Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease, with a median survival as short as 3 years from the time of diagnosis and no pharmacological therapies yet approved by the U.S. Food and Drug Administration. To address the great unmet need for effective IPF therapy, a number of new drugs have recently been, or are now being, evaluated in clinical trials. The rationales for most of these therapeutic candidates are based on the current paradigm of IPF pathogenesis, in which recurrent injury to the alveolar epithelium is believed to drive aberrant wound healing responses, resulting in fibrosis rather than repair. Here we discuss drugs in recently completed or currently ongoing phase II and III IPF clinical trials in the context of their putative mechanisms of action and the aberrant repair processes they are believed to target: innate immune activation and polarization, fibroblast accumulation and myofibroblast differentiation, or extracellular matrix deposition and stiffening. Placed in this context, the positive results of recently completed trials of pirfenidone and nintedanib, and results that will come from ongoing trials of other agents, should provide valuable insights into the still-enigmatic pathogenesis of this disease, in addition to providing benefits to patients with IPF.
Keywords: idiopathic pulmonary fibrosis, pathogenesis, investigational treatments
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease in which normal lung parenchyma is progressively replaced with fibrotic tissue, leading to dyspnea, cough, impaired lung function, and death (1). The annual U.S. incidence has been estimated to be between 6.8 and 16.3 cases per 100,000 persons (2). IPF has a poor prognosis, with a median survival of approximately 3 years from the time of diagnosis (1), and great associated morbidity, with wide-ranging negative effects on quality of life (3). Despite promising preclinical data for a variety of therapeutic approaches, clinical trials conducted over more than a decade yielded negative or inconsistent results. More recently, evolving understanding of the pathogenesis of IPF has led to the identification of a new set of potential therapies, two of which, pirfenidone and nintedanib, have recently been shown to be effective at slowing disease progression (4, 5) and have New Drug Applications under review at the U.S. Food and Drug Administration. Here we review drugs evaluated as treatments for IPF in recently completed or ongoing phase II or III clinical trials, discussing their proposed mechanisms of action in the context of the current paradigm of IPF pathogenesis. Multiple other therapies targeting the repetitive lung injury or aberrant repair processes believed to drive IPF are in earlier stages of development, but this review focuses on those therapies that have already progressed to phase II or III trials.
Current Paradigm of IPF Pathogenesis
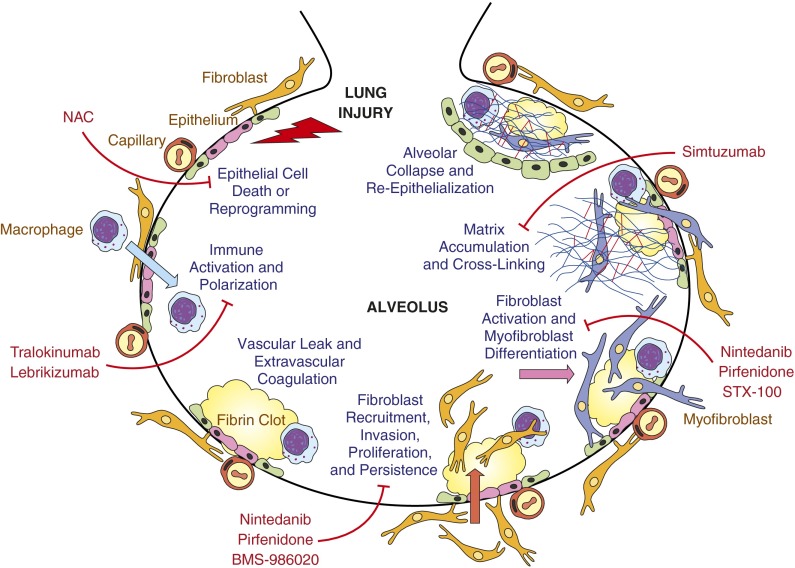
According to the prevailing paradigm of IPF, fibrosis develops as a consequence of aberrant wound healing responses to repetitive lung injury (6). This injury appears to primarily target alveolar epithelial cells (AECs), and their death triggers wound healing responses including vascular leak and extravascular coagulation; innate immune activation; fibroblast recruitment, proliferation, and activation; and extracellular matrix synthesis and cross-linking (Figure 1). When these responses are appropriate in duration and magnitude, reepithelialization, fibroblast apoptosis, and matrix reabsorption restore normal lung structure and function. However, when these injury responses are deregulated or overexuberant, persistent fibroblast activation and matrix synthesis result in progressive lung fibrosis and loss of function. Fibrosis in IPF appears to predominantly expand the interstitial compartment, but the aberrant repair processes driving IPF progression, and the fibrosis they produce, may occur in both the interstitium and the airspaces. Although the synthetically active fibroblasts in fibroblastic foci are typically covered by poorly adherent hyperplastic alveolar epithelium in IPF lungs, immunohistochemical and electron microscopy studies indicate that these foci are frequently located distal to the original alveolar basal laminae or their remnants (7, 8). When followed by alveolar collapse and the coalescence of alveolar septal walls (Figure 1), airspace fibrosis can produce the thickened fibrotic bands characteristic of IPF. For purposes of figure clarity, we have depicted the development of fibrosis in the airspaces in Figure 1, but the same processes can occur in the interstitium.
Figure 1.
Investigational therapies for idiopathic pulmonary fibrosis (IPF): targeting aberrant responses to injury. This schematic indicates the sequential profibrotic processes implicated in the currently prevailing paradigm of IPF pathogenesis, in which recurrent or persistent injury to the alveolar epithelium is believed to drive aberrant wound-healing responses, resulting in fibrosis rather than repair. Drug candidates evaluated in recently completed or ongoing phase II and III clinical trials in IPF are placed in the context of the profibrotic process(es) they are believed to target. Although fibrosis in IPF appears to predominantly expand the interstitial compartment, the aberrant repair processes driving IPF progression, and the fibrosis they produce, may occur in both the interstitium and the airspaces. For purposes of figure clarity, we have depicted the development of fibrosis in the airspaces in this figure. NAC = N-acetylcysteine. Inspired by Reference 6.
Epithelial Cell Injury and Vulnerability to Injury due to Aging and Genetics
The consequences of AEC injury in IPF are manifest as increased AEC death and phenotypic alterations of surviving cells that may reflect a “reprogramming” of these cells that predisposes them to further injury and to promote abnormal repair (9–12). The causes of AEC injury in IPF remain to be identified, but viral infections, gastroesophageal reflux, cigarette smoke, inhaled particulates, or other environmental exposures may contribute (6, 13, 14). IPF occurs in only a small fraction of persons subject to such common injurious stimuli, however, suggesting that these exposures may only cause fibrosis in individuals who are particularly vulnerable (15), possibly due to aging and/or genetic predisposition. Multiple hallmarks of aging have been identified, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (16), each of which may plausibly contribute to alveolar epithelial vulnerability to injury (17). As recently pointed out, many of these same hallmarks of aging could contribute to the development of chronic obstructive pulmonary disease rather than IPF in older persons’ lungs that are injured by smoking, suggesting that genetic mutations and/or epigenetic modifications that augment alveolar epithelial injury may also be needed for IPF development (18). Mutations causing misfolding of surfactant protein C and A2 have been identified in kindreds with familial pulmonary fibrosis and persons with sporadic IPF (19–22). Accumulation of these misfolded proteins in the endoplasmic reticulum of AECs can produce endoplasmic reticulum stress, making AECs more susceptible to apoptosis when exposed to other insults, such as viral infections (23). Mutations in the genes encoding the two principle components of telomerase, the telomerase reverse transcriptase (TERT) and the telomerase RNA component (TERC), have also been identified in familial and sporadic pulmonary fibrosis (24–26). Loss of telomerase function accelerates the telomere shortening that occurs with aging, and telomere erosion triggers DNA damage responses leading to cell senescence or apoptosis (27).
Vascular Leak and Extravascular Coagulation
The early phases of wound healing after tissue injury are characterized by increased vascular permeability (28). Extravascular coagulation produced by the resulting extravasation of clotting factors into tissues provides a provisional wound matrix of fibrin, fibronectin, and platelets, into which inflammatory cells, new blood vessels, and fibroblasts migrate (28). Consistent with the occurrence of repetitive lung injury in patients with IPF, alveolar-capillary permeability is abnormally increased in the lungs within IPF and predicts disease progression and mortality (29, 30). Potentially consistent with a role for extravascular coagulation in the pathogenesis of IPF, the presence of a prothrombotic state, defined as the presence of an inherited or acquired mechanism of hypercoagulability or a marker of ongoing fibrinolysis, adversely affects survival in patients with IPF (31). Additionally, this type of profibrotic state is more than four times more common in patients with IPF than in matched control subjects (31). Intraalveolar fibrin deposition does not itself appear to be required for pulmonary fibrosis, at least in animal models, because fibrinogen-null mice develop lung fibrosis after bleomycin lung injury (32, 33). Coagulation proteases, including thrombin and Factor Xa, appear to have important profibrotic activities independent of their fibrin-generating ability, however, through the activation of proteinase-activated receptors on epithelial cells and fibroblasts. Proteinase-activated receptor signaling promotes lung fibrosis in animal models (34–36) by inducing activation of latent transforming growth factor-β (TGF-β) and the expression of other profibrotic mediators (35, 37, 38), and may provide a mechanistic link between vascular leak, extravascular coagulation, and fibrosis in IPF.
Immune System Activation
After injury, activation of the innate immune system by danger-associated molecular patterns produced by dead or dying cells is well recognized (39), and macrophages, which are central to innate immune responses, have well-established roles in wound healing (40). Different classes of macrophages have functions that would be expected to be pro- or antifibrotic in IPF. Alternatively activated M2a-like macrophages have several functions that would be expected to promote IPF progression (41–43). M2a-like macrophages secrete large amounts of profibrotic cytokines and growth factors, including TGF-β, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) 2, insulin-like growth factor–binding protein 5, CCL18, and Galectin-3. In addition, arginase expressed by these cells promotes the production of hydroxyproline, enabling fibroblasts to increase collagen synthesis. Classically activated M1 macrophages can also promote fibrosis by extending tissue injury through reactive oxygen species (ROS) production and by the elaboration of profibrotic cytokines as well (43). In contrast, regulatory macrophages (Mreg/M2c-like macrophages) can promote the resolution of fibrosis through multiple mechanisms, including the production of suppressive cytokines such as IL-10 (43). The pace of IPF progression therefore may be strongly influenced by the prevailing macrophage phenotype(s) present in the lung (41, 42). Whether induced by recognition of danger-associated molecular patterns or pathogen-associated molecular patterns, innate immune activation promotes the development of adaptive immune responses (44). The role of adaptive immunity in pulmonary fibrosis is complex: Th2- and Th17-type immunity may be profibrotic, whereas Th1-type immunity and regulatory T cells may have antifibrotic activities (45). Although glucocorticoid and cytotoxic therapy directed at adaptive immune responses was recently demonstrated to worsen outcomes in patients with IPF (46), beneficial manipulation of adaptive responses in IPF may be possible with more selective strategies.
Fibroblast Activation, Myofibroblast Differentiation, Matrix Accumulation, and Cross-Linking
In wound healing, the accumulation and activation of fibroblasts transforms cellular, edematous “granulation tissue” into paucicellular scar tissue composed largely of dense collagen (28). In pathological fibrosis, these cells similarly are the principle source of collagens and other extracellular matrix components. Fibroblasts in both wound healing and fibrotic diseases characteristically differentiate into myofibroblasts (47), which are distinguished by their acquisition of contractile features of smooth muscle cells, such as the expression of α-smooth muscle actin (48), and are marked by their ability to secrete increased levels of matrix components (49). Myofibroblast differentiation is driven by both biochemical mediators and biomechanical signals (50). Biochemical mediators that induce myofibroblast differentiation are activated or produced by earlier steps in the pathogenesis of fibrosis (Figure 1), including epithelial injury, activation of coagulation proteases, and innate immune activation. Biomechanical signals that induce myofibroblast differentiation are delivered by the increased rigidity, or stiffness, that characterizes fibrotic tissues (51–55). Increased matrix stiffness in fibrosis may result from quantitative differences in matrix components (i.e., increased accumulation of matrix proteins), qualitative differences in matrix components (i.e., accumulation of different matrix proteins), or differences in the structural ordering of matrix components (i.e., increased cross-linking of matrix proteins) (56). The ability of increased matrix stiffness to amplify myofibroblast differentiation, coupled to the ability of myofibroblasts to increase matrix stiffness through collagen synthesis and cross-linking, creates a feed-forward loop that by itself might drive fibrosis progression (55).
Therapeutic Targets in IPF
Many compounds with apparent promise as treatments for IPF have not shown efficacy when evaluated in phase II and III clinical trials (summarized in Table 1), and consequently a great need for effective IPF therapy has remained. Ongoing and recently completed phase II and III clinical trials (Table 2) have focused on drug candidates that target epithelial cell injury and death, or aberrant wound healing responses, including immune system dysregulation, fibroblast accumulation and myofibroblast differentiation, and extracellular matrix deposition and stiffening (Figure 1). Recent positive results of phase III trials of pirfenidone and nintedanib demonstrate that agents targeting the biologic processes that drive fibrosis can be effective in IPF (4, 5). Below we discuss the biologic targets and putative mechanisms of action of drug candidates evaluated in ongoing or recently completed phase II or III trials in IPF, in terms of the aberrant wound healing responses they are believed to address. Developing therapies to target different biologic processes believed to contribute to IPF pathogenesis may be needed to create effective treatment options for all patients with this disease, as IPF likely has significant biological heterogeneity (10, 57, 58) (i.e., different profibrotic pathways may be more or less activated in individual patients with IPF).
Table 1.
Products That Have Failed in Phase II/III Trials in Idiopathic Pulmonary Fibrosis
| Agent | Hypothesized Mechanism of Action | Clinical Trial, Name and clinicaltrials.gov Identifier | Study Design/Sample Size, Planned or Enrolled | Primary Endpoint | Outcome (Reference) |
|---|---|---|---|---|---|
| Warfarin | Anticoagulant | ACE-IPF NCT00957242 | Phase III, randomized, double-blind, placebo-controlled trial; n = 256 | Composite of time to death, hospitalization or ≥10% decline FVC | Terminated due to increased deaths in warfarin arm at interim analysis (134) |
| Bosentan | Dual endothelin-receptor antagonist | BUILD-1 NCT00071461 | Phase III, randomized, double-blind, placebo-controlled trial; n = 158 | Δ 6MWD at 12 mo | No significant difference between groups (135) |
| BUILD-3 NCT00391443 | Phase III, randomized, double-blind, placebo-controlled trial; n = 616 | Time to IPF worsening* or death at 8–32 mo | No significant difference between groups (136) | ||
| Macitentan | Dual endothelin-receptor antagonist | MUSIC NCT00903331 | Phase II, randomized, double-blind, placebo-controlled trial; n = 178 | Δ FVC at 12 mo | No significant difference between groups (137) |
| Ambrisentan | Endothelin A receptor antagonist | ARTEMIS-IPF NCT00768300 | Phase III, randomized, double-blind, placebo-controlled trial; n = 660 | Time to disease progression† or death over 4 yr | Terminated due to futility at interim analysis (138) |
| Interferon (IFN-γ1b) | Immunoregulatory cytokine | NCT00047645 | Phase III, randomized, double-blind, placebo-controlled trial; n = 330 | Progression-free survival‡ over 48 wk | No significant difference between groups (139) |
| INSPIRE NCT00075998 | Phase III, randomized, double-blind, placebo-controlled trial; n = 826 | Overall survival at 90–96 wk | Terminated due to futility at second interim analysis (140) | ||
| Sildenafil | Phosphodiesterase-5 inhibitor | STEP-IPF NCT00517933 | Phase III, randomized, double-blind, placebo-controlled trial; n = 180 | Proportion subjects with ≥20% increase in 6MWD at 12 wk | No significant difference between groups (141) |
| Imatinib mesylate | Tyrosine kinase inhibitor | Imatinib-IPF NCT00131274 | Phase II–III, randomized, double-blind placebo-controlled trial; n = 119 | Time to disease progression§ or death over 96 wk | No significant difference between groups (142) |
| Octreotide | Somatostatin analog | FIBROSAND NCT00463983 | Phase II, open-label study of octreotide only; n = 25 | Treatment failure|| or death over 48 wk | Trend decline in FVC and DlCO compared with historical control subjects (143) |
| Etanercept | TNF-α inhibitor | NCT00063869 | Phase II, randomized, double-blind, placebo-controlled trial; n = 87 | Δ FVC, DlCO% predicted corrected for Hb and P(A–a)O2 at rest at 48 wk | No significant difference between groups (144) |
| Carlumab (CNTO 888) | Anti-CCL2 antibody | NCT00786201 | Phase II, randomized, double-blind, placebo-controlled trial; n = 126 | Δ FVC / 4-wk interval over 52 wk | Terminated due to futility at interim analysis at 24 wk (145) |
| QAX576 | Anti–IL-13 monoclonal antibody | NCT01266135 | Phase II, randomized, double-blind, placebo-controlled trial; n = 60 | Safety, tolerability and Δ FVC at 52 wk | Terminated; no information available |
| CC-930 | JNK inhibitor | NCT01203943 | Phase II, randomized, double-blind, placebo-controlled trial; n = 28 | Type, frequency, severity and relationship of adverse events over 4 wk | Terminated as benefit/ risk profile did not support continuation |
| Prednisone/azathioprine/NAC | Immunosuppression | PANTHER-IPF NCT00650091 | Phase III, randomized, double-blind, placebo-controlled trial; n = 77 on triple therapy vs. n = 78 on placebo | Δ FVC at 60 wk | Interim analysis showed excess numbers of deaths, hospitalizations, and serious adverse events in the triple therapy group (46) |
| NAC | Antioxidant | PANTHER-IPF NCT00650091 | Phase III, randomized, double-blind, placebo-controlled trial; n = 133 on NAC vs. n = 131 on placebo | Δ FVC at 60 wk | No significant difference between groups (64) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ACE-IPF = Anticoagulant Effectiveness in Idiopathic Pulmonary Fibrosis; ARTEMIS-IPF = A Placebo-Controlled Trial of Ambrisentan in Idiopathic Pulmonary Fibrosis; BUILD = Bosentan Use in Interstitial Lung Disease; DlCO = diffusing capacity of the lung for carbon monoxide; FIBROSAND = Treatment of Idiopathic Pulmonary Fibrosis with Long Acting Octreotide; INSPIRE = Effect of Interferon Gamma-1b on Survival in Patients with Idiopathic Pulmonary Fibrosis; IPF = idiopathic pulmonary fibrosis; JNK = c-Jun N-terminal kinase; MUSIC = Macitentan for the Treatment of Idiopathic Pulmonary Fibrosis; NAC = N-acetylcysteine; P(A–a)O2 = alveolar to arterial oxygen pressure difference; PANTHER-IPF = Prednisone, Azathioprine, and N-Acetylcysteine: A Study That Evaluates Response in Idiopathic Pulmonary Fibrosis; STEP-IPF = Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis; TNF = tumor necrosis factor.
Acute exacerbation of IPF or ≥10% decrease in FVC plus ≥15% decrease in absolute DlCO from baseline (confirmed by two tests conducted ≥4 wk apart).
Respiratory hospitalization or categorical decrease in lung function from baseline (≥10% decrease in FVC plus ≥5% decrease in DlCO or a ≥15% decrease in DlCO plus ≥5% decrease in FVC).
Progression defined as ≥10% decrease in FVC or ≥5 mm Hg increase in P(A–a)O2 at rest from baseline (confirmed by a second test 4–14 wk later).
At least 10% decrease in FVC from baseline.
At least 10% decrease in FVC from baseline between consecutive measurements (with a ≥12-wk interval between them).
Table 2.
Ongoing and Recently Completed Phase II/III Trials in Idiopathic Pulmonary Fibrosis
| Agent | Hypothesized Mechanism of Action | Clinical Trial, Name and clinicaltrials.gov Identifier | Study Design/Sample Size, Planned or Enrolled | Primary Endpoint | Status | Estimated Primary Completion |
|---|---|---|---|---|---|---|
| Tralokinumab | IL-13 monoclonal antibody | NCT01629667 | Phase II, randomized, double-blind, dose-ranging, placebo-controlled trial; n = 302 | Δ FVC % predicted at 72 wk | Recruiting | January 2016 |
| Lebrikizumab | IL-13 monoclonal antibody | NCT01872689 | Phase II, randomized, double-blind, placebo-controlled trial; n = 250 | Progression-free survival at 130 wk | Recruiting | August 2016 |
| Pirfenidone | Antifibrotic, antiinflammatory, antioxidant | ASCEND NCT01366209 | Phase III, randomized, double-blind, placebo-controlled trial; n = 555 | Δ FVC % predicted at 52 wk | Completed (5) | N/A |
| Nintedanib | Tyrosine kinase inhibitor targeting VEGFR, FGFR, PDGFR | INPULSIS-1 NCT01335464, INPULSIS-2 NCT01335477 | Phase III, randomized, double-blind, placebo-controlled trials; n = 1,066 | Annual rate of decline in FVC over 52 wk | Completed (4) | N/A |
| STX-100 | Integrin αvβ6 monoclonal antibody | NCT01371305 | Phase II, randomized, double-blind, dose-escalation study; n = 32 | Incidence and severity of adverse events | Recruiting | Unknown |
| FG-3019 (FibroGen) | CTGF inhibitor | NCT01262001 | Phase II, open-label, dose-escalation study; n = 42 | Safety and tolerability | Ongoing (fully recruited) | April 2018 |
| NCT01890265 | Phase II, randomized, double-blind, placebo-controlled trial; n = 90 | Δ FVC % predicted at 48 wk | Recruiting | July 2016 | ||
| BMS-986020 | Lysophosphatidic acid receptor antagonist | NCT01766817 | Phase II, randomized, double-blind, placebo-controlled trial; n = 300 | Δ FVC % predicted at 26 wk | Recruiting | April 2016 |
| Simtuzumab | LOXL2 monoclonal antibody | NCT01769196 | Phase II, randomized, double-blind, placebo-controlled trial; n = 500 | Progression-free survival* up to 182 wk | Recruiting | February 2018 |
Definition of abbreviations: ASCEND = Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis; CTGF = connective tissue growth factor; FGFR = fibroblast growth factor receptor; INPULSIS = Efficacy and Safety of BIBF 1120 at High Dose in Idiopathic Pulmonary Fibrosis Patients; PDGFR = platelet-derived growth factor receptor; VEGFR = vascular endothelial growth factor receptor.
Progression defined as a categorical decrease in FVC % predicted (>10% relative decrease in FVC or >5% absolute decrease in FVC).
Targeting Lung Injury and Epithelial Cell Death
Antioxidant Therapy
Oxidative stress, the imbalance between a tissue’s burden of ROS and its ability to detoxify ROS or repair the damage they produce, may contribute to the nonresolving lung epithelial injury believed to drive IPF. ROS may promote AEC apoptosis through the mitochondria-mediated “intrinsic” pathways or the death receptor–mediated “extrinsic” pathways (59). Oxidative stress can have profound effects on gene expression. For example, at least some pathways leading to activation of the transcription factor nuclear factor (NF)-κB are ROS dependent, and at least some NF-κB–dependent profibrotic gene expression could therefore be mitigated by antioxidants (60). Lungs of patients with IPF exhibit a high oxidant burden and are deficient in glutathione, the major antioxidant of the lung (61), providing a rationale for augmenting lung antioxidant defenses with N-acetylcysteine (NAC) as a therapeutic strategy in IPF (62). The IFIGENIA (Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual) trial had demonstrated that NAC added to prednisone and azathioprine reduced decline in lung function in patients with IPF compared to treatment with prednisone and azathioprine alone (63). Subsequent evaluation of NAC, azathioprine, and prednisolone compared with placebo in the PANTHER-IPF (Prednisone, Azathioprine, and N-Acetylcysteine: A Study That Evaluates Response in Idiopathic Pulmonary Fibrosis) trial, however, found that this triple combination therapy was associated with a significant increase in mortality, hospitalizations, and serious adverse events, but no differences in lung function (46), leading to early termination of this arm of the trial. The comparison of NAC monotherapy to placebo was completed, but NAC did not demonstrate evidence of benefit (64) (Table 1).
Targeting Immune Activation and Polarization
IL-13 Inhibition
IL-13, the predominant profibrotic cytokine of Th2-type immunity (45), is one of the drivers of profibrotic alternative M2a-like macrophage activation. IL-13 induces macrophage TGF-β expression both directly (65) and indirectly by stimulating macrophage CCL2 (66). In addition to its effects on macrophages, IL-13 directly induces profibrotic gene expression by normal human lung fibroblasts, including CCL2 and IL-6 (67). IL-13 also directly increases fibroblast α-smooth muscle actin and collagen expression, and this effect was dramatically increased in fibroblasts isolated from IPF lungs compared with control lungs (68). IL-13 is overexpressed in patients with IPF (69), and therefore therapies targeting IL-13 could be effective in IPF by inhibiting alternative macrophage activation and TGF-β production as well as by directly inhibiting fibroblast activation and myofibroblast differentiation. Although prior phase II trials of an anti–IL-13 antibody (QAX576) and an anti-CCL2 antibody (CNTO888) were negative (Table 1), two phase II randomized, double-blind, placebo-controlled trials of different monoclonal antibodies against IL-13, tralokinumab (MedImmune, Gaithersburg, MD) and lebrikizumab (Roche, Basel, Switzerland) are recruiting subjects (Table 2).
Targeting Fibroblast Accumulation and Myofibroblast Differentiation
Fibroblasts are the cells most commonly targeted by the therapies currently under evaluation as potential treatments for IPF. These therapies aim to inhibit the processes that contribute to the accumulation of fibroblasts (e.g., their recruitment and proliferation) and/or the differentiation of these cells into myofibroblasts.
Pirfenidone
Pirfenidone (InterMune, Brisbane, CA) has been licensed in multiple countries (but not in the United States) for the treatment of IPF. It has pleiotropic antifibrotic, antiinflammatory, and antioxidant effects in animal and cell-based models (70, 71), but its mechanism of action is unknown. In animal models of lung fibrosis, pirfenidone has been shown to decrease the production of profibrotic cytokines, chemokines, and growth factors, including TGF-β, basic fibroblast growth factor, tumor necrosis factor-α, IL-1β, IL-6, CXCL12, and CCL2 (72, 73). Pirfenidone has also been demonstrated to reduce markers of oxidative stress in animal models, including lipid peroxidation and advanced lipoxidation end products, and superoxide dismutase and myeloperoxidase activity (74, 75). In studies on cells in vitro, pirfenidone has been shown to inhibit multiple profibrotic behaviors of fibroblasts, including their proliferation, differentiation to myofibroblasts, and synthesis of collagen, suggesting that at least some of pirfenidone’s antifibrotic activity is attributable to its actions on these cells (70, 72, 75–77). Four phase III studies of pirfenidone in IPF have now been completed—one in Japan (78), and three multinational trials: CAPACITY (Clinical Studies Assessing Pirfenidone in Idiopathic Pulmonary Fibrosis: Research of Efficacy and Safety Outcomes) 1 and 2 (79), and ASCEND (Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis) (5)—of which three were positive. The Japanese, CAPACITY 2, and ASCEND trials showed reductions in IPF progression with pirfenidone, although CAPACITY 1 did not.
Tyrosine Kinase Inhibition
Many of the mediators believed to drive fibroblast recruitment, proliferation, activation, and collagen synthesis in IPF signal through receptor tyrosine kinases. Nintedanib (formerly known as BIBF 1120; Boehringer Ingelheim, Ingelheim, Germany) is a tyrosine kinase inhibitor that targets the PDGF receptors α/β, FGF receptors 1 to 3, and vascular endothelial growth factor (VEGF) receptors 1 to 3 (80, 81), which have been implicated in the pathogenesis of IPF (82). Although PDGF and FGF drive profibrotic behaviors of fibroblasts, VEGF affects the endothelial compartment. VEGF increases lung vascular permeability (83), which can contribute to fibrosis as described above. VEGF also drives angiogenesis, which is involved in the vessel remodeling that is present in the fibrotic lung. The roles and consequences of dysregulated angiogenesis in pulmonary fibrosis, however, appear to be quite complex and remain to be fully elucidated (84). Nintedanib has been shown to have antifibrotic and antiinflammatory activity in animal models of lung fibrosis and to inhibit the proliferation and differentiation to myofibroblasts of primary human lung fibroblasts in vitro (81). Two multinational phase III studies of nintedanib 150 mg twice daily in IPF (INPULSIS [Efficacy and Safety of BIBF 1120 at High Dose in Idiopathic Pulmonary Fibrosis Patients]-1 and -2) have recently been completed (4), after evidence of efficacy with this dose in the phase II TOMORROW (To Improve Pulmonary Fibrosis with BIBF 1120) trial. Both these trials were positive, showing reductions in IPF progression with nintedanib.
TGF-β Inhibition
The pleiotropic cytokine TGF-β has long been implicated as a central mediator in the pathogenesis of IPF and other fibrotic diseases (85–89). Because TGF-β is also believed to participate in homeostatic functions such as tumor suppression and regulation of inflammation (90), global TGF-β inhibition may have adverse effects, and a more focused approach targeting TGF-β only at sites of progressive fibrosis may be desirable. The activity of this cytokine is primarily regulated by the post-translational activation of latent TGF-β complexes (91), through several mechanisms including interaction with cell-surface integrins (92). The αvβ6 integrin is required for the activation of latent TGF-β during the development of pulmonary fibrosis, and genetic deficiency or antibody inhibition of this integrin protects against the development of fibrosis in animal models (92–94). αvβ6 expression is minimal in normal lungs and up-regulated specifically at sites of lung injury, suggesting that targeting this integrin produces local blockade of TGF-β activation in IPF lungs without global TGF-β inhibition (93). A humanized monoclonal antibody against αvβ6, STX-100 (Biogen Idec, Cambridge, MA), is being evaluated in a phase II study in patients with IPF (Table 2).
Connective Tissue Growth Factor Inhibition
Connective tissue growth factor (CTGF) is a matricellular protein that serves as an adaptor molecule connecting cell surfaces and extracellular matrix. As such, CTGF regulates fibroblast behaviors that are directly dependent on their attachment to the matrix, such as migration, as well as their proliferation, differentiation, matrix production, and apoptosis (95). CTGF is minimally expressed in adult tissues under homeostatic conditions but is strongly up-regulated in tissues subjected to mechanical stresses (96, 97), which are believed to be present in fibrotic tissues (55). Activation of fibroblasts by TGF-β also induces CTGF expression, and CTGF appears responsible for at least some of TGF-β’s effects on these cells (98). There is increased CTGF expression in the lung tissue and bronchoalveolar lavage (BAL) fluid of patients with IPF, and tissue expression localizes to proliferating type II alveolar epithelial cells and activated fibroblasts (99, 100). The efficacy of a CTGF-neutralizing antibody, FG-3019 (FibroGen, San Francisco, CA), is being evaluated in a phase II, randomized, double-blind, placebo-controlled trial (Table 2).
Lysophosphatidic Acid Receptor-1 Inhibition
Lysophosphatidic acid (LPA) is a bioactive lipid mediator that promotes multiple wound-healing responses that are believed to contribute to pulmonary fibrosis, including fibroblast migration, activation, and persistence, as well as epithelial cell apoptosis and loss of endothelial cell barrier function (101). LPA signals through multiple specific receptors, several of which may be involved in its profibrotic effects. Targeting LPA’s signaling specifically through LPA1 has been shown to confer significant protection in animal models of multiple fibrotic diseases, including pulmonary fibrosis (102–105). LPA levels are increased in BAL fluid from patients with IPF, and LPA-LPA1 signaling appears to be responsible for most of the fibroblast chemoattractant activity of BAL fluid (102). An LPA1 receptor antagonist, BMS-986020 (Bristol-Myers Squibb, New York City, NY), is being evaluated in a phase II trial in IPF (Table 2).
Targeting the Matrix
Lysyl Oxidase-like 2 Inhibition
In addition to increases in their quantity or changes in their composition, the increased cross-linking of matrix proteins could contribute to the pathologically increased matrix stiffness present in fibrotic diseases, including IPF (50). This increase in matrix stiffness promotes myofibroblast differentiation and matrix production, further driving fibrosis progression. Matrix protein cross-linking can be catalyzed by a series of enzymes including lysyl oxidases, transglutaminases, and prolyl hydroxylases (106). Expression of the cross-linking enzyme lysyl oxidase-like 2 (LOXL2) is increased in the lungs of patients with IPF (107), and simtuzumab (Gilead, Foster City, CA), a monoclonal antibody directed against LOXL2, is being evaluated in a phase II trial in IPF (Table 2).
Comorbidities in the Context of IPF Pathogenesis
Pending U.S. Food and Drug Administration approval of drugs for IPF, the care of patients with this disease in the United States focuses on supportive care, lung transplantation for patients who are candidates, and the treatment of comorbidities. Several comorbidities that are frequently associated with IPF, including gastroesophageal reflux disease (GERD), obstructive sleep apnea (OSA), and pulmonary hypertension (PH) (108–110), can also be understood in the context of the recurrent injury and aberrant repair paradigm of IPF pathogenesis, in terms of contributing to and/or resulting from recurrent injury to the alveolar epithelium.
GERD is frequently present in patients with IPF, with a prospective study finding reflux in 87% of patients (111). Although it is possible that GERD may occur in patients with IPF as a consequence of decreases in intrathoracic pressure, microaspiration of refluxed gastric acid may be a cause of repetitive lung injury. Evidence consistent with GERD having a causal or exacerbating role in fibrosis in at least some patients with IPF includes correlation between fibrosis and reflux severity (112), increased levels of pepsin in the BAL of patients with an acute exacerbation of IPF (113), several case series demonstrating that medical or surgical treatment of GERD is associated with stabilization or even improvement of lung function in patients with IPF (114–116), and a retrospective analysis showing that lung function of patients with IPF taking anti-GERD therapy declines more slowly than that of patients not taking antireflux medications (117).
OSA is also prevalent in patients with IPF, being present, for example, in 88% in a representative sample of 50 patients from the IPF clinic population at a major U.S. center. In this population, body mass index did not correlate strongly with OSA severity (118). Rather, the severity of OSA in patients with IPF has been correlated with their degree of lung restriction (119). Whether mechanistic links exist between OSA and IPF is unknown, but hypothetical connections include: (1) repetitive forced inspirations against a closed glottis in OSA leading to excessive alveolar stretch and injury (120, 121), and/or (2) cyclic hypoxia-reoxygenation during OSA leading to increased oxidative stress (121). Nocturnal oxygen desaturation has been associated with decreased survival in IPF (122), but the effects of treating OSA on IPF prognosis have not yet been determined.
PH is estimated to be present in one-third to one-half of patients with advanced IPF when evaluated for lung transplantation (123, 124) and has been associated with increased morbidity and mortality (124, 125). Although regional hypoxia due to destruction of lung parenchymal architecture may contribute to PH in IPF through hypoxic vasoconstriction, many of the biological processes believed to drive IPF may similarly drive PH (126, 127). The increased oxidative stress present in the IPF lung may induce endothelial cell as well as AEC apoptosis. Apoptotic endothelial cells in turn may release growth factors active on vascular smooth muscle cells, augmenting pulmonary artery muscularization. Mediators contributing to the progression of fibrosis in the surrounding lung, including TGF-β, PDGF, FGF-2, and angiotensin II, could contribute to pathological changes in all three vascular layers of pulmonary arteries, including intima proliferation and fibrosis, media thickening, and adventitia fibrosis (127). Therapies inhibiting these mediators may be able to concurrently target both lung fibrosis and PH.
Conclusions
IPF remains a disease with a poor prognosis and an incompletely understood pathogenesis. However, recent progress in unraveling IPF pathogenesis has led to the identification of specific biological processes and mediators that can serve as rational targets for drug therapies. As we have reviewed, multiple drug candidates based on the prevailing paradigm of IPF pathogenesis have recently been or are currently being evaluated in phase II and III clinical trials. The recent positive results of the pirfenidone and nintedanib phase III trials demonstrate that agents targeting the biologic processes that drive fibrosis can reduce the progression of IPF. Many other therapies are in earlier phases of development for IPF, and the rationales for many if not most of these can similarly be understood in terms of the prevailing paradigm of IPF pathogenesis (i.e., in terms of the abilities of these therapies to reduce epithelial cell injury, or reduce one or more of the aberrant repair processes induced by that injury). For example, to reduce epithelial injury, drugs are being developed to inhibit one of the key sources of epithelial cell-injuring ROS in IPF, the ROS-generating nicotinamide adenine dinucleotide phosphate reduced oxidase (NOX) enzymes, in particular NOX4 (128–130). Another example of a therapeutic strategy to selectively manipulate immune activation in IPF that is at an early stage of development, having recently completed a phase I study, is recombinant Pentraxin-2 (PRM-151; Promedior, Lexington, MA), which promotes monocyte differentiation into Mreg/M2c-like macrophages while inhibiting M2a-like or M1 macrophage differentiation (131). The rationale for cell-based therapies being explored for IPF may also be understood in terms of their effects on epithelial injury and aberrant repair. For example, human bone marrow–derived mesenchymal stem cells may be found to have beneficial effects in IPF due to their ability to facilitate lung epithelial cell wound closure (132, 133).
The prevailing paradigm of IPF pathogenesis can thus provide IPF researchers, caregivers, and patients with this disease a framework for understanding new therapies being developed and evaluated for IPF. We believe that the positive results already produced in the pirfenidone and nintedanib trials, coupled to the strong biological rationales for drug candidates in ongoing trials or at earlier phases of development, give strong grounds for optimism that new IPF therapies will improve the outlook for patients with this devastating disease.
Footnotes
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants T32HL116275 (N.A.), K08HL105656 (B.S.S.), R01HL095732, and R01HL108975 (A.M.T.), and a grant from the Nirenberg Center for Advanced Lung Disease and the Scleroderma Research Foundation (A.M.T.). Medical writing assistance, supported financially by Boehringer Ingelheim Pharmaceuticals, Inc., was provided by Wendy Morris of Fleishman-Hillard Group, Ltd during the preparation of this manuscript.
Author Contributions: N.A., B.S.S., and A.M.T. wrote the manuscript.
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version of the review, which reflects the authors’ interpretation and conclusions. Boehringer Ingelheim Pharmaceuticals, Inc., was given the opportunity to check the data used in the review article for factual accuracy only.
Originally Published in Press as DOI: 10.1164/rccm.201403-0509PP on August 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60:588–594. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn C, III, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 8.Basset F, Ferrans VJ, Soler P, Takemura T, Fukuda Y, Crystal RG. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986;122:443–461. [PMC free article] [PubMed] [Google Scholar]
- 9.Borok Z. Alveolar epithelium: beyond the barrier. Am J Respir Cell Mol Biol. 2014;50:853–856. doi: 10.1165/rcmb.2014-0089PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, et al. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med. 2014;189:214–222. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:911–921. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 13.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake GW, Schwarz MI. Does current knowledge explain the pathogenesis of idiopathic pulmonary fibrosis? A perspective. Proc Am Thorac Soc. 2007;4:449–452. doi: 10.1513/pats.200702-036MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thannickal VJ. Mechanistic links between aging and lung fibrosis. Biogerontology. 2013;14:609–615. doi: 10.1007/s10522-013-9451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med. 2014;189:1161–1172. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 19.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 20.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, van Es HW, van den Bosch JM, Grutters JC. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 23.Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta. 2013;1832:940–947. doi: 10.1016/j.bbadis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 25.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, Taniguchi H, Kubo M, Kamatani N, Nakamura Y Pirfenidone Clinical Study Group. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45:654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 27.Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. doi: 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 29.Mogulkoc N, Brutsche MH, Bishop PW, Murby B, Greaves MS, Horrocks AW, Wilson M, McCullough C, Prescott M, Egan JJ Greater Manchester Pulmonary Fibrosis Consortium. Pulmonary (99m)Tc-DTPA aerosol clearance and survival in usual interstitial pneumonia (UIP) Thorax. 2001;56:916–923. doi: 10.1136/thorax.56.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J. 2009;33:77–84. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 31.Navaratnam V, Fogarty AW, McKeever T, Thompson N, Jenkins G, Johnson SR, Dolan G, Kumaran M, Pointon K, Hubbard RB. Presence of a prothrombotic state in people with idiopathic pulmonary fibrosis: a population-based case-control study. Thorax. 2014;69:207–215. doi: 10.1136/thoraxjnl-2013-203740. [DOI] [PubMed] [Google Scholar]
- 32.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ploplis VA, Wilberding J, McLennan L, Liang Z, Cornelissen I, DeFord ME, Rosen ED, Castellino FJ. A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am J Pathol. 2000;157:703–708. doi: 10.1016/S0002-9440(10)64582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogatkevich GS, Ludwicka-Bradley A, Nietert PJ, Akter T, van Ryn J, Silver RM. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 2011;63:1416–1425. doi: 10.1002/art.30255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers RC, Laurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans. 2002;30:194–200. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 40.Stefater JA, III, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 45.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 48.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 49.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 50.Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol. 2013;25:92–100. doi: 10.1097/BOR.0b013e32835b13cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 52.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 53.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 57.du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 2010;9:129–140. doi: 10.1038/nrd2958. [DOI] [PubMed] [Google Scholar]
- 58.Maher TM. Beyond the diagnosis of idiopathic pulmonary fibrosis; the growing role of systems biology and stratified medicine. Curr Opin Pulm Med. 2013;19:460–465. doi: 10.1097/MCP.0b013e328363f4b7. [DOI] [PubMed] [Google Scholar]
- 59.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 62.Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2285–2287. doi: 10.1056/NEJMe058210. [DOI] [PubMed] [Google Scholar]
- 63.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 64.Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G Idiopathic Pulmonary Fibrosis Clinical Research Network. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2002;168:2953–2962. doi: 10.4049/jimmunol.168.6.2953. [DOI] [PubMed] [Google Scholar]
- 67.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol. 2001;25:474–485. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- 68.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff HL, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–2182. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 70.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc) 2010;46:473–482. doi: 10.1358/dot.2010.46.7.1488336. [DOI] [PubMed] [Google Scholar]
- 72.Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 73.Inomata M, Kamio K, Azuma A, Matsuda K, Kokuho N, Miura Y, Hayashi H, Nei T, Fujita K, Saito Y, et al. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir Res. 2014;15:16. doi: 10.1186/1465-9921-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289:211–218. [PubMed] [Google Scholar]
- 75.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125:779–785. [PubMed] [Google Scholar]
- 76.Hirano A, Kanehiro A, Ono K, Ito W, Yoshida A, Okada C, Nakashima H, Tanimoto Y, Kataoka M, Gelfand EW, et al. Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge. Am J Respir Cell Mol Biol. 2006;35:366–377. doi: 10.1165/rcmb.2005-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–373. [PubMed] [Google Scholar]
- 78.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, et al. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 79.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 80.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 81.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 82.Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res. 2002;3:13. doi: 10.1186/rr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 84.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest. 2012;142:200–207. doi: 10.1378/chest.11-1962. [DOI] [PubMed] [Google Scholar]
- 85.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 86.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 87.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 90.Pardali K, Moustakas A. Actions of tgf-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 92.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 93.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 94.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 96.Chaqour B, Whitbeck C, Han JS, Macarak E, Horan P, Chichester P, Levin R. Cyr61 and CTGF are molecular markers of bladder wall remodeling after outlet obstruction. Am J Physiol Endocrinol Metab. 2002;283:E765–E774. doi: 10.1152/ajpendo.00131.2002. [DOI] [PubMed] [Google Scholar]
- 97.Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 98.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 99.Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999;21:693–700. doi: 10.1165/ajrcmb.21.6.3719. [DOI] [PubMed] [Google Scholar]
- 100.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 101.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc. 2012;9:102–110. doi: 10.1513/pats.201201-005AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 103.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pradère JP, Klein J, Grès S, Guigné C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18:3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 106.J Kolb MR, Gauldie J. Idiopathic pulmonary fibrosis: the matrix is the message. Am J Respir Crit Care Med. 2011;184:627–629. doi: 10.1164/rccm.201107-1282ED. [DOI] [PubMed] [Google Scholar]
- 107.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 108.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 109.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, King TE, Jr, Collard HR. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pihtili A, Bingol Z, Kiyan E, Cuhadaroglu C, Issever H, Gulbaran Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013;17:1281–1288. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 111.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, II, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 112.Savarino E, Carbone R, Marabotto E, Furnari M, Sconfienza L, Ghio M, Zentilin P, Savarino V. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur Respir J. 2013;42:1322–1331. doi: 10.1183/09031936.00101212. [DOI] [PubMed] [Google Scholar]
- 113.Lee JS, Song JW, Wolters PJ, Elicker BM, King TE, Jr, Kim DS, Collard HR. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129:794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 115.Linden PA, Gilbert RJ, Yeap BY, Boyle K, Deykin A, Jaklitsch MT, Sugarbaker DJ, Bueno R. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 116.Hoppo T, Jarido V, Pennathur A, Morrell M, Crespo M, Shigemura N, Bermudez C, Hunter JG, Toyoda Y, Pilewski J, et al. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146:1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 117.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mermigkis C, Stagaki E, Tryfon S, Schiza S, Amfilochiou A, Polychronopoulos V, Panagou P, Galanis N, Kallianos A, Mermigkis D, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14:387–390. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 120.Toumpanakis D, Kastis GA, Zacharatos P, Sigala I, Michailidou T, Kouvela M, Glynos C, Divangahi M, Roussos C, Theocharis SE, et al. Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med. 2010;182:1129–1136. doi: 10.1164/rccm.201001-0116OC. [DOI] [PubMed] [Google Scholar]
- 121.Lederer DJ, Jelic S, Bhattacharya J, Basner RC.Is obstructive sleep apnea a cause of idiopathic pulmonary fibrosis? Arch Pathol Lab Med 2012136470author reply 470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kolilekas L, Manali E, Vlami KA, Lyberopoulos P, Triantafillidou C, Kagouridis K, Baou K, Gyftopoulos S, Vougas KN, Karakatsani A, et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9:593–601. doi: 10.5664/jcsm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 124.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 125.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 126.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 127.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 128.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ.Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance Sci Transl Med 20146231ra247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dillingh MR, van den Blink B, Moerland M, van Dongen MG, Levi M, Kleinjan A, Wijsenbeek MS, Lupher ML, Jr, Harper DM, Getsy JA, et al. Recombinant human serum amyloid P in healthy volunteers and patients with pulmonary fibrosis. Pulm Pharmacol Ther. 2013;26:672–676. doi: 10.1016/j.pupt.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Mora AL, Rojas M. Adult stem cells for chronic lung diseases. Respirology. 2013;18:1041–1046. doi: 10.1111/resp.12112. [DOI] [PubMed] [Google Scholar]
- 133.Akram KM, Samad S, Spiteri MA, Forsyth NR. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res. 2013;14:9. doi: 10.1186/1465-9921-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, Kaner RJ, Olman MA Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet) A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stähler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 136.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 137.Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J MUSIC Study Group. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42:1622–1632. doi: 10.1183/09031936.00104612. [DOI] [PubMed] [Google Scholar]
- 138.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, et al. ARTEMIS-IPF Investigators. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 139.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE, Jr Idiopathic Pulmonary Fibrosis Study Group. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 140.King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, et al. INSPIRE Study Group. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 141.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR Imatinib-IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 143.Crestani B, Chapron J, Wallaert B, Bergot E, Delaval P, Israel-Biet D, Lacronique J, Monnet I, Reynaud-Gaubert M, Tazi A, et al. Octreotide treatment of idiopathic pulmonary fibrosis: a proof-of-concept study. Eur Respir J. 2012;39:772–775. doi: 10.1183/09031936.00113011. [DOI] [PubMed] [Google Scholar]
- 144.Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, Thomeer M, Utz JP, Khandker RK, McDermott L, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 145.Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Well AU, Lancaster L, Gibson KF, Haddad T, Agarwal P, et al. A phase II, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study of the safety and efficacy of CNTO 888 (carlumab) in patients with idiopathic pulmonary fibrosis [abstract] Am J Respir Crit Care Med. 2013;187:A3376. [Google Scholar]