Cox proportional hazards regression for the overall cohort. OS was adjusted by cytogenetics, PB blast presence, and treatment.
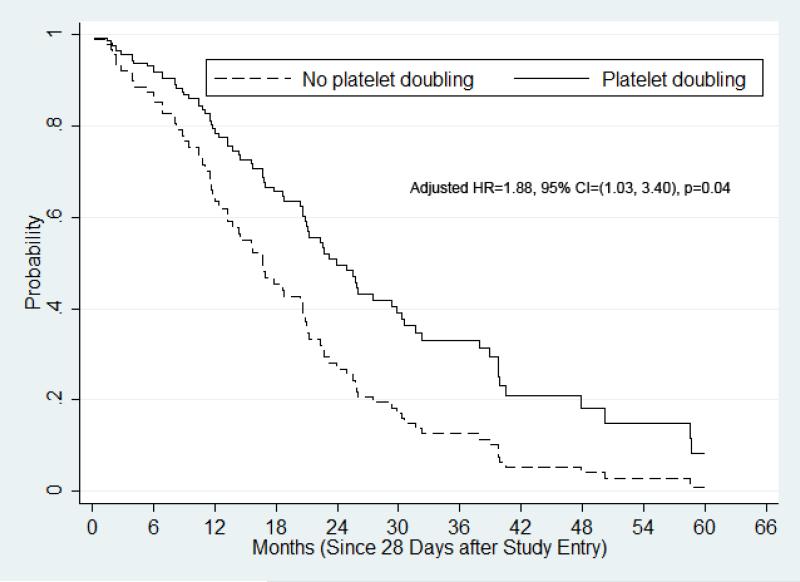
Figure 3.
Adjusted overall survival by Cox proportional hazards regression for the MDS or oligoblastic AML cohort. OS was adjusted by cytogenetics, PB blast presence, and treatment.
For the subcohort of patients with MDS or oligoblastic AML (n=102), univariate analysis similarly showed a statistically-insignificant trend for OS prolongation in patients who achieved platelet count doubling (n=23, median OS, 21.0 months) compared to their counterparts (n=79, median OS, 16.7 months, HR [no/yes]=1.38, 95% CI, 0.83-2.28, P=0.21). In the adjusted survival analysis of this subcohort, there was a statistically significant reduction in risk of death for patients who achieved platelet count doubling (adjusted HR (no/yes)=1.88, 95% CI, 1.03-3.40, P=0.04, Figure 3).
Adding prognostic impact of the platelet count doubling to the prognostic scores
We used the AIC to analyze whether adding the binary variable of platelet count doubling at start of cycle 2 to the revised International Prognostic Scoring System (IPSS-R) (Greenberg et al, 2012) or the French Prognostic Scoring System (FPSS) (Itzykson et al, 2011b, Itzykson et al, 2012) improves survival prediction over either score alone in patients for whom data were available to both calculate the score and assess for platelet doubling status. As noted in Table 3, we did not observe any significant incremental improvement in the power of the IPSS-R or the FPSS to predict OS by accounting for platelet count doubling at start of cycle 2 of therapy.
Table 3.
Akaike's information criterion (AIC) for various models.
| Cohort | Model | N | AIC |
|---|---|---|---|
| Overall | Null | 101 | 714.40 |
| IPSS-R | 101 | 707.78 | |
| IPSS-R & platelet doubling | 101 | 707.03 | |
| Null | 96 | 668.46 | |
| FPSS | 96 | 663.79 | |
| FPSS & platelet doubling | 96 | 665.67 | |
| MDS/oligoblastic AML | Null | 83 | 551.96 |
| IPSS-R | 83 | 544.45 | |
| IPSS-R & platelet doubling | 83 | 542.69 | |
| Null | 80 | 525.52 | |
| FPSS | 80 | 522.08 | |
| FPSS & platelet doubling | 80 | 524.05 |
Note: Given the same set of observations, a model with a smaller AIC value indicates a better model.
IPSS-R: Revised International Prognostic Scoring System, FPSS: French Prognostic Scoring System, MDS: Myelodysplastic syndromes, AML Acute myeloid leukemia.
Discussion and conclusions
Identification of MDS patients with low probability of achieving benefit from azacitidine at baseline or shortly after initiation of therapy would allow help avoiding subjecting such patients to several months of ineffective therapy and its associated toxicity and expense, and would allow directing such patients for clinical trials or more intensive therapies at an earlier point in their disease course (Zeidan & Komrokji, 2013). Baseline biomarkers or clinical variables that consistently predict clinical benefit from azacitidine therapy in patients with MDS have not been identified despite extensive investigation (Steensma, 2012). Therapy with azacitidine currently remains indiscriminate with many IPSS higher-risk (HR) receiving this agent for prolonged periods without real benefit. One approach of identifying differential probabilities of benefit from azacitidine therapy is combining baseline clinical prognostic schemes (e.g. IPSS-R or FPSS) with baseline genetic, epigenetic, or other molecular markers (e.g. TET2 mutations or methylation signals), but no such prediction rule has been validated (Zeidan & Komrokji, 2013).
Another approach is to use post-treatment variables (e.g. change in platelet count after one cycle of therapy) in combination with clinical prognostic schemes. Compared to the compassionate named-program from which the Dutch cohort by van der Helm (van der Helm et al, 2011) was selected, our cohort was larger (n=126 vs. 90 patients), North American (rather than European), and treated with a lower-dose, more prolonged administration of azacitidine. Additionally, our cohort had a longer median follow-up (49 vs. 8 months) and the data was collected prospectively in the context of a large clinical trial. Nonetheless, we reached similar conclusions and confirmed the findings of van der Helm et al that doubling of platelet count after one cycle of azacitidine therapy in patients with MDS and oligoblastic AML is significantly and independently associated with achieving eventual objective response and a reduced risk of death after adjustment for important confounders (cytogenetic prognostic group and presence of circulating blasts). Although platelet count doubling was associated with achieving response in unadjusted regression analysis, the platelet count doubling was not associated with reduced risk of death in unadjusted analysis. This observation could potentially have resulted from unequal distribution of significant predictive factors for survival between the 2 groups (e.g. unfavorable cytogenetics were significantly more prevalent in patients who achieved platelet count doubling).
There is no clear explanation for the statistically significant observation of higher prevalence of unfavorable karyotypes among patients who achieved platelet count doubling. Baseline platelet counts among patients with unfavorable cytogenetics were not statistically significantly different from those of patients with other cytogenetics (median baseline platelet count 52×109/L versus 45×109/L, respectively; P=0.86). Therefore, lower baseline platelet counts in patients with unfavorable cytogenetics making it more feasible for the platelet count to double could not have accounted for this observation. It should be noted that azacitidine has been shown to be equally effective in MDS patients with unfavorable karyotypes including monosomy 7.
The IPSS and the revised IPSS (IPSS-R) are the most widely used prognostic tool for MDS (Greenberg et al, 1997, Greenberg et al, 2012). Although both the IPSS and IPSS-R were developed using cohorts of untreated patients, both models were shown to be prognostic for survival among treated MDS patients, including those treated with azacitidine (Lamarque et al, 2012, Vosoet al, 2013, Mishra et al, 2013, Savic et al, 2013, Neukirchen et al, 2014). The recently described FPSS has been also shown to separate azacitidine-treated patients with HR-MDS and oligoblastic AML into 3 groups with significantly different median OS based on 4 baseline clinical and laboratory parameters: the Eastern Cooperative Oncology Group performance status, karyotype, presence of PB blasts, and red blood cell transfusion-dependence (Itzykson et al, 2011b, Itzykson et al, 2012).
Similar to the Dutch investigators, we have previously validated the prognostic potential of the FPSS using the E1905 patient cohort but also showed that the FPSS did not have an advantage in the prognostic discrimination over the more widely-used IPSS-R (Zeidan et al, 2014). In this current analysis, we found that the addition of post-treatment platelet count doubling did not improve the survival prediction offered by either the IPSS-R or the FPSS in azacitidine-treated patients. The components which constitute the IPSS-R and FPSS likely are stronger survival predictors compared with early platelet doubling. Platelet doubling likely identifies a small number of patients whose survival improves with azacitidine, but does not account for the entire cohort of patients with improved survival.
Our analysis has several limitations. Although the use of other levels of platelet count increment (e.g. tripling) or exceeding an absolute cutoff (e.g. 50 × 109/L or 100 × 109/L) at start of cycle 2 might provide better survival prediction, such analysis would be exploratory in nature and will need further confirmation. Therefore we chose to examine the association using the same count doubling threshold used by the Dutch group. Secondly, while 74.1% of patients with platelet count doubling have eventually achieved a PR or CR, 46.5% of patients without platelet count doubling after first cycle of azacitidine therapy have also subsequently achieved a PR or CR. Therefore, patients who do not achieve platelet count doubling after the first cycle of azacitidine therapy might still achieve benefit from the drug and early termination of azacitidine therapy if this threshold is not crossed should not be recommended at this time. Third, the issue of platelet count doubling at the start of second cycle of azacitidine for patients with very low baseline platelet counts (e.g. 1 × 109/L) and those who are platelet-transfusion dependent can cloud the validity of these results. Fourth, our patients received a prolonged lower-dose regimen of azacitidine therapy instead of the approved and commonly-used 75 mg/day 7-day cycles, and half of the patients additionally received a histone deacetylase inhibitor (entinostat).
Despite these limitations, our results confirm that doubling of platelet count after one cycle of azacitidine therapy is an early predictor of achieving response and survival benefit among patients with MDS. Identification of reliable and consistent predictors for clinical benefit (or lack of) for azacitidine therapy remains an unmet medical need for MDS patients and should continue to constitute a high research priority.
Acknowledgments
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA16116, CA20176, CA27525, CA14958, CA31946, CA32102, CA17145, CA27057 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Author contributions
AMZ conceived and designed the study, performed the research, analyzed the data and wrote the manuscript. SDG, TP, PG designed the study, performed the research, analyzed the data and critically reviewed the manuscript. JWL and ZS designed the study, performed the research, analyzed the data, conducted the statistical analysis and critically reviewed the manuscript. MJ, MRS, EP, JG, HPE, and MST provided patients and critically reviewed the manuscript. All authors reviewed the final version and approved submission.
Disclosures: Amer Zeidan is supported by a Young Investigator Award (YIA) from the American Society of Clinical Oncology (ASCO) and by an ’Evans Fellow’ award from the MDS Clinical Research Consortium.
References
- Faltas B, Zeidan A, Gergis U. Myelodysplastic syndromes: Toward a risk-adapted treatment approach. Expert Review of Hematology. 2013;6:611–624. doi: 10.1586/17474086.2013.840997. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR, International Vidaza High-Risk MDS Survival Study Group Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. The Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzykson R, Thepot S, Quesnel B, Dreyfus F, Recher C, Wattel E, Gardin C, Ades L, Fenaux P. Long-term outcome of higher-risk MDS patients treated with azacitidine: An update of the GFM compassionate program cohort. Blood. 2012;119:6172–6173. doi: 10.1182/blood-2012-04-422204. [DOI] [PubMed] [Google Scholar]
- Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, Quesnel B, Vey N, Gelsi-Boyer V, Raynaud S, Preudhomme C, Ades L, Fenaux P, Fontenay M, Groupe Francophone des Myelodysplasies (GFM) Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia : Official Journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2011a;25:1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P, Groupe Francophone des Myelodysplasies(GFM) Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011b;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- Lamarque M, Raynaud S, Itzykson R, Thepot S, Quesnel B, Dreyfus F, Rauzy OB, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Visanica S, Stamatoullas A, Fenaux P, Ades L. The revised IPSS is a powerful tool to evaluate the outcome of MDS patients treated with azacitidine: The GFM experience. Blood. 2012;120:5084–5085. doi: 10.1182/blood-2012-09-453555. [DOI] [PubMed] [Google Scholar]
- Mishra A, Corrales-Yepez M, Ali NA, Kharfan-Dabaja M, Padron E, Zhang L, Epling-Burnette PK, Pinilla-Ibarz J, Lancet JE, List AF, Komrokji RS. Validation of the revised international prognostic scoring system in treated patients with myelodysplastic syndromes. American Journal of Hematology. 2013;88:566–570. doi: 10.1002/ajh.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirchen J, Lauseker M, Blum S, Giagounidis A, Lubbert M, Martino S, Siragusa S, Schlenk RF, Platzbecker U, Hofmann WK, Gotze K, Palumbo GA, Magrin S, Kundgen A, Aul C, Hildebrandt B, Hasford J, Kobbe G, Haas R, Germing U. Validation of the revised international prognostic scoring system (IPSS-R) in patients with myelodysplastic syndrome: A multicenter study. Leukemia Research. 2014;38:57–64. doi: 10.1016/j.leukres.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, Dreyfus F, Rauzy OB, Recher C, Ades L, Quesnel B, Beach CL, Fenaux P, Vey N. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg PL, Herman J, Juckett M, Smith MR, Malick L, Paietta E, Czader M, Litzow M, Gabrilove J, Erba HP, Gore SD, Tallman MS. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: Results of the US leukemia intergroup trial E1905. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2014;32:1242–1248. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic A, Marisavljevic D, Kvrgic V, Stanisavljevic N. Validation of the revised international prognostic scoring system for patients with myelodysplastic syndromes. Acta Haematologica. 2013;131:231–238. doi: 10.1159/000354840. [DOI] [PubMed] [Google Scholar]
- Sekeres MA, Tiu RV, Komrokji R, Lancet J, Advani AS, Afable M, Englehaupt R, Juersivich J, Cuthbertson D, Paleveda J, Tabarroki A, Visconte V, Makishima H, Jerez A, Paquette R, List AF, Maciejewski JP. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–4951. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, Larson RA, Cancer and Leukemia Group B Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the cancer and leukemia group B. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- Steensma DP. Can hypomethylating agents provide a platform for curative therapy in myelodysplastic syndromes? Best Practice & Research.Clinical Haematology. 2012;25:443–451. doi: 10.1016/j.beha.2012.10.007. [DOI] [PubMed] [Google Scholar]
- van der Helm LH, Alhan C, Wijermans PW, van Marwijk Kooy M, Schaafsma R, Biemond BJ, Beeker A, Hoogendoorn M, van Rees BP, de Weerdt O, Wegman J, Libourel WJ, Luykx-de Bakker SA, Minnema MC, Brouwer RE, Croon-de Boer F, Eefting M, Jie KS, van de Loosdrecht AA, Koedam J, Veeger NJ, Vellenga E, Huls G. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the dutch azacitidine compassionate named patient programme. British Journal of Haematology. 2011;155:599–606. doi: 10.1111/j.1365-2141.2011.08893.x. [DOI] [PubMed] [Google Scholar]
- Voso MT, Fenu S, Latagliata R, Buccisano F, Piciocchi A, Aloe-Spiriti MA, Breccia M, Criscuolo M, Andriani A, Mancini S, Niscola P, Naso V, Nobile C, Piccioni AL, D'Andrea M, D'Addosio A, Leone G, Venditti A. Revised international prognostic scoring system (IPSS) predicts survival and leukemic evolution of myelodysplastic syndromes significantly better than IPSS and WHO prognostic scoring system: Validation by the gruppo romano mielodisplasie italian regional database. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2013;31:2671–2677. doi: 10.1200/JCO.2012.48.0764. [DOI] [PubMed] [Google Scholar]
- Zeidan AM, Komrokji RS. There's risk, and then there's RISK: The latest clinical prognostic risk stratification models in myelodysplastic syndromes. Current Hematologic Malignancy Reports. 2013;8:351–360. doi: 10.1007/s11899-013-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan AM, Linhares Y, Gore SD. Current therapy of myelodysplastic syndromes. Blood Reviews. 2013a;27:243–259. doi: 10.1016/j.blre.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan AM, Kharfan-Dabaja MA, Komrokji RS. Beyond hypomethylating agents failure in patients with myelodysplastic syndromes. Current Opinion in Hematology. 2013b;21:123–130. doi: 10.1097/MOH.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan AM, Faltas B, Douglas Smith B, Gore S. Myelodysplastic syndromes: What do hospitalists need to know? Journal of Hospital Medicine: An Official Publication of the Society of Hospital Medicine. 2013c;8:351–357. doi: 10.1002/jhm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan AM, Lee JW, Prebet T, Greenberg P, Sun Z, Juckett M, Smith MR, Paietta E, Gabrilove J, Erba HP, Tallman MS, Gore SD, the Eastern Cooperative Oncology Group (ECOG) and North American Leukemia intergroup Comparison of the prognostic utility of the revised international prognostic scoring system and the french prognostic scoring system in azacitidine-treated patients with myelodysplastic syndromes. British Journal of Haematology. 2014 Apr 9; doi: 10.1111/bjh.12884. 2014 doi: 10.1111/bjh.12884. [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]