Abstract
Cancer remains one of the most dreadful diseases. Whereas most treatment regimens for various cancers have resulted in improved clinical responses and sometimes cures, unfortunately, subsets of cancer patients are either pre-treatment resistant or develop resistance following therapy. These subsets of patients develop cross-resistance to unrelated therapeutics and usually succumb to death. Thus, delineating the underlying molecular mechanisms of resistance of various cancers and identifying molecular targets for intervention are the current main focus of research investigations. One approach to investigate cancer resistance has been to identify pathways that regulate resistance and develop means to disrupt these pathways in order to override resistance and sensitize the resistant cells to cell death. Hence, we have identified one pathway that is dysregulated in cancer, namely, the NF-κB/Snail/YY1/RKIP loop, that has been shown to regulate, in large part, tumor cell resistance to apoptosis by chemotherapeutic and immunotherapeutic cytotoxic drugs. The dysregulated resistant loop is manifested by the overexpression of NF-κB, Snail and YY1 activities and the underexpression of RKIP. The induction of RKIP expression results in the downregulation of NF-κB, Snail and YY1 and the sensitization of resistant cells to drug-induced apoptosis. These findings identified RKIP, in addition to its anti-proliferative and metastatic suppressor functions, as an anti-resistance factor. This brief review describes the role of RKIP in the regulation of drug sensitivity via disruption of the NF-κB/Snail/YY1/RKIP loop that regulates resistance in cancer cells.
Keywords: chemotherapy, immunotherapy, NF-κB, resistance, RKIP, Snail, stem cells, YY1
I. INTRODUCTION
An aspect in the pathogenesis of cancer is the recognition that the majority of cancers arises and persists as a consequence of the activity of oncogenes and tumor suppressor genes and that such oncogenes dysregulate anti-apoptotic pathways involved in the resistance. The genomic characterization of tumors has highlighted the pivotal role of “driver” somatic mutations and tumor dependency called “oncogenic addiction”.1 Further, “non-oncogenic addiction” was also caused to define dependences that are not manifested by somatic cancer gene modifications.2 Other tumor dependencies include hormone dependency, lineage dependency and metabolic dependency.2,3 Clinically, several drugs were developed to target tumor dependency factors.4
We have experienced three waves of drug development, namely, a wave of drugs targeting DNA replication and cell division, a wave of drugs targeting signaling intermediates that contribute to cancer growth, and a wave of drugs that target cellular mechanisms essential for tumor growth and survival.5 The first wave of drugs, namely, chemotherapeutic drugs, still represents the vast majority of clinically used chemotherapeutic drugs today in several cancers (examples platinum derivatives, topoisomerase inhibitors, nucleoside analogs, vinca alkaloids, and taxanes). The second wave of drugs are developed and targets genetic alterations in cancer cells and ascribed for tumor survival and “oncogene addiction” and other targets that are not genetically altered “non-oncogenic addiction”. Many of the drugs have been used for the treatment of variety of cancers. In addition, another form of a second wave drugs is monoclonal antibodies targeting cell surface receptors. The third wave consists of drugs that have developed to target factors that regulate tumor growth and survival and are not directly involved in DNA replication or cell division.5
Although the above therapies resulted in significant clinical responses, however, many patients experience drug resistance. The phenomenon of drug resistance dates back for several decades. For instance, Brockman reported in a long review chapter the mechanisms of resistance that were known at that time, primarily emphasizing the biochemical basis of resistance, that the resistant cancer cells differed biochemically from the sensitive cancer cells in their response to chemotherapeutics.6 However, the insights made by Brockman were not validated in clinical practice. The malignant variants that emerged after the initial therapy developed cross-resistance against the drugs used and several unrelated classes of drugs.7,8 This phenomenon of multiple drug resistance (MDR) was analyzed by the use of various inhibitors to reverse the MDR phenotype, however, such analyses were not translated in the clinic.9 In addition, the drugs used were under the rationale that once inside the tumor cells they should inhibit cell proliferation and kill the cells; however, it is clear nowadays that drug resistance is a complex and a multi-faceted system consisting of biochemical, molecular and genetic alterations that the tumor cells exploit to avoid cytotoxic drugs.
Drug resistance represents a major barrier in the eradication of cancer and its cure by all available therapeutic regimens. The failure to recognize the underlying mechanisms of drug resistance in relapsed patients and their treatments with drugs that are not targeted to these mechanisms results in dismal response rates. Our understanding on the mechanisms of resistance remains incomplete. Hence, emphasis nowadays is directed at the development of targeted agents at factors that regulate resistance that may differ for each type of cancer.
Several cancer non-specific conventional and more specific targeted therapies are currently being used in the treatment of a variety of cancers. A common denominator for all of these strategies is that there is an excellent initial clinical response for primary tumors and seldom with metastatic tumors with improvement of progress-free survival and seldom cures. Another common denominator is that a subset of patients does not respond to these treatments and another subset of responding patients develops cross-resistance to further treatments. The former is called “intrinsic resistance” and the latter “acquired resistance.” Clearly, tumor cells thrive to survive, proliferate, and metastasize and, thus, develop several mechanisms to escape the cytotoxic effects of the various therapeutics that are currently being used.
The intrinsic resistance signifies that prior to receiving therapy, a subset of tumor cells and their microenvironment are regulated by resistant factors that make the tumor unresponsive. In contrast, acquired resistance may develop during treatment of the tumor cells that were initially sensitive. However, it may also be considered that due to tumor heterogeneity, that intrinsic resistance may be in a subset, such as cancer stem/initiated cells, that is highly drug resistant. Also, acquired resistance may consist of selective pressure to novel drug-induced oncogenic mutations.An example of acquired resistance is the mutation within the BCR-ABL oncoprotein in CML treated with the ABL inhibitor imatinib in which a residual subset was selected by the treatment and exhibited drug resistance.10 An example of intrinsic resistance is found in approximately 10% of the BRAFv600E mutation in melanoma following treatment with the BRAF inhibitor vemarufenib.11
II. MECHANISMS OF CANCER RESISTANCE TO CYTOTOXIC DRUGS
Mechanisms of drug resistance include, but not as yet unknown other mechanisms, increased rates of drug efflux, DNA repair, alteration in drug metabolism, mutations of drug targets, activation of survival anti-apoptotic pathways, epigenetic changes, the tumor microenvironment, the presence of cancer stem cells and the molecular and genetic hallmarks of this tumor population. These above various mechanisms have been reviewed extensively recently.12
The mechanisms of resistance regulated by downstream factors are due to constitutively activated cell survival/anti-apoptotic pathways. Since the main objective of cancer treatment is to kill the tumor cells, clearly, the tumor cells, in turn, exhibit intrinsic adaptive responses that counteract death signals and promote cell survival. During the transformation process, pathways that regulate apoptosis are dysregulated and often malfunctional.13 Examples of a few gene products that regulate apoptosis and their role in certain cancers14 are briefly summarized below: 1) the anti-apoptotic members of the Bcl-2 family are, in large part, regulated by constitutively activated NF-κB and STAT3 transcription factors. For instance, overexpression of Bcl-2 correlates with resistance of leukemic cells to drug therapy.15 Since several members regulate the mitochondrial membrane permeability and subsequent activation of the type II apoptotic pathway, the relative activity of pro-and anti-apoptotic gene products of this family dictates the ultimate fate of the tumor cells.16 A correlation between a mitochondrial response to chemotherapy and a clinical response was reported.17 Similar findings of the implication of Bcl-2 family members in resistance to targeted therapy have been reported. The BH3-only protein BIM was reported to play a central role in imatinib-induced apoptosis in CML and gefitinib and erlotinib-induced apoptosis in EGFR-mutated NSCLC.18-20
Several mechanisms of drug resistance to small molecule inhibitors have been reported including MDR1.21-23 Monoclonal antibodies are also not immune to the development of resistance.5,24 The cellular machines that were targets by the first wave chemotherapeutics included DNA replication, DNA repair and cell division. Analysis revealed that these machines are required for tumor cell proliferation and survival. The existence of chronic stress conditions in tumors and the ensuing nononcogene addiction of cancer cells are now recognized as “hallmarks of cancer”.2,13 Examples are heat shock proteins (HSP90).25 Targeting the proteasome function increases the proteotoxic stress in tumors. Also, targeting chromatin modification has been investigated.5
III. ROLE OF RKIP IN THE REGULATION AND REVERSAL OF RESISTANCE
The Raf kinase inhibitor protein (RKIP) is a member of the phophatidylethylnolamine-binding protein (PEBP) family, which was shown, initially, to play a role in lipid metabolism and phospholipid membrane biogenesis.26 Yeung et al. cloned the RKIP gene and reported its inhibitory activity on both the Raf/MEK/ERK pathway (through which RKIP’s name was derived) and the NF-κB survival pathways.27,28 RKIP was shown to be inactivated via phosphorylation at Serine153 by PKC.29 Lorenz et al. reported that phosphorylated RKIP inhibits the G-protein coupled receptor (GPCR) kinase (GRK-2) and facilitates the crosstalk between the EGF and GPCR signaling pathways.30 Ectopic expression of RKIP suppresses invasion and metastasis of human prostate cancer cells in mice31 and, in contrast, the downregulation of RKIP led to invasiveness of tumor cells in vitro.32
RKIP has been classified as a tumor metastatic suppressor gene and was found to be downregulated during the metastatic process.31,33-36 In contrast to cervival cancer, in many other tumors the loss of RKIP was of poor prognostic significance.33,35,37-42
A. Role of RKIP in Inhibiting Resistance to Chemotherapeutic Drugs
Chatterjee et al.43 were the first to report on the role of RKIP in the regulation of drug resistance-mediated apoptosis. The induction or overexpression of RKIP in drug-resistant human cancer cell lines sensitized the cells to various chemotherapeutic drugs-induced apoptosis. Due to the original findings by Yeung et al.27.28 on the role of RKIP in the inhibition Raf/MEK/ERK and NF-κB pathways, clearly, several upregulated anti-apoptotic gene products and downregulated pro-apoptotic products that are transcriptionally regulated by these pathways will result in opposing effects by overexpression of RKIP. Hence, the tumor cells will experience a lowering of the threshold of resistance and become sensitized to apoptosis by cytotoxic drugs. 43-47,48 RKIP depletion is associated with radio and chemoresistance .Al-Mulla et al.48 reported that depletion of RKIP in HEK-293 cells resulted in the induction of a reactive oxidative stress response. Oxidative stress-induced NRF2 (NF-E2 related nuclear factor 2) activation to the ubiquitination of KEAP-1 (Kelch-like ECH-associated protein 1), which binds and inhibits NRF2 nuclear translocation.49 NRF2 activation confers cell survival and its expression is upregulated in greater than 90% of head and neck cancer patients50 and determines chemoresistance.51 Al-Mulla et al.52 reported that KEAP-1 expression in colorectal cancer is associated with RKIP stability and that KEAP-1-NFR2 is a novel target in RKIP-induced resistance. The authors suggested that in the presence of RKIP, NRF2 is kept in the cytoplasm by KEAP-1 binding. In the absence of RKIP, inactivation/ubiquitination of KEAP-1 takes place and NRF2 is stabilized and active in the nucleus where its transcripts of anti-oxidants correlated with drug resistance.
A previously reported study analysis by microarray revealed that RKIP was one of the genes that is differentially expressed between tumor samples of cervical cancer patients.53 And in a large series of patients, RKIP protein expression was markedly downregulated in cervical cancer and lymph nodes metastasis.54 Martenko et al.33 investigated the expression levels of RKIP protein in nonmalignant tumor cervical samples and clinical outcome and the role of RKIP in chemotherapy response. RKIP was highly expressed in the cytoplasm of benign tissues, but significantly reduced in cervical cancer tissues as examined by IHC. Further, using human cervical cancer cell lines, there was a correlation between the resistance to cisplatinum and low RKIP expression. In drug sensitive lines, downregulation of RKIP resulted in the resistance to several chemotherapeutic drugs.
There was a correlation between phosphorylated RKIP and STAT3 activity in chemo-resistant colon cancer. Reports in the literature have examined the clinical resistance in colon cancer patients.55,56 In Duke’s B CRC patients, it was reported that RKIP may be a biomarker identifying patients with high resistant aggressive CRC.57,58 Cross-Knoll et al.59 demonstrated that treatment of cell lines with IL-6 resulted in the activation of STAT3 concomitant with phosphorylation of RKIP in vitro. The drugs OXP and CPT (used in the clinic for CRC) inhibited IL-6-induced STAT3 activation and pRKIP through the inhibition of STAT3 interaction with the IL-6 receptor ß subunit, namely, gp130. Stage II colon cancer patients with low levels of nuclear pRKIP experienced longer occurrence free survival compared to patients with high levels of pRKIP.
The cell survival signaling triggered by IL-6 and STAT3 activation is abolished by treatment with OXP or CPT and this treatment also resulted in the inhibition of pRKIP and pSTAT3. JAK-1 and JAK-2 overexpression resulted in the increase of STAT3 transcription and was associated with increased pRKIP.60 The above findings implied the role of RKIP in the sensitization of drug-resistant tumor cells via drug-induced inhibition of phosphorylation of STAT3 and RKIP.
The enhancer of zeste homolog 2 (EZH2) is overexpressed in breast and prostate cancers.61,62 EZH2 exerts oncogenic activity and treatment of prostate cancer cells with shRNA EZH2 inhibited proliferation of cancer cells in vitro.61 Overexpression of EZH2 supports tumor invasiveness and metastasis and underlies the suppression of various genes involved, such as PRC1 proteins,63 Kruppel-like factor 2,64 and the EMT suppressor gene CHH1 that encodes E-cadherin.65 Noteworthy, the suppression of E-cadherin by the repressor Snail requires the participation of EZH2.66,67 E-cadherin shares its metastatic suppressor activity with the metastatic suppressor RKIP.26 Loss of RKIP is associated with EMT induction, enhanced invasion, as well as chemoresistance. The repressor activity of EZH2 on RKIP transcription requires the presence of EMT-inducer Snail. Snail is one of the recruiters of EZH2 to the RKIP promoter and links the induction of RKIP expression.68
B. RKIP Disrupts the Dysregulated NF-κB/Snail/YY1/RKIP Loop Resulting in the Reversal of Drug/Immune Resistance
We have reported the existence of a dysregulated NF-κB/Snail/YY1/RKIP loop in cancer cells that is intimately involved in cell survival and resistance to cytotoxic stimuli. The resistant cancer cells exhibit primarily a constitutively hyperactivated NF-κB pathway and downstream targets, Snail, and YY1 and underexpression of RKIP Snail-induced repression. The inhibition of NF-κB, for example, results in the inhibition of Snail and YY1 and induction of RKIP through the inhibition of the RKIP repressor, Snail. These various modifications result in overriding resistance and the cells become sensitive to both chemo- and immuno-therapeutic drugs.69
The hyperactivation of NF-κB in cancer cells results downstream in the transcription of gene products involved in cell growth and proliferation, apoptotic pathways, EMT and metastasis.70 NF-κB regulates the transcription of Snail, a repressor of RKIP, as well as it regulates YY1 that in turn regulates Snail.71 Snail, in turn, represses both RKIP and PTEN.72 Hence, inhibition of NF-κB results in the inhibition of Snail and YY1 expressions and the induction of both RKIP and PTEN. The upregulation of RKIP accentuates the inhibition of NF-κB and downstream both Snail and YY1 and, therefore, it acts in a feedback mechanism. Likewise, the repression of PTEN results in the inhibition of the PI3K-AKT pathway and this also results in the inhibition of NF-κB via a crosstalk between the two pathways.73
1. Reversal of chemoresistance through the RKIP-disruption of the loop
YY1 is a multi-functional DNA-binding protein that can activate, repress, or initiate transcription depending on the context in which it binds (directly or indirectly) through the formation of a complex with other DNA-binding proteins.74 There is an inverse relationship between YY1 and RKIP. This is the result of both direct and indirect underlying mechanisms. Indirectly, since YY1 regulates positively the transcription of Snail and since Snail represses RKIP, therefore, it will also regulate negatively RKIP. Inhibition of YY1 will result in the inhibition of Snail and derepression of RKIP, leading to its upregulation. Also, transfection of cells with siRNA YY1 resulted in the upregulation of RKIP. There are also preliminary findings demonstrating that YY1 binds to the RKIP promoter as assessed by chromatin-immunoprecipitation (unpublished results). In addition, YY1 represses PTEN and also PTEN suppresses YY1 through its induction of HIF-2-α transcription activity.75
Inhibition of YY1 by various agents sensitizes drug-resistant tumor cells to drug-induced apoptosis.76 It is not clear whether the inhibition of YY1 and its sensitizing activity are the direct role of YY1 or whether they are the consequence indirectly of the induction of RKIP and PTEN and their respective inhibition of the NF-κB and the PI3K-AKT anti-apoptotic pathways.
Beach et al.77 reported that RKIP transcription was under the control of the repressor transcription factor Snail in tumor cell lines. Snail is a member of the Snail superfamily of the zinc-finger transcription factors that plays a role in embryonic development and cell survival.78 Snail is transcriptionally regulated, in part, by NF-κB79 and by itself.80 To upregulate RKIP expression and sensitization of resistant tumor cells to drugs, as reported by Chatterjee et al.,43 we examined 2 proteasomal inhibitors, Bortezomib and NPI-0052 (marizomib), which inhibit NF-κB and downstream inhibit Snail and resulting in the derepression of RKIP transcription and expression of RKIP. Treatment of human prostate cancer cell lines with these inhibitors resulted in their sensitization to chemotherapeutic drugs-induced apoptosis. The sensitization was the result of the induction of RKIP. These findings demonstrated that the dysregulated NF-κB /Snail/YY1/RKIP loop is modified by the proteasome inhibitors and resulting in the inhibition of NF-κB, Snail and YY1 and in the induction of RKIP and resulting in the reversal of chemoresistance.45
In addition to the proteasome inhibitors, we have also reported that treatment of tumor cells with the NO donor DETANONOate sensitized tumor cells to chemotherapeutic drugs. The NO treatment resulted in the inhibition of NF-κB and downstream inhibition of Snail and YY1 along with the induction of RKIP, findings similar to those obtained above with the proteasome inhibitors.81
2. Reversal of immunoresistance through the RKIP disruption in the loop
The role of RKIP expression in target cells and their response to cytotoxic lymphocytes have been examined using the cytotoxic ligands FasL and TRAIL. The innate (such as NK cells) and adaptive (such as CTL) cytotoxic immune systems have evolved to fight infections and transformed cells like cancer. The human cytotoxic lymphocytes (such as NK and CTL) mediate their killing mechanisms by both apoptotic and necrotic mechanisms. The necrotic mechanism is mediated by the perforin/granzyme system in which perforin perforates holes on the membrane resulting in changes in the osmotic pressure and lysis of the cells. The apoptotic mechanisms consist primarily of the interaction of membrane expression of ligands on the lymphocytes (consisting primarily of TNF-α, FasL, and TRAIL) with their corresponding receptors (TNFR-1/2, Fas, DR4 and DR5), respectively, expressed on the surface of the target cells. Sensitive target cells in contact with the cytotoxic lymphocytes are triggered via ligand receptors-interactions which lead to activation of the apoptotic pathways (Type I and/or Type II) and resulting in cell death by apoptosis. However, often, a subset of infected/cancer cells is resistant to apoptotic stimuli due to a dysregulation of the apoptotic pathways in favor of anti-apoptotic mechanisms.82,83 Several mechanisms of immune resistance have been postulated and reviewed extensively elsewhere.69,84-87
Tumor cells are inherently resistant to TRAIL apoptosis or selected for resistance by immune selection.88 Resistance to apoptosis can be mediated by inhibitory proteins such as the Bcl-2 family of anti-apoptotic proteins89 cFLIP short,90 and inhibitors of apoptosis91 all of which are targets of constitutively activated NF-κB. Also, the poor expression or functional signaling by DR5 in tumor cells also results in failure to TRAIL apoptosis.92,93 Treatment of resistant tumor cells with subtoxic concentrations of chemotherapeutic dugs sensitized the cells to TRAIL apoptosis.44,94 We have also reported that drug sensitization to TRAIL was accompanied by upregulation of DR5.44
TRAIL binds to four receptors, namely, DR4, DR5, DcR1 and DcR2. Only DR4 and DR5 signal the cells for apoptosis.95,96 It was reported that TRAIL-resistant cancer cells can be sensitized to TRAIL apoptosis by overexpression of RKIP. We have reported that both Fas and DR5 are under the negative regulation of the transcription factor repressor YY1, a target of NF-κB.44,97 We hypothesized that RKIP-induced sensitization to TRAIL may be due to the inhibition of YY1 and upregulation of DR5 as a consequence of RKIP-mediated inhibition of NF-κB that regulates YY1. The direct effect of RKIP-induced sensitization to TRAIL apoptosis was corroborated in cells treated with siRNA RKIP and resulting in resistance to TRAIL apoptosis.76 Overexpression of RKIP, however, resulted in inhibition of YY1 and upregulation of DR5. RKIP induced sensitization to TRAIL was the result of activation of both type I and type II apoptotic pathways.
Both YY1 reporter and expression were inhibited by RKIP. Inhibition of NF-κB by chemical inhibitors mimics RKIP overexpression and sensitized the cells to TRAIL apoptosis. The findings observed with TRAIL can also be extrapolated to sensitization to FasL since Fas is negatively repressed by YY1. The findings above support the role of RKIP in the immunosurveillance of cancer.
IV. INDUCITON OF RKIP BY VARIOUS AGENTS AND REVERSAL OF RESISTANCE BY SENSITIZATION TO DRUGS
A. NO-Mediated Upregulation of RKIP
We have reported that treatment of drug-immune resistant tumor cells with the NO donor, DETANONOate, sensitized the tumor cells to both chemotherapeutic drugs and immune cytotoxic ligands-mediated apoptosis.81,97,98 Treatment with the NO donor disrupted the NF-κB/Snail/YY1/RKIP loop and S-nitrosylated both the NF-κB subunits (p50 and p65) and Snail, and resulted in the inhibition of NF-κB, Snail and YY1 and the induction of RKIP.69 We have reported on the role of NO-mediated sensitization to CDDP and TRAIL, as examples of chemotherapeutic drugs and immune ligands, respectively. Treatment with NO sensitized the resistant tumor cells to CDDP and TRAIL apoptosis. The combination treatment was synergistic. The inhibition by DETANONOate of NF-κB, Snail, and YY1 and induction of RKIP suggested strongly that each member of this loop is involved (directly and indirectly) in the sensitization through its participation in the loop.69
The direct role of NF-κB in sensitization to CDDP and TRAIL by DETANONOate was corroborated by the use of specific NF-κB inhibitors (e.g. DHMEQ) which mimicked DETANONOate in inhibiting NF-κB, Snail and YY1 and the induction of RKIP concomitantly with sensitization and apoptosis.
The direct role of Snail inhibition by DETANONOate and its role in sensitization was shown by the demonstration that DETANONOate nitrosylates Snail and thus, inhibits its transcriptional repressor activity on RKIP. In addition, using Snail silencing by siRNA resulted in upregulation of RKIP and chemoimmunosensitization of resistant tumor cells.
Treatment of tumor cells with DETANONOate inhibited YY1, a target gene of NF-κB as assessed by inhibition of its DNA-binding activity through direct S-nitrosylation.98-100 Since YY1 transcriptionally activates Snail, its inhibition results in the inhibition of Snail transcriptional expression and derepression of RKIP and upregulation of RKIP expression. In addition, treatment of cells with YY1 siRNA sensitized the cells to CDDP and TRAIL apoptosis.44
Overall, treatment with DETANONOate resulted in the inhibition of NF-κB, Snail and YY1 and the induction of RKIP. The direct role of RKIP in sensitization was shown by treatment of tumor cells with an RKIP expression vector. Cells overexpressing RKIP were sensitized to CDDP and TRAIL apoptosis. Overexpression of RKIP was correlated with the inhibition of Snail and YY1 as a result, in part, of RKIP-mediated inhibition of NF-κB and downstream its targets Snail and YY1.45
B. NPI-0052 (Marizomib)-mediated induction of RKIP and chemo-immunosensitization
We investigated the underlying molecular mechanism by which the proteasome inhibitor NPI0052 (marizomib) sensitized drug-immune resistant tumor cell lines to apoptosis. Our hypothesis was based on the findings that proteasome inhibitors inhibit NF-κB activity on one hand and our findings on the dysregulated NF-κB/Snail/YY1/RKIP loop in cancer cells. Hence, we postulated that treatment with NPI-0052 of resistant tumor cells will result in the inhibition of NF-κB and downstream targets Snail and YY1 along with the induction of RKIP. Such modifications will correlate with the reversal of chemo-immune resistance. This hypothesis was investigated and the findings validated the hypothesis.45 We demonstrated that treatment with NPI-0052 inhibited NF-κB, Snail, and YY1 and induced the expression of RKIP and resulted in the reversal of resistance to CDDP and TRAIL.45 This mechanism was an extension of previous reports demonstrating that several proteasome inhibitors sensitize tumor cells to apoptotic stimuli.101-104
Several lines of evidence support the role of NF-κB/Snail/ and YY1 inhibition and induction of RKIP by NPI-0052 in the sensitization of resistant tumor cells. The NF-κB inhibitor DHMEQ, when used for treatment of tumor cells, mimicked NPI 0052 in chemo immunosensitization and inhibited several anti-apoptotic gene products.105 Treatment with DHMEQ inhibited Snail and induced RKIP expression. The direct role of RKIP induction in chemoimmunosensitization was shown by both overexpression of RKIP that resulted in the reversal of resistance and, in contrast, silencing RKIP induced resistance. The mechanism by which NPI-0052 sensitized the cells to apoptosis resulted from activation to the type II mitochondrial pathway of apoptosis.45
C. Induction of RKIP by anti-CD20 antibody and chemosensitization
Rituximab (anti-CD20, chimeric monoclonal antibody) is being used for the treatment of BNHL as mono-therapy or in combination with chemotherapy.106 In addition to its activity by effector cells (NK-mediated ADCC) and complement mediated cytotoxicity, it has also been reported to signal the cells and also to sensitize drug-resistant tumor cells to apoptosis by chemotherapeutic drugs.107 Treatment of B-NHL cell lines with another anti-CD20 antibody, BM-ca, resulted in the inhibition of constitutively activated NF-κB and p38 MAPK pathways. This inhibition was accompanied by the induction of RKIP and inhibition of its repressor Snail. In addition, there was inhibition downstream of several anti-apoptotic gene products such as Mcl-1 and the induction of a pro apoptotic gene product such as Bax. These various gene modifications by anti-CD20 antibodies resulted in the sensitization to drug-induced apoptosis.108 These findings are in agreement with another report by Daniel et al.109
D. Induction of RKIP expression by photodynamic therapy
Photodynamic therapy (PDT) has been applied as a therapeutic approach to treat some cancers. It consists of the interplay of three components, namely, the photosensitizer, light, and oxygen. Combined, they produce ROS and/or a singlet oxygen (1O2). Cell death occurs by multiple mechanisms, such as, apoptosis, necrosis and autophagy.110,111 It has been reported that the level of NO in the tumor environment influences the response to PDT.112,113 PDT induces iNOS in tumor cells in vivo and also activates inflammatory cells to produce NO.114 As a result of the above findings and the role of NO in PDT, Rapozzi et al.115 hypothesized that the efficacy of PDT inhibitory activity may be enhanced by the addition of an exogenous NO donor such as DETANONOate.116 The findings by Rapozzi et al.,115 using the B78-H1 amelanotic melanoma cells both in vitro and in vivo, demonstrated the effectiveness of the treatment with both PDT and DETANONOate. Treatment with the photosensitizer Pba induced iNOS and inhibited the antiapoptotic effects by interfering with the dysregulated NF-κB/Snail/RKIP loop, namely, inhibition of NF-κB and Snail and induction of RKIP. Low doses of NO resulted in opposing effects by low dose PDT and activated NF-κB and Snail and inhibited RKIP expression. The high level of NO resulted in the S-nitrosylation of p65117 and induction of RKIP.43 The findings by Rapozzi et al.115 demonstrated, for the first time, the in vivo antitumor activity of PDT combined with DETANONOate and the implied role of the induction of RKIP in the reversal of resistance.
V. ROLE OF RKIP IN THE INHIBITION OF DRUG-RESISTANT CELLS WITH THE EMT PHENOTYPE
The evaluation of tumor specimens and in vitro studies revealed the resistance to EGFR inhibitors in lung cancer and revealed that EMT may underlie the EGFR pathway-independent mechanism of resistance.118-120 A link between EMT and the acquisition of cancer stemness and the hallmark of CSC and drug resistance have been reported.121
In several examined solid tumors, metastasis has been shown to be the result of cells acquiring the EMT phenotype. EMT results from a number of constitutively activated survival pathways such as the NF-κB112-124 and the inhibitors of NF-κB suppress EMT.125-127 The NF-κB’s target gene product, Snail, is a metastasis inducer via its repressive activity on E-cadherin, a metastatic suppressor gene product.127,128 Snail, in turn, represses a metastatic suppressor RKIP.77
We have reported that RKIP induction inhibits the EMT phenotype. Treating EMT positive tumor cells with siRNA Snail inhibited both Snail and NF-κB43 and induced RKIP concomitantly with inhibition of EMT. Similar findings were observed following treatment with the proteasome inhibitor NPI-0052 or the NO donor DETANONOate.129 The direct role of RKIP-induced expression in the inhibition of EMT was corroborated by several lines of evidence, namely, overexpression of RKIP, silencing Snail, and inhibition of NF-κB. In contrast, the induction of EMT was demonstrated by the inhibition of RKIP or the overexpression of Snail. In a reported study, it was demonstrated the mechanism by which RKIP regulates EMT through a signaling cascade involving the MAPK, Myc, lin28, let7, and downstream let7 targets.130
We have reported that treatment of EMT positive tumor cells with the proteasome inhibitor NPI-0052 or bortezomib resulted in the inhibition of NF-κB activity and downstream the metastatic inducer Snail concurrently with the induction of RKIP, originally repressed by Snail.131
Since proteasome inhibitors have been reported to inhibit NF-κB132 and NF-κB is involved in EMT, we tested the effect of NPI-0052 on the EMT phenotype in cancer cell lines. Evidence was presented that demonstrated the treatment of the tumor cells that NPI-0052 inhibited NF-κB and downstream Snail concomitantly with induction of RKIP and E-cadherin. The suppression of Snail expression by NPI-0052 resulted in the downregulation of mesenchymal markers and the induction of epithelial markers. Treatment with NPI-0052 resulted in the upregulation of RKIP mRNA and protein levels. Also, overexpression of RKIP resulted in the inhibition of EMT-related gene products such as vimentin and fibronectin and upregulation of epithelial gene products related to suppression of metastasis including E-cadherin and cytokeratin 18.131 These findings supported previous findings by Fu et al.,31 in studies done both in vitro and in vivo in mice bearing human tumor xenografts. Hence, RKIP overexpression inhibits the drug resistance of cells of the EMT phenotype.
VI. ROLE OF RKIP EXPRESSION IN DRUG RESISTANT STEM CELLS
Evidence has been reported that cancer initiated cells (CICs) also cancer stem cells (CSCs) are responsible, in part, for tumor relapse and unresponsiveness to treatments. They also contribute to tumor dormancy, metastasis, and relapse.133,134 The ability of CSCs to exhibit highly tumorigenic properties correlated with a high degree of drug resistance.121,135 The identification of CSCs in tumors relied primarily on phenotypic markers for certain tumors.136,137 However, none of these have resulted as targets for therapeutic intervention to reverse drug resistance. A recent report by Sequin et al.138 have identified α3β3 integrin, a protein associated with poor outcome and high incidence of metastasis, in a variety of epithelial tumors139 and its expression reported in leukemic cancer stem cells.140 Of note, α3β3 integrin can trigger anchorage-independent cell survival and metastasis in the absence of ligand binding.139 The new finding by Sequin et al.138 demonstrated that αvβ3 expression and its upregulation on the surface of various epithelial tumor cells that were exposed to receptor tyrosine kinases (RTK) inhibitors were also associated with drug resistance. In that study, the role of CD61 (integrin ß3) was found to be both necessary and sufficient to promote tumor stem cell properties and resistance to RTK inhibitors. They demonstrated that integrin avß3 acts in a complex with KRAS and that KRAS is necessary for resistance. By using a combination of bortezomib (inhibitor of NF-κB) and RTK inhibitors it resulted in the reversal of stemness and drug resistance. Based on the above findings on the induction of RKIP by proteasome inhibitors, these studies suggest that these inhibitors may induce RKIP expression and play a role in the reversal of resistance in CSCs.
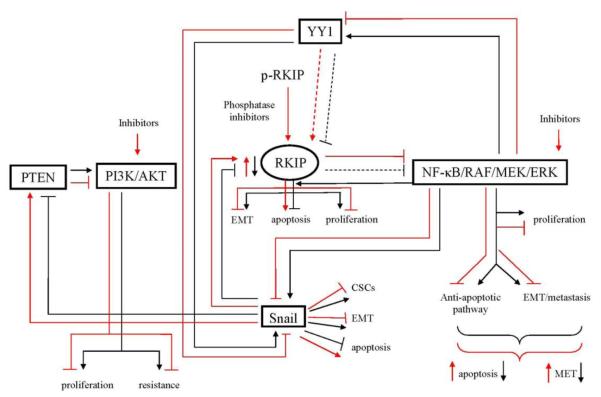
VII. CONCLUSIONS AND FUTURE DIRECTIONS (See scheme in Figure 1)
Figure 1.
Schematic diagram demonstrating the role of RKIP in the regulation of tumor cell drug/immune resistance (black lines). Tumor cells exhibit a constitutively hyperactivated NF-κB pathway which regulates, in large part, the survival, growth, metastasis and resistance of tumor cells. Among many target genes that NF-κB regulates, there exists a dysregulated NF-κB/Snail/YY1/PTEN/RKIP loop that is responsible, in part, for NF-κB-mediated above tumor manifestations. The hyperactivated NF-κB pathway regulates cell proliferation, anti-apoptotic pathways, EMT, metastasis and resistance. These events are the results of NF-κB activation of (1) the metastasis inducer and resistance transcription factor Snail, which regulates CSCs, EMT and anti-apoptotic pathways and (2) the drug/immune resistant transcription factor YY1, which also regulates the transcription of Snail. The overexpression of Snail results in the repression of the metastasis suppressor/drug sensitizers, RKIP and PTEN. The repression of RKIP minimally represses the NF-κB pathway directly and indirectly by the repression of PTEN, which does not inhibit the PI3K/AKT pathway that regulates NF-κB. Overall, the tumor cells exhibit a dysregulated NF-κB /Snail/YY1/PTEN/RKIP loop with the overexpression of NF-κB, Snail and YY1 and under expression of RKIP and PTEN and resulting in tumor cell survival, metastasis and drug resistance (red lines). Inhibition of the dysregulated loop products, on each of the overexpressed NF-κB, YY1, Snail, PI3K/AKT, results in the derepression of RKIP and PTEN and resulting in the inhibition of cell growth, survival, EMT, metastasis, and activation of proapoptotic pathways resulting in sensitization to drug-induced apoptosis. There are many classes of inhibitors that may be used and some examples are illustrated in the scheme, for instance, the expression of the active form of RKIP in its phosphorylated form can be rendered active by the use of phosphatase inhibitors. Inhibitors of NF-κB will have both direct and indirect effects on NF-κB target genes such as Snail and YY1 and derepression of RKIP and PTEN. The direct inhibition of Snail and YY1 will have similar effects as the NF-κB inhibitors. Likewise, inhibitors of the PI3K/AKT pathway will have direct and indirect effects on the inhibition of the NF-κB pathway and its targets Snail and YY1.
The development of drugs to target drug resistance is based on in vitro studies that result in answering fundamental questions such as 1) Is the candidate effector molecule required to maintain a response phenotype 2) Is the candidate effector sufficient to confer resistance? 3) Does the candidate effector mediate the downstream resistant signaling pathway and 4) Is the candidate effector dysregulated in drug-resistance in patient-derived specimens.4
Currently, numerous studies have been developed to generate targeted drugs that inhibit cell proliferation and induce cell death. These include DNA replication, the mitotic apparatus, protein chaperones (ex. HSPS), the proteasome, the chromatin, etc. (Reviewed by Dobbles and Moll).5 The expression level of RKIP may identify tumors requiring a specific drug for its activation to reverse resistance. It may also serve as an important prognostic biomarker. The combination of RKIP-inducing agents with other drugs that target different pathways may result in a significant synergy. Clearly, the resistant mechanisms are less likely to lead to a dominant resistant clone when two drugs are targeted against different cell pathways.
In many tumors, genomic alterations lead to dysregulation of signaling pathways. For example, resistance to MEK and Raf inhibitors have been studied. Activating mutations of MEK and NRAS are observed in tumors that progress in the presence of vemurafenib treatment in melanoma and also confers resistance to Raf inhibitors in vitro.141 MEK signaling becomes uncoupled from the inhibitor B2AF oncoprotein. Erlotinib resistance to lung cancer showed dysregulated NF-κB signaling associated with patient survival.142 It is important for any resistant mechanism that its information and validation must be relevant in cancer patients. The examination of tumor specimens from patients who relapsed upon exposure to therapy are valuable to establish correlations with the observed in vitro and in vivo pre-clinical data.
The demonstration that the metastasis suppressor and drug sensitizer RKIP is involved in the dysregulation of the NF-κB/Snail/YY1/RKIP loop is reminiscent of another loop, namely, KRAS/RalB-NF-κB, that is induced by a3ß3 expression. Seguin et al.138 have reported that the expression of a3ß3 and the resulting KRAS/RalB-NF-κB pathway were both necessary and sufficient for tumor metastasis, anchorage independence, self-renewal and resistance to erlotinib. Disruption of ß3 downstream signaling leads to a reversal of drug resistance. The implication of NF-κB in NF-κB/Snail/YY1/RKIP and the K-Ras/RalB-NF-κB loops suggests that these two loops may cross-talk and that RKIP may be involved in both loops. It would be interesting to demonstrate that RKIP is involved in the regulation of a3ß3-mediated metastasis and resistance. Hence, it is reasonable to assume that RKIP may be a universal gene product that is involved in the regulation of cell survival, cell growth, metastasis and resistance.
ACKNOWLEDGEMENTS
The author acknowledges the laboratory personels at UCLA and collaborators outside of UCLA whose reported research investigations in the RKIP field have been used in the preparation of this review. These investigators are doctors: Baritaki S (UCLA), Berenson J (Institute for Myeloma and Bone Marrow Cancer Research, Hollywood, CA), Chapman A (UCLA), Chatterjee D (Brown University), Huerta-Yepez S (UCLA and Hospital Infantil de Mexico, Mexico City), Jazirehi AR (UCLA), Katsman A (UCLA), Palladino M (Nereus Pharmaceuticals, San Diego), Rapozzi V (University of Udine, Italy), Spandidos D (University of Crete, Greece), Vega MI (UCLA and Hospital de Infectologia CMN La Raza, Mexico City), Wu K (UCLA), Yeung KC (Medical College of Ohio).
The author also acknowledges the research supports that were funded for the reported RKIP publications used in the review and include: the Jonsson Comprehensive Cancer Center at UCLA; the University of California Gene Medicine Program; the UCLA AIDS Institute; the UCLA Fogarty International Center Fellowship (D43. TW0013-14); UC-MEXUS-CONACYT; Bodasaki Foundation (Greece); NCI-RO1-CA133479; NCICA107023-02S1; NCICA05715213S1; NIHR21149938.
The assistance of Melissa Cao, Daphne Liang, and Kathy Nguyen is acknowledged in the preparation of this manuscript. Philip Postovoit is also acknowledged for his assistance with the computer software.
ABBREVIATIONS
- CPT
carboplatin
- CRC
colorectal cancer
- CSC
cancer stem cell
- CTL
cytotoxic T lymphocyte
- DETANONOate
diethylenetriamine NONOate
- DcR1
decoy receptor 1
- DcR2
decoy receptor 2
- DR4
death receptor 4
- DR5
death receptor 5
- EGFR
epidermal growth factor receptor
- EMT
epithelial mesenchymal transition
- EZH2
enhancer of zeste homolog 2
- FasL
Fas ligand
- GPCR
G-protein coupled receptor
- HSP
heat shock protein
- KEAP-1
Kelch-like ECH-associated protein 1
- MDR
multiple drug resistance
- NK
natural killer
- NPI-0052
proteasome inhibitor
- NRF2
NF-E2 related nuclear factor 2
- OXP
oxiplatin
- PEBP
phosphatidyl ethylenolamine binding protein
- PDT
photodynamic therapy
- Rituximab
chimeric antiCD20 mAb
- RKIP
Raf kinase inhibitor protein
- YY1
Yin-Yang 1
REFERENCES
- 1.Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and nononcogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- 4.Garraway LA, Jänne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2:214–26. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- 5.Dobbelstein M, Moll U. Targeting tumour-supportive cellular machineries in anticancer drug development. Nat Rev Drug Discov. 2014;13:179–96. doi: 10.1038/nrd4201. [DOI] [PubMed] [Google Scholar]
- 6.Brockman RW. Mechanisms of resistance to anticancer agents. Adv Cancer Res. 1963;7:129–234. doi: 10.1016/s0065-230x(08)60983-5. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Garraway LA, Chabner B. MDR1 inhibition: less resistance or less relevance? Eur J Cancer. 2002;38:2337–40. doi: 10.1016/s0959-8049(02)00490-2. [DOI] [PubMed] [Google Scholar]
- 10.Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–70. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nature Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 15.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 16.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K, Nakazawa S, Hirai H, Ozawa K, Inaba T. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Mol Cell Biol. 2004;24:6172–6183. doi: 10.1128/MCB.24.14.6172-6183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, Tenen DG, Kobayashi S. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–1679. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–7527. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- 22.Sierra JR, Cepero V, Giordano S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol Cancer. 2010;9:75. doi: 10.1186/1476-4598-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DM, 3rd, Simeonov A. Small molecule inhibitors of DNA repair nuclease activities of APE1. Cell Mol Life Sci. 2010;67:3621–31. doi: 10.1007/s00018-010-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villamor N, Montserrat E, Colomer D. Mechanism of action and resistance to monoclonal antibody therapy. Seminars Oncol. 2003;30:424–433. doi: 10.1016/s0093-7754(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 25.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nature Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res. 2008;18:452–7. doi: 10.1038/cr.2008.43. [DOI] [PubMed] [Google Scholar]
- 27.Yeung K, Seitz T, Li S, Janosch P, McFerrah B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. ppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 28.Yeung KC, Rose DW, Dhillon AS, Yaros D, Gastafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and TAK1 and inhibits NF-κB activation. Mol Cell Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;78:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 31.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–89. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 32.Li HZ, Wang Y, Gao Y, Shao J, Zhao XL, Deng WM, Liu YX, Yang J, Yao Z. Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res. 2008;6:917–928. doi: 10.1158/1541-7786.MCR-08-0093. [DOI] [PubMed] [Google Scholar]
- 33.Martinho O, Pinto F, Granja S, Miranda-Gonçalves V, Moreira MA, Ribeiro LF, di Loreto C, Rosner MR, Longatto-Filho A, Reis RM. RKIP inhibition in cervical cancer is associated with higher tumor aggressive behavior and resistance to cisplatin therapy. PLoS One. 2013;8:e59104. doi: 10.1371/journal.pone.0059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, García JJ, Kolch W. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res. 2005;11:7392–7397. doi: 10.1158/1078-0432.CCR-05-0283. [DOI] [PubMed] [Google Scholar]
- 35.Al-Mulla F, Hagan S, Behbehani AI, Bitar MS, George SS, Going JJ, Garcia JJ, Scott L, Fyfe N, Murray GI, Kolch W. Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol. 2006;24:5672–5679. doi: 10.1200/JCO.2006.07.5499. [DOI] [PubMed] [Google Scholar]
- 36.Minoo P, Zlobec I, Baker K, Tornillo L, Terracciano L, Jass JR, Lugli A. Loss of raf-1 kinase inhibitor protein expression is associated with tumor progression and metastasis in colorectal cancer. Am J Clin Pathol. 2007;127:820–827. doi: 10.1309/5D7MM22DAVGDT1R8. [DOI] [PubMed] [Google Scholar]
- 37.Maresch J, Birner P, Zakharinov M, Toumangelova-Uzeir K, Natchev S, Guentchev M. Additive effect on survival of Raf kinase inhibitor protein and signal transducer and activator of transcription 3 in high-grade glioma. Cancer. 2011;117:2499–504. doi: 10.1002/cncr.25799. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Kim GY, Lim SJ, Kim YW. Loss of Raf-1 kinase inhibitory protein in pancreatic ductal adenocarcinoma. Pathology. 2010;42:655–60. doi: 10.3109/00313025.2010.522172. [DOI] [PubMed] [Google Scholar]
- 39.Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX, Cai MY, Ju MJ, Zhou J, Zhang BH, Fan J. PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J Hepatol. 2010;53:872–9. doi: 10.1016/j.jhep.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Chatterjee D, Sabo E, Tavares R, Resnick MB. Inverse association between Ras Kinase Inhibitory Protein and signal transducer and activators of transcription 3 expression in gastric adenocarcinoma patients: implications for clinical outcome. Clin Cancer Res. 2008;14:2994–3001. doi: 10.1158/1078-0432.CCR-07-4496. [DOI] [PubMed] [Google Scholar]
- 41.Zlobec I, Baker K, Terracciano L, Peter S, Degen L, Beglinger C, Lugli A. Two-marker protein profile predicts poor prognosis in patients with early rectal cancer. Br J Cancer. 2008;99:1712–7. doi: 10.1038/sj.bjc.6604729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66:248–256. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, Braastad C, Sun Y, Mukhopadhyay A, Aggarwal BB, Darnowski J, Pantazis P, Wyche J, Fu Z, Kitagwa Y, Keller ET, Sedivy JM, Yeung KC. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem. 2004 Apr 23;279:17515–23. doi: 10.1074/jbc.M313816200. [DOI] [PubMed] [Google Scholar]
- 44.Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007;6:1387–1399. doi: 10.1158/1535-7163.MCT-06-0521. [DOI] [PubMed] [Google Scholar]
- 45.Baritaki S, Yeung K, Palladino M, Berenson J, Bonavida B. Pivotal roles of snail inhibition and RKIP induction by the proteasome inhibitor NPI-0052 in tumor cell chemoimmunosensitization. Cancer Res. 2009;69:8376–8385. doi: 10.1158/0008-5472.CAN-09-1069. [DOI] [PubMed] [Google Scholar]
- 46.Wu K, Bonavida B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol. 2009;29:241–254. doi: 10.1615/critrevimmunol.v29.i3.40. [DOI] [PubMed] [Google Scholar]
- 47.Woods Ignatoski KM, Grewal NK, Markwart SM, Vellaichamy A, Chinnaiyan AM, Yeung K, Ray ME, Keller ET. Loss of Raf kinase inhibitory protein induces radioresistance in prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:153–160. doi: 10.1016/j.ijrobp.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Mulla F, Bitar MS, Al-Maghrebi M, Behbehani AI, Al-Ali W, Rath O, Doyle B, Tan KY, Pitt A, Kolch W. Raf kinase inhibitor protein RKIP enhances signaling by glycogen synthase kinase-3beta. Cancer Res. 2011;71:1334–1343. doi: 10.1158/0008-5472.CAN-10-3102. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 50.Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR, Freeman ML. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–818. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 51.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Mulla F, Bitar MS, Feng J, Park S, Yeung KC. A new model for raf kinase inhibitory protein induced chemotherapeutic resistance. PLoS One. 2012;7:e29532. doi: 10.1371/journal.pone.0029532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biewenga P, Buist MR, Moerland PD, Ver Loren van Themaat E, van Kampen AH, ten Kate FJ, Baas F. Gene expression in early stage cervical cancer. Gynecologic Oncology. 2008;108:520–6. doi: 10.1016/j.ygyno.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Hu CJ, Zhou L, Zhang J, Huang C, Zhang GM. Immunohistochemical detection of Raf kinase inhibitor protein in normal cervical tissue and cervical cancer tissue. J Int Med Res. 2011;39:229–237. doi: 10.1177/147323001103900125. [DOI] [PubMed] [Google Scholar]
- 55.Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 56.Plasencia C, Rooney PH, Taron M, Martinez-Balibrea E, McLeod HL, Abad A. Chromosomal imbalance maps of human 5FU-resistant colorectal cancer cell lines: implications in the analysis of 5FU-acquired resistance mechanisms. Int J Oncol. 2003;22:945–953. doi: 10.3892/ijo.22.5.945. [DOI] [PubMed] [Google Scholar]
- 57.Doyle B, Hagan S, Al-Mulla F, Scott L, Harden S, Paul J, Mulcahy H, Murray GI, Sheahan K, O’Sullivan J, Kolch W. Raf kinase inhibitor protein expression combined with peritoneal involvement and lymphovascular invasion predicts prognosis in Dukes’ B colorectal cancer patients. Histopathology. 2013;62:505–510. doi: 10.1111/his.12014. [DOI] [PubMed] [Google Scholar]
- 58.Koelzer VH, Karamitopoulou E, Dawson H, Kondi-Pafiti A, Zlobec I, Lugli A. Geographic analysis of RKIP expression and its clinical relevance in colorectal cancer. Br J Cancer. 2013;108:2088–2096. doi: 10.1038/bjc.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cross-Knorr S, Lu S, Perez K, Guevara S, Brilliant K, Pisano C, Quesenberry PJ, Resnick MB, Chatterjee D. RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer. 2013;13:463. doi: 10.1186/1471-2407-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yousuf S, Duan M, Moen EL, Cross-Knorr S, Brilliant K, Bonavida B, LaValle T, Yeung KC, Al-Mulla F, Chin E, Chatterjee D. Raf kinase inhibitor protein (RKIP) blocks signal transducer and activator of transcription 3 (STAT3) activation in breast and prostate cancer. PLoS One. 2014;9:e92478. doi: 10.1371/journal.pone.0092478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 62.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, Kim JH, Brenner JC, Jing X, Cao X, Wang R, Li Y, Dahiya A, Wang L, Pandhi M, Lonigro RJ, Wu YM, Tomlins SA, Palanisamy N, Qin Z, Yu J, Maher CA, Varambally S, Chinnaiyan AM. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–99. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE, Shinomura Y, Imai K, Esteller M. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene. 2012;31:1988–94. doi: 10.1038/onc.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, Kleer CG, Varambally S, Chinnaiyan AM. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herranz N, Pasini D, Díaz VM, Francí C, Gutierrez A, Dave N, Escrivà M, Hernandez-Muñoz I, Di Croce L, Helin K, García de Herreros A, Peiró S. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–81. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, Liu TH, Bian XW, Guan XY, Lin MC, Zeng MS, Zeng YX, Kung HF, Xie D. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit Ecadherin. Oncogene. 2012;31:583–94. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 68.Ren G, Baritaki S, Marathe H, Feng J, Park S, Beach S, Bazeley PS, Beshir AB, Fenteany G, Mehra R, Daignault S, Al-Mulla F, Keller E, Bonavida B, de la Serna I, Yeung KC. Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res. 2012;72:3091–104. doi: 10.1158/0008-5472.CAN-11-3546. [DOI] [PubMed] [Google Scholar]
- 69.Bonavida B, Baritaki S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry. Crit Rev Oncog. 2011;16:211–26. doi: 10.1615/critrevoncog.v16.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 70.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 71.Palmer MB, Majumder P, Cooper JC, Yoon H, Wade PA, Boss JM. Yin yang 1 regulates the expression of snail through a distal enhancer. Mol Cancer Res. 2009;7:221–229. doi: 10.1158/1541-7786.MCR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barberà MJ, Puig I, Domínguez D, Julien-Grille S, Guaita-Esteruelas S, Peiró S, Baulida J, Francí C, Dedhar S, Larue L, García de Herreros A. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 73.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of BRAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 75.Petrella BL, Brinckerhoff CE. PTEN suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null renal-cell carcinoma. Cancer Biol Ther. 2009;8:1389–1401. doi: 10.4161/cbt.8.14.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baritaki S, Katsman A, Chatterjee D, Yeung KC, Spandidos DA, Bonavida B. Regulation of tumor cell sensitivity to TRAIL-induced apoptosis by the metastatic suppressor Raf kinase inhibitor protein via Yin Yang 1 inhibition and death receptor 5 up-regulation. J Immunol. 2007;179:5441–5453. doi: 10.4049/jimmunol.179.8.5441. [DOI] [PubMed] [Google Scholar]
- 77.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–2248. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 79.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 80.Peiro S, Escriva M, Puig I, Barberà MJ, Dave N, Herranz N, Larriba MJ, Takkunen M, Francí C, Muñoz A, Virtanen I, Baulida J, García de Herreros A. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–84. doi: 10.1093/nar/gkl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huerta-Yepez S, Baritaki S, Baay-Guzman G, Hernandez-Luna MA, Hernandez-Cueto A, Vega MI, Bonavida B. Contribution of either YY1 or BclXL-induced inhibition by the NO-donor DETANONOate in the reversal of drug resistance, both in vitro and in vivo. YY1 and BclXL are overexpressed in prostate cancer. Nitric Oxide. 2013;29:17–24. doi: 10.1016/j.niox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Jager MJ, Hurks HM, Levitskaya J, Kiessling R. HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol. 2002;63:444–51. doi: 10.1016/s0198-8859(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 83.Frost PJ, Butterfield LH, Dissette VB, Economou JS, Bonavida B. Immunosensitization of melanoma tumor cells to non-MHC Fas-mediated killing by MART-1-specific CTL cultures. J Immunol. 2001;166:3564–73. doi: 10.4049/jimmunol.166.5.3564. [DOI] [PubMed] [Google Scholar]
- 84.Classen CF, Falk CS, Friesen C, Fulda S, Herr I, Debatin KM. Natural killer resistance of a drug-resistant leukemia cell line, mediated by up-regulation of HLA class I expression. Haematologica. 2003;88:509–21. [PubMed] [Google Scholar]
- 85.Huang Y, Obholzer N, Fayad R, Qiao L. Turning on/off tumor-specific CTL response during progressive tumor growth. J Immunol. 2005;175:3110–6. doi: 10.4049/jimmunol.175.5.3110. [DOI] [PubMed] [Google Scholar]
- 86.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 87.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 89.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 90.Tschopp J, Irmler M, Thome M. Inhibition of fas death signals by FLIPS. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 91.Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 92.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 93.Soderstrom TS, Poukkula M, Holmstrom TH, Heiskanen KM, Eriksson JE. Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol. 2002;169:2851–2860. doi: 10.4049/jimmunol.169.6.2851. [DOI] [PubMed] [Google Scholar]
- 94.Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–86. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- 95.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. 2000. [DOI] [PubMed] [Google Scholar]
- 96.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–3. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 97.Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor Yin-Yang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J Immunol. 2001;167:75–81. doi: 10.4049/jimmunol.167.1.75. [DOI] [PubMed] [Google Scholar]
- 98.Huerta-Yepez S, Vega M, Escoto-Chavez SE, Murdock B, Sakai T, Baritaki S, Bonavida B. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide. 2009;39:52. doi: 10.1016/j.niox.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Vega MI, Jazirehi AR, Huerta-Yepez S, Bonavida B. Rituximab-induced inhibition of YY1 and Bcl-xL expression in Ramos non-Hodgkin’s lymphoma cell line via inhibition of NF-kappa B activity: role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectively. J Immunol. 2005;175:2174–2183. doi: 10.4049/jimmunol.175.4.2174. [DOI] [PubMed] [Google Scholar]
- 100.Hongo F, Garban H, Huerta-Yepez S, Vega M, Jazirehi AR, Mizutani Y, Miki T, Bonavida B. Inhibition of the transcription factor Yin Yang 1 activity by S-nitrosation. Biochem Biophys Res Commun. 2005;336:692–701. doi: 10.1016/j.bbrc.2005.08.150. [DOI] [PubMed] [Google Scholar]
- 101.Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–64. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Sterz J, von Metzler I, Hahne JC, Lamottke B, Rademacher J, Heider U, Terpos E, Sezer O. The potential of proteasome inhibitors in cancer therapy. Expert Opin Investig Drugs. 2008;17:879–95. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- 104.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Schlossman R, Munshi NC, Hideshima T, Anderson KC. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 105.Sevilla L, Zaldumbide A, Pognonec P, Boulukos KE. Transcriptional regulation of the bcl-x gene encoding the anti-apoptotic Bcl-xL protein by Ets, Rel/NFkappaB, STAT and AP1 transcription factor families. Histol Histopathol. 2001;16:595–601. doi: 10.14670/HH-16.595. [DOI] [PubMed] [Google Scholar]
- 106.Coiffier B. Monoclonal antibodies in the management of newly diagnosed, aggressive B-cell lymphoma. Curr Hematol Rep. 2003;2:23–29. [PubMed] [Google Scholar]
- 107.Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24:2121–43. doi: 10.1038/sj.onc.1208349. [DOI] [PubMed] [Google Scholar]
- 108.Vega MI, Huerta-Yepez S, Martinez-Paniagua M, Martinez-Miguel B, Hernandez-Pando R, González-Bonilla CR, Chinn P, Hanna N, Hariharan K, Jazirehi AR, Bonavida B. Rituximab-mediated cell signaling and chemo/immuno-sensitization of drug-resistant BNHL is independent of its Fc functions. Clin Cancer Res. 2009;15:6582–94. doi: 10.1158/1078-0432.CCR-09-1234. [DOI] [PubMed] [Google Scholar]
- 109.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 110.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;4:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford MR, Chaplin DJ. Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy. Br J Cancer. 2000;82:1835–1843. doi: 10.1054/bjoc.2000.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reeves KJ, Reed MW, Brown NJ. Is nitric oxide important in photodynamic therapy?, J Photochem. Photobiol. B. 2009;95:141–147. doi: 10.1016/j.jphotobiol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 114.Gupta S, Ahmad N, Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58:1785–8. [PubMed] [Google Scholar]
- 115.Rapozzi V, Pietra ED, Zorzet S, Zacchigna M, Bonavida B, Xodo LE. Nitric Oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide. 2013;30:26–35. doi: 10.1016/j.niox.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Keefer LK, Nims RW, Davies KM, Wink DA. NONOates (1-substituted diazen-1-ium-1,2-diolates) as nirtric oxide donors: convenient nitric oxide dosage form. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 117.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NFjB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 118.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–61. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 120.Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, Kenner L, Sordella R. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107:15535–40. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–44. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 123.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu WS, Wu JR, Hu CT. Signal cross talks for sustained MAPK activation and cell migration: the potential role of reactive oxygen species. Cancer Metastasis Rev. 2008;27:303–14. doi: 10.1007/s10555-008-9112-4. [DOI] [PubMed] [Google Scholar]
- 125.Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L, Chen W, Li X. A novel anticancer effect of butein: inhibition of invasion through the ERK1/2 and NF-kappa B signaling pathways in bladder cancer cells. FEBS Lett. 2008;582:1821–8. doi: 10.1016/j.febslet.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 127.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 128.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 129.Baritaki S, Huerta-Yepez S, Sahakyan A, Karagiannides I, Bakirtzi K, Jazirehi A, Bonavida B. Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: inhibition of the metastasis-inducer Snail and induction of the metastasis-suppressor RKIP. Cell Cycle. 2010;9:4931–40. doi: 10.4161/cc.9.24.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostatic cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 2009;28:3573–85. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- 132.Miller CP, Ban K, Dujka ME, McConkey DJ, Munsell M, Palladino M, Chandra J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–77. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patel P, Chen EI. Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol. 2012;3:125. doi: 10.3389/fendo.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumour growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 135.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 136.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, Yebra M, Mielgo A, Lowy AM, Husain H, Cascone T, Diao L, Wang J, Wistuba II, Heymach JV, Lippman SM, Desgrosellier JS, Anand S, Weis SM, Cheresh DA. An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–68. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Järås M, Puram RV, Puissant A, Callahan KP, Ashton J, McConkey ME, Poveromo LP, Cowley GS, Kharas MG, Labelle M, Shterental S, Fujisaki J, Silberstein L, Alexe G, Al-Hajj MA, Shelton CA, Armstrong SA, Root DE, Scadden DT, Hynes RO, Mukherjee S, Stegmaier K, Jordan CT, Ebert BL. In vivo RNAi screening identifies a leukemia-specific dependence on Integrin Beta 3 signaling. Cancer Cell. 2013;24:45–58. doi: 10.1016/j.ccr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bivona TG, Hieronymus H, Parker J, Chang K, Taron M, Rosell R, Moonsamy P, Dahlman K, Miller VA, Costa C, Hannon G, Sawyers CL. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–6. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]