Abstract
Purpose.
We determined the ultrastructure of mouse adenovirus keratitis, a model for human adenovirus keratitis.
Methods.
Adenovirus keratitis was induced in C57Bl/6j mice by intrastromal injection of human adenovirus species D type 37 (HAdV-D37) with a heat-pulled, glass, micropipette needle under compressed air. At select time points after infection, mice were euthanized and their corneas removed, fixed, and sectioned at 70-nm thickness for electron microscopy.
Results.
Injection of HAdV-D37 into the mouse corneal stroma placed virus predominantly in the pericellular corneal stromal matrix. Virus was seen bound to and entering stromal cells at 1 and 2 hours after infection, respectively. Cell membrane transit by virus was seen to involve two distinct structures resembling caveolae and macropinosomes. However, later during infection intracellular virus was not seen within membrane-bound organelles. By 8 hours after infection, intracellular virus had accumulated into densely packed, perinuclear arrays. Virus disassembly was not obvious at any time point after infection. Infiltrating neutrophils seen by one day after infection had engulfed degraded stromal cells by 4 days after infection.
Conclusions.
By transmission electron microscopy, injected HAdV-D37 readily enters stromal cells in the C57Bl/6j mouse cornea and induces stromal inflammation, as was shown previously by light microscopy. However, electron microscopy also revealed dense, static arrays of intracytoplasmic virus, suggesting a block in viral capsid disassembly and viral DNA nuclear entry. These findings may explain why human adenoviruses do not replicate in the mouse corneal stroma.
Keywords: adenovirus, keratitis, mouse, electron microscopy
In the mouse adenovirus keratitis model, human adenoviruses enter corneal cells but do not replicate. This study demonstrates the intracytoplasmic accumulation of virus in the corneal stroma, suggesting that a block in virus capsid disassembly in mouse cells leads to reduced nuclear entry by adenovirus DNA.
Introduction
Human adenovirus (HAdV) infects all human mucosal epithelia, including most notably those of the respiratory, gastrointestinal, genitourinary, and ocular surfaces, and can be associated with significant mortality and morbidity.1–3 Infection of the cornea by HAdV species D types, 8, 37, 53, 54, 56, and 64 causes adenovirus keratitis,4–8 manifest as highly distinctive, multifocal, leukocyte infiltrates of the subepithelial corneal stroma, with onset 7 to 10 days after resolution of ocular surface epithelial infection (epidemic keratoconjunctivitis). The HAdVs do not cause productive infection in mice, nor are mouse adenoviruses known to cause murine keratitis in the wild. However, HAdV-C5 in high titer (1010 plaque-forming units) can infect the mouse respiratory tract and cause pneumonitis,9 and similarly, high titer (≥5 × 104 infectious units) of virus injected into the mouse corneal stroma induces keratitis.7,10–12 In the keratitis model, HAdV-D37 has been shown to infect mouse cells in the corneal stroma, expresses early but not late viral genes and does not replicate, and persists with very slow loss of titer over time.10
To better understand the pathogenesis of keratitis in mouse cornea in the absence of viral replication, we performed thin-section transmission electron microscopy of the cornea at specific times after infection.
Methods
Mouse Adenovirus Keratitis Model
The HAdV-D37 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown on A549 cells (ATCC) before cesium chloride gradient purification and titering. Cell cultures and viral preparations were tested and found free of Mycoplasma and endotoxin contamination. Adenovirus keratitis was induced in 8- to 12-week-old, female, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) as described previously,10 with approval by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary, and as per the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Briefly, 105 infectious units of purified HAdV-D37 (or dialysis buffer as a control) in 1 μL volume were injected into the right corneal stroma of anesthetized mice with a heat-pulled, glass, micropipette needle using a compressed air injection system and under direct visualization with an operating microscope, and the mice allowed to recover from anesthesia.
Transmission Electron Microscopy
Mice were euthanized at select time points (0 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 1 day, 2 days, and 4 days) after infection, and their corneas removed and fixed overnight in 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M cacodylate buffer with 2.5 mM CaCl2. Corneas then were postfixed for 1.5 hours in 2% aqueous osmium tetroxide (OsO4), dehydrated in graded ethanols, transitioned in propylene oxide, infiltrated with propylene oxide and epon mixtures, embedded in epon, and cured for 48 hours at 60°C. Sections (1 μm) were cut on a Leica Ultracut microtome (Buffalo Grove, IL, USA), and stained with 1% toluidine blue in 1% borate buffer for light microscopic determination of the correct site for ultrathin sectioning. Ultrathin sections were cut at 70 nm and stained with saturated, aqueous uranyl acetate and Sato's lead stain. Photomicrographs were taken on a Philips CM-10 electron microscope (FEI, Hillsboro, OR, USA) operating at 80 kv and fitted to a CCD camera.
Results
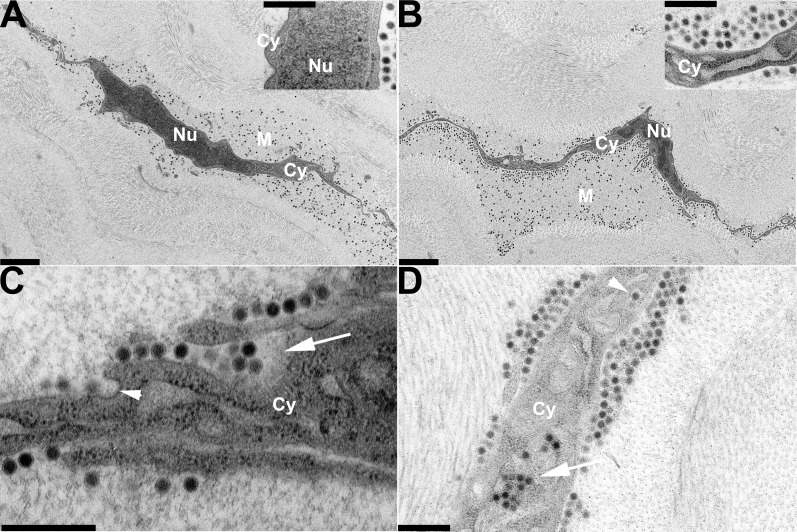
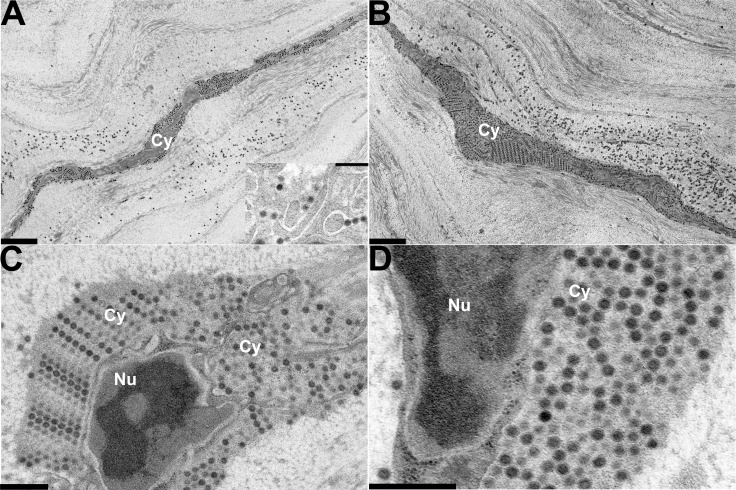
Keratocytes comprise the major cellular component in the stroma, and are fully interconnected by gap junctions to form a cellular web floating between dense rows of collagen fibrils within a relatively looser extracellular matrix.13 In corneas removed and processed for ultrastructural study immediately after injection of HAdV-D37 into the corneal stroma, virus was seen mostly in this less dense pericellular matrix (Fig. 1A), suggesting a path of least resistance to pressurized injection into the corneal stroma, with some viruses on cell membrane surfaces. At 30 minutes after infection, virus had accumulated closer to the cell membrane(s) (Fig. 1B). By 1 hour after infection, cellular binding and entry was apparent (Fig. 1C) with some viruses appearing to enter by macropinocytosis,14,15 and other viruses within flask-shaped invaginations of the cell membrane reminiscent of caveolae. By 2 hours after infection, virus was apparent within cell cytoplasm in endosomes and macropinosomes (Fig. 1D). At 4 hours after infection, more virus is seen in the cells (Fig. 2A), while at 8 hours after infection, cells showed prominent intracytoplasmic accumulations of virus, often in regular packed arrays adjacent to the nuclei and interpolated between cellular organelles (Figs. 2B–D). Nuclear entry by viral DNA into corneal stromal cells of the mouse was previously suggested by experiments showing early viral gene expression (E1A and E1B 19k) in vivo within 4 hours after infection.10 However, at the ultrastructural level, virus disassembly with nuclear entry by viral DNA could not be verified.
Figure 1.
Thin-section electron microscopy of C57Bl/6j mouse corneal stroma at early time points after intrastromal injection of HAdV-D37. Micrographs show areas of corneal stroma (A) immediately, (B) 30 minutes, (C) 1 hour, and (D) 2 hours after injection. Intracellular structures of corneal stromal cells are labeled as follows: Cy, cytoplasm; Nu, nucleus; M, pericellular matrix. Viruses are visible as electron dense black dots under lower magnification in (A) and (B) (×3400), and as spherical structures with denser cores in insets (A, B) and ([D], ×19,000), and in ([C], ×34,000). The arrowhead in (C), at 1 hour after infection, points to a caveolae-like structure; the arrow points to a membrane ruffle that contains multiple viruses (incipient macropinosome). By 2 hours after infection (D), some viral entry has occurred, and virus can be seen in an endosome (single virus particle, arrowhead) and macropinosome (multiple viruses, arrow). Scale bars: 2 μm for (A) and (B), and 0.5 μm for insets (A) and (B), and (C) and (D).
Figure 2.
Thin-section electron microscopy of C57Bl/6j mouse corneal stroma at intermediate time points after intrastromal injection of HAdV-D37. Micrographs show areas of corneal stroma (A) 4 hours, and (B–D) 8 hours after injection. Intracellular structures are labeled as follows: Cy, cytoplasm; Nu, nucleus. The inset in (A) shows a higher magnification of intracellular virus 4 hours after injection. Viruses are visible as electron dense black dots under lower magnification in (A) and ([B], ×3400), and as spherical structures with denser cores in the inset (×19,000) in (A), and in (C) and ([D], ×19,000 and ×34,000, respectively). All micrographs show densely packed intracellular viral arrays. Intracellular virus is seen interspersed directly among and between intracellular organelles in (C), and adjacent to the nuclear membrane in (D). Scale bars: 2 μm for (A) and (B), and 0.5 μm for (C) and (D), and the inset in (A).
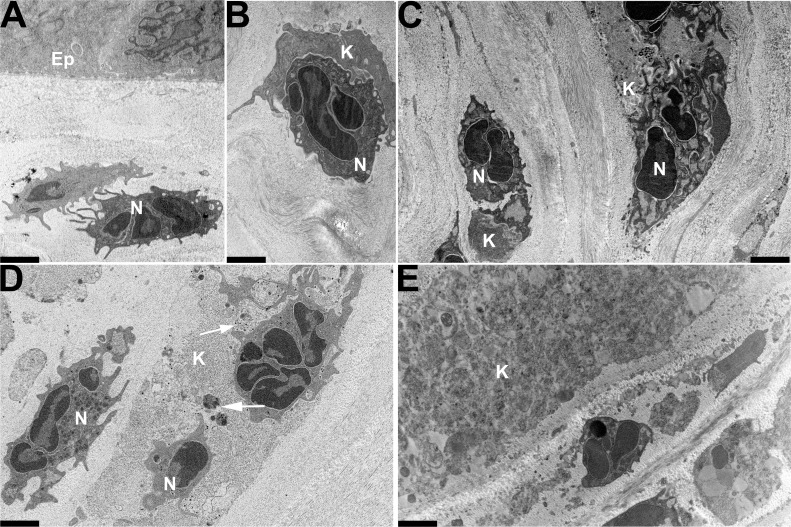
The onset of clinically evident stromal inflammation in the mouse adenovirus keratitis model typically appears at 24 hours after infection, and by flow cytometry, polymorphonuclear neutrophils form the initial burst of infiltrating leukocytes.10,12 By ultrastructural analysis performed 24 hours after infection, polymorphonuclear neutrophils were seen infiltrating the cornea (Fig. 3A), closely interacting with (Fig. 3B), and engulfing (Fig. 3C) infected cells. Morphologically intact viruses still could be observed within the cytosol of some corneal stromal cells. At 48 hours after infection, most of the stromal cells appeared to have lost their structural integrity (Fig. 3D), suggestive of cell death, and intracellular viral arrays were no longer evident. At 4 days after infection, residual cellular debris was seen as electron-dense patches (Fig. 3E).
Figure 3.
Thin-section electron microscopy of C57Bl/6j mouse cornea at late time points after intrastromal injection of HAdV-D37. Micrographs show areas of corneal stroma (A–C) 1 day, (D) 2 days, and (E) 4 days after injection. Ep, epithelium; N, neutrophil; K, keratocyte. Neutrophils are evident (A) infiltrating the cornea, (B) closely interacting with, and (C) engulfing resident stromal cells. Morphologically intact viruses still could be observed within the cytosol of some resident corneal stromal cells (arrows in [D]). Cellular disintegration and debris (electron dense material) are evident at 2 and 4 days after injection (D, E), respectively. All micrographs were taken at ×3400. Scale bars: 2 μm.
Discussion
Adenovirus keratitis in the mouse is induced by pressurized injection of high titer HAdV-D into the corneal stroma, a methodology designed to bypass the intrinsically nonpermissive mouse corneal epithelium.16 We have previously shown evidence for viral entry into mouse corneal stromal cells in this model, based on the observation of fluorescently-labeled virus capsid in the perinuclear region of cells, and by demonstrating early viral gene expression in the infected corneas.10 The latter can occur only if viral DNA is transported successfully into the cell nucleus. Inflammation in the mouse cornea infected with HAdV occurs due to innate immune responses to virus-associated molecular patterns principally on the viral capsid, less so the viral nucleic acid, and viral replication in the stroma is not a prerequisite.12
In the current study, thin-section transmission electron microscopy of corneas harvested immediately after injection show virus predominantly in the pericellular corneal stroma. The pericellular matrix is the least densely packed and, therefore, likely a path of least resistance to injection. Biomechanical stress due to blinking and eye movements then may be responsible for what appears to be “migration” of intrinsically nonmotile virus toward cell membranes within the first few hours after infection, combined with the “stickiness” of the stromal cells for virus due to virus-receptor binding (flypaper effect). The precise receptors for HAdV-D on corneal stromal cells in the mouse remain unknown. In human cells, the HAdV-D fiber knob (primary virus–host interaction) binds to either CD46 or GD1a glycan,17,18 and the five-sided penton base protein's arginine-glycine-aspartic acid motif binds to αvβ1, αvβ3, and αvβ5 (secondary virus-host interaction) to induce intracellular signaling and viral internalization by endocytosis.19–25
Once bound to corneal stromal cells, we observed two morphologically distinct mechanisms of possible viral entry by HAdV-D37. One means of entry was through cylindrical invaginations into the cell membrane suggestive of macropinocytosis. Macropinosomes form upon actin polymerization, membrane ruffling, and eventually, closure of lamellipodia to capture a sample of the extracellular compartment. Macropinocytosis is receptor independent and is triggered by various signaling pathways, including some previously implicated in HAdV-D entry, such as the PI3 and rho family kinases.21–23,25–27 The HAdV-C2 was previously shown to trigger αv integrin, protein kinase C, rho-GTPase, and F-actin–dependent macropinocytosis in HeLa cells.28
In addition to macropinocytosis, we also observed viral entry into flask-shaped vesicles at the cell membranes suggestive of caveolae. Lipid raft–mediated, caveolin-1–associated viral entry was demonstrated recently for HAdV-D37 in cultured human keratocytes,29 in which the presence of caveolin-1 was confirmed in virus-containing endosomes by immuno-electron microscopy. However, caveolin-associated HAdV entry remains controversial,30 and viral entry for HAdVs appears to be cell and viral type specific. For example, HAdV-C2, a nonocular-tropic virus, entered epithelial cells by clathrin-coated pits.29 Confirmation of a role for caveolin in HAdV-D37 in the mouse model of adenovirus keratitis would require immunoelectron microscopy.
A second question addressed by these studies is how and why HAdV-37 can persist in the mouse cornea but does not replicate.10 At 1 week after infection, intact virus in the denser portions of the corneal stroma was still evident (data not shown), which could explain viral persistence with only modest loss of titer previously observed in the mouse model. As a nonenveloped virus without apparent intrinsic chemotactic properties, the adenovirus is inherently stable under a wide range of environmental conditions.31–33 For example, fecal adenoviruses can persist even in treated water under conditions where Escherichia coli may not.34
Early but not late viral gene expression has been shown previously in the mouse keratitis model under identical conditions to those in the current study,10 demonstrating indirectly that some viral DNA enters the nuclei of corneal stromal cells of the mouse, and is transcribed. By electron microscopy, we observed densely packed arrays of virus in the cytoplasm of resident stromal cells. Molecular interactions between adenovirus hexon protein and nuclear pore components are critical to final virus uncoating and delivery of DNA into the nucleus,35–37 and might be faulty in a mouse cell with a human adenovirus. The packed symmetry of intracytoplasmic viral arrays suggests retention of an intact icosahedral capsid structure, that is, that those viruses retained in the cytoplasm have not uncoated, preventing the passage of their DNA into the nucleus. Therefore, on the basis of these ultrastructural observations and those previously published,10,16 we now have evidence for at least two obstacles to efficient HAdV replication in the mouse: impaired viral DNA delivery to the nucleus and failure of transition from early to late viral gene expression. The specific cellular receptors and entry pathways used by different adenovirus types can lead to differences in intracellular trafficking which in turn directly impact downstream processes, including inflammatory and immune responses to infection.38 The intracytoplasmic accumulation of HAdV-D37 in mouse corneal cells could derive from a cellular entry pathway that less efficiently delivers virus to the perinuclear region for uncoating. Although virus was observed early in infection at corneal stromal cell membranes in flask-shaped indentations resembling caveolae, at later time points we did not observe virus within cellular endosomes or bound by any intracellular membrane compartment. These observations suggested that the means by which HAdV-D37 enters mouse cells leads to inefficient uncoating and delivery of viral DNA to the nucleus.
Acknowledgments
The authors thank Elizabeth A. Benas for technical support.
Supported by National Institutes of Health (Bethesda, MD, USA) Grants EY013124, EY021558, and P30EY014104, a Senior Scientific Investigator Award (JC) from Research to Prevent Blindness, Inc., New York, NY, the Massachusetts Lions Eye Research Fund, and the Falk Foundation.
Disclosure: S. Mukherjee, None; X. Zhou, None; J. Rajaiya, None; J. Chodosh, None
References
- 1. Harding SP, Mutton KJ, van der Avoort H, Wermenbol AG. An epidemic of keratoconjunctivitis due to adenovirus type 37. Eye. 1988; 2: 314–317. [DOI] [PubMed] [Google Scholar]
- 2. Wood DJ. Adenovirus gastroenteritis. Br Med J. 1988; 296: 229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dingle JH, Langmuir AD. Epidemiology of acute, respiratory disease in military recruits. Am Rev Respir Dis. 1968; 97 (suppl): 1–65. [DOI] [PubMed] [Google Scholar]
- 4. Robinson CM, Shariati F, Gillaspy AF, Dyer DW, Chodosh J. Genomic and bioinformatics analysis of human adenovirus type 37: new insights into corneal tropism. BMC Genomics. 2008; 9: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson CM, Shariati F, Zaitshik J, Gillaspy AF, Dyer DW, Chodosh J. Human adenovirus type 19: genomic and bioinformatics analysis of a keratoconjunctivitis isolate. Virus Res. 2009; 139: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson CM, Singh G, Henquell C, et al. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 2011; 409: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh MP, Chintakuntlawar A, Robinson CM, et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One. 2009; 4: e5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou X, Robinson CM, Rajaiya J, et al. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest Ophthalmol Vis Sci. 2012; 53: 2804–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ginsberg HS, Moldawer LL, Sehgal PB, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991; 88: 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci. 2007; 48: 781–788. [DOI] [PubMed] [Google Scholar]
- 11. Chintakuntlawar AV, Chodosh J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res. 2009; 29: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 2010; 6: e1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995; 36: 2557–2567. [PubMed] [Google Scholar]
- 14. Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009; 10: 364–371. [DOI] [PubMed] [Google Scholar]
- 15. Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011; 89: 836–843. [DOI] [PubMed] [Google Scholar]
- 16. Chodosh J. Human adenovirus type 37 and the BALB/c mouse: progress toward a restricted adenovirus keratitis model (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006; 104: 346–365. [PMC free article] [PubMed] [Google Scholar]
- 17. Wu E, Trauger SA, Pache L, et al. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol. 2004; 78: 3897–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nilsson EC, Storm RJ, Bauer J, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011; 17: 105–109. [DOI] [PubMed] [Google Scholar]
- 19. Huang S, Kamata T, Takada Y, Ruggeri ZM, Nemerow GR. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996; 70: 4502–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol. 2001; 75: 5405–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998; 72: 8806–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998; 72: 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li E, Stupack DG, Brown SL, Klemke R, Schlaepfer DD, Nemerow GR. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000; 275: 14729–14735. [DOI] [PubMed] [Google Scholar]
- 24. Natarajan K, Rajala MS, Chodosh J. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J Immunol. 2003; 170: 6234–6243. [DOI] [PubMed] [Google Scholar]
- 25. Rajala MS, Rajala RV, Astley RA, Butt AL, Chodosh J. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol. 2005; 79: 12332–12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000; 10: 839–848. [DOI] [PubMed] [Google Scholar]
- 27. Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996; 135: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier O, Boucke K, Hammer SV, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002; 158: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yousuf MA, Zhou X, Mukherjee S, et al. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One. 2013; 8: e77462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engel S, Heger T, Mancini R, et al. Role of endosomes in simian virus 40 entry and infection. J Virol. 2011; 85: 4198–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nauheim RC, Romanowski EG, Araullo-Cruz T, et al. Prolonged recoverability of desiccated adenovirus type 19 from various surfaces. Ophthalmology. 1990; 97: 1450–1453. [DOI] [PubMed] [Google Scholar]
- 32. Gordon YJ, Gordon RY, Romanowski E, Araullo-Cruz TP. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro. Ophthalmology. 1993; 100: 1835–1839, discussion 1839–1840. [DOI] [PubMed] [Google Scholar]
- 33. Kowalski RP, Romanowski EG, Waikhom B, Gordon YJ. The survival of adenovirus in multidose bottles of topical fluorescein. Am J Ophthalmol. 1998; 126: 835–836. [DOI] [PubMed] [Google Scholar]
- 34. Kauppinen A, Ikonen J, Pursiainen A, Pitkanen T, Miettinen IT. Decontamination of a drinking water pipeline system contaminated with adenovirus and Escherichia coli utilizing peracetic acid and chlorine. J Water Health. 2012; 10: 406–418. [DOI] [PubMed] [Google Scholar]
- 35. Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997; 16: 5998–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saphire AC, Guan T, Schirmer EC, Nemerow GR, Gerace L. Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J Biol Chem. 2000; 275: 4298–4304. [DOI] [PubMed] [Google Scholar]
- 37. Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001; 3: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 38. Teigler JE, Kagan JC, Barouch DH. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol. 2014; 88: 10354–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]